|
Size: 17609
Comment:
|
← Revision 13 as of 2014-12-02 09:56:06 ⇥
Size: 17596
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 31: | Line 31: |
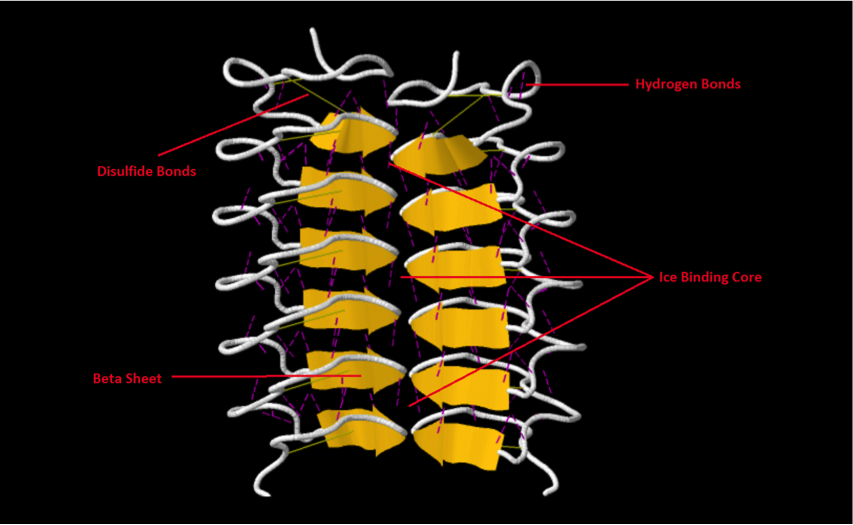
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image.png|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''AFP of Tenebrio Molitor beetle (Protein Data Bank)'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image.png|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''AFP of Tenebrio Molitor beetle (Protein Data Bank)'' || |
| Line 37: | Line 37: |
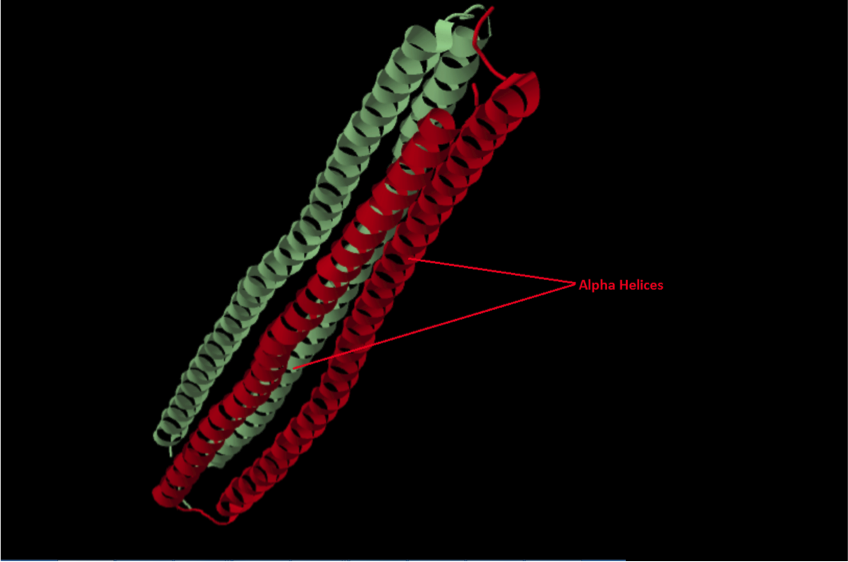
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image1.png|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''AFP of winter flounder (Protein Data Bank)'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image1.png|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''AFP of winter flounder (Protein Data Bank)'' || |
| Line 44: | Line 44: |
| Antifreeze proteins (AFPs) function via an adsorption-inhibition mechanism (DeLuca et al.,1998). The protein itself binds (adsorbs) to the surface of ice crystals and from there has an influence upon the ice growth; they achieve this by their thermal hysteresis activity (Dolev et al., 2013). | Antifreeze proteins (AFPs) function via an adsorption-inhibition mechanism (!DeLuca et al.,1998). The protein itself binds (adsorbs) to the surface of ice crystals and from there has an influence upon the ice growth; they achieve this by their thermal hysteresis activity (Dolev et al., 2013). |
| Line 51: | Line 51: |
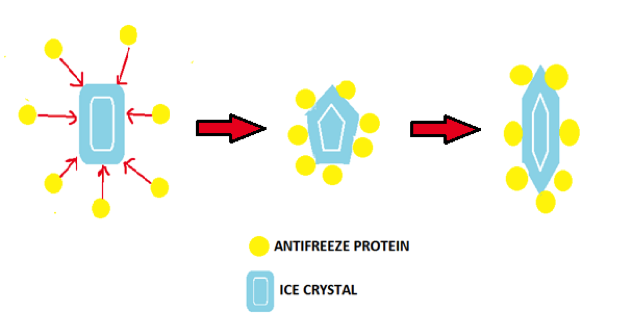
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image2.png|pop-up text}} <<BR>>'''Fig 3.'''<<BR>>''How AFPs depress ice formation by altering the shape of ice crystals'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image2.png|pop-up text}} <<BR>>'''Fig 3.'''<<BR>>''How AFPs depress ice formation by altering the shape of ice crystals'' || |
| Line 67: | Line 67: |
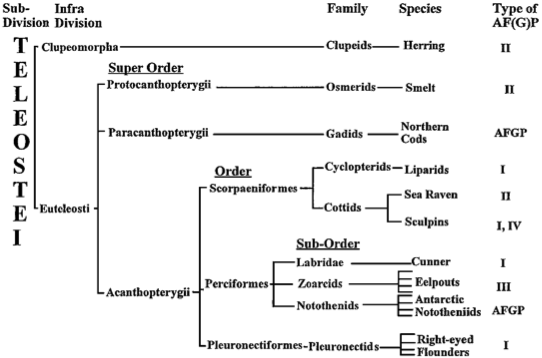
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image3.png|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''Taxonomic distribution of AFP types (Fletcher et al., 2001)'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:image3.png|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''Taxonomic distribution of AFP types (Fletcher et al., 2001)'' || |
| Line 84: | Line 84: |
| DeLuca, C., Comley, R & Davies, P. (1998). Antifreeze Proteins Bind Independently to ice. ''Biophysical Journal'', 74: (3) 1502-1508.143 | !DeLuca, C., Comley, R & Davies, P. (1998). Antifreeze Proteins Bind Independently to ice. ''Biophysical Journal'', 74: (3) 1502-1508.143 |
| Line 106: | Line 106: |
| Figure 1 - PDB ID: 1EZG Liou, Y.C., Tocilj, A., Davies, P.L., Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. |
Figure 1 - PDB ID: 1EZG |
| Line 109: | Line 108: |
| . Liou, Y.C., Tocilj, A., Davies, P.L., Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. | |
| Line 110: | Line 110: |
| Figure 2 - PDB ID: 4KE2 | |
| Line 111: | Line 112: |
| Figure 2 - PDB ID: 4KE2 Sun, T., Lin, F.H., Campbell, R.L., Allingham, J.S., Davies, P.L. An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters. |
. Sun, T., Lin, F.H., Campbell, R.L., Allingham, J.S., Davies, P.L. An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters. |
The Effects of Antifreeze Proteins
This essay discusses a specific type of ice binding protein known as antifreeze proteins. Antifreeze proteins play an important role in the life of many species of fish, arthropods and plants by reducing the freezing point and preventing ice formation during acclimatization.
Contents
General Overview
Antifreeze proteins are a subtype of ice binding proteins (IBPs). IBPs are proteins that directly interact with the surface of ice, inhibiting its crystallisation by adhesion mechanisms and also by altering the freezing point (Drori et al., 2014).
IBPs are found in various locations that require some kind of influence over the growth of ice. They are found frequently in nature, enabling the survival of many organisms that live in sub-zero temperatures. In this context they are found in so called Antifreeze Proteins (AFPs). The AFPs possess hydrophilic side chains which present ice binding surfaces (IBS), which adsorb water molecules on the surface of ice and prevent them from freezing and therefore growing (Yang et al., 1998).
Developing resistance to freezing via these antifreeze proteins has been vital for the survival of many species. The freezing point of water is 0 degrees Celsius; once environmental temperature drops to this it causes water to become ice crystals and these ice crystals then form hydrogen bonds between each other resulting in a solid ice lattice. IBPs and in particular AFPs are key to the survival of organisms in lower climates, as water is the most important component of the inner environment of the body. The water content of blood is 90%, liver, muscles and brain 70-75%, bones 25% and fat 10%. If certain animals living in cold habitats did not possess AFPs the water content of their bodies would freeze, resulting in the demise of most, if not all of their vital bodily functions due to a complete upset of their homeostasis and ultimately this would lead to their death.
This is the main field of ice binding proteins that will be the focus of this essay, however, the use of ice binding proteins can also be found in agriculture to produce freeze proof crops, medicine and industry (Dolev et al., 2013).
Structure Of Antifreeze Proteins
AFPs like all proteins are composed of primary, secondary, tertiary and quaternary structures. It’s important to understand what these terms are before delving into the types and structures of the AFP macromolecule.
Primary structures of any protein consist of amino acids (AA). These are the building blocks of proteins and there are 20 different types. They all contain the common chemical backbone HOOCCH(NH2)R. The R group is the side chain that differentiates them, and forms various groups based on their chemical structure. The linear sequence of AA is what a primary structure is, and each AA is attached to its neighbour via peptide bonds (N terminus and C terminus of opposing AA form the covalent peptide bond). A polypeptide can range from 50 – 2000+ AA residues all linked together.
The secondary structure is based on the primary one. It involves the polypeptide chain forming regular structures known as alpha helices and beta pleated sheets. The alpha helix is comparable to a spiral staircase and with each turn there is a point where one of the AA residues will be closer to the AA directly below it than any other AA in the same turn of the helix; at this point there is hydrogen bonding which is the main force of stabilisation in secondary structures. The hydrogen bonding is between the carbonyl and the amide group. The beta pleated sheet is different segments of the primary structure lying parallel and stabilised once again by hydrogen bonds.
Tertiary structures are just alpha helices and beta sheets folded into a globular structure and quaternary structures are multiple subunits folding together and forming a compact 3 dimensional structure.
AFPs are amphiphilic proteins, which means they contain both hydrophilic and hydrophobic side chains (Yang et al., 1998). Individual AFPs vary in structure depending on the type and context within which they are found, however, the general and rather basic configuration is that they consist of an alpha helix or beta pleated sheet, a hydrophilic side chain and a hydrophobic side chain. The hydrophilic residues within the alpha helices/beta pleated sheets of the AFPs give them a rigid nature, which is a general characteristic of all AFPs (Yang et al.,1998). This means that although there are many forms of AFPs, their ice binding sites all share a similar morphology.
The structure of the Tenebrio molitor beetles AFP is one of the most regularly structured AFPs observed to date. It is an 8.4kDa protein that consists of two identical subunits. Each subunit consists of 12 beta pleated sheets which are composed predominantly of threonine and cysteine residues. The protein has an extensive ice binding site and is reinforced by numerous hydrogen and disulphide bonds (Liu et al., 2000).
|
The AFP of the winter flounder is a four alpha-helical structure with two identical subunits. It is an alanine rich protein and although each of the helixes don’t directly come into contact with each other, the carbonyl backbones retain water and this is what stabilises the protein. These retained water molecules within the protein core then actively take part in ice binding (Sun et al., 2014).
|
Mechanism Of Antifreeze Proteins
Antifreeze proteins (AFPs) function via an adsorption-inhibition mechanism (DeLuca et al.,1998). The protein itself binds (adsorbs) to the surface of ice crystals and from there has an influence upon the ice growth; they achieve this by their thermal hysteresis activity (Dolev et al., 2013).
Thermal hysteresis is a behaviour of a temperature dependent property; and in the case of AFPs results in a lowering of the freezing point and a separation to develop between the freezing and melting point of ice (Lee et al., 2012). It can lower the freezing point of water by up to 5-7 degrees Celsius.
AFPs have both hydrophilic and hydrophobic subunits. The hydrophilic part of the protein is the part that actual ‘sticks’ the protein to the surface of the ice, whereas the hydrophobic part repels the water molecules (Garnham et al., 2011). Despite the hydrophobic region of the protein taking no part in the actual bond between the AFP and the ice, it does significantly support the process. It does this by forcing all the water molecules it comes into contact with into an ‘ice cage’. Here the water molecules are aligned and create a strong connection between the ice and the hydrophilic part of the protein (Garnham et al., 2011).
The variability amongst AFPs is vast in regard to their amino acid sequences and structures. Despite this fact, they all exhibit similar behaviour in regards to binding ice, suggesting that the ice binding sites of the different types of antifreeze proteins are somewhat uniform. From this we can conclude that the behaviour of the protein with regards to ice binding is independent of its overall structure, whether it is primary, secondary or tertiary. Therefore, we can assume the same general mechanism for all antifreeze proteins (Yang et al., 1998).
|
IBPs are classified as either hyperactive or moderate according to their thermal hysteresis activity, which is the gap between their melting and freezing point (Celika et al., 2013). There is an interesting difference between the IBPs of insects as opposed to fish. The IBPs of insects are of the hyperactive nature and are substantially more effective at depressing the freezing point and inhibiting ice growth. Fish on the other hand possess moderate IBPs which only act to lower the freezing point by 1 degree Celsius (Dolev et al., 2013)). Both of these types of IBPs work by shaping ice crystals into different characteristic forms in order to prevent the growth of ice into larger, more dangerous crystals which would have the ability to cause freeze injuries at a cellular level that would ultimately result in death of the organism. Moderate IBPs form bi-pyramidal crystals that are elongated and have well defined faces, while hyperactive IBPs form crystals in varying shapes, such as ‘lemon like’ crystals, which are characteristic of the insect Tenebrio molitor (yellow mealworm beetle) (Dolev et al., 2013).
Occurrence In Nature
Antifreeze proteins have been extensively researched in marine teleosts. Additionally, AFPs have been found in many terrestrial arthropods including spiders, mites, centipedes and insects as well as in algae and certain plant species (Dunam et al., 2004).
Some species of teleosts live at higher latitudes where they often encounter ice-laden waters that can be lower than the freezing point of their body fluids. In order to avoid freezing these fish produce AFPs to adsorb ice and halt its growth (Fletcher et al., 2001). The liver as well as the skin, scales, fins and gills are all responsible for secreting AFPs. In teleosts the secretion of AFPs is controlled by growth hormone, and in certain species follows a seasonal cycle (Fletcher et al., 2001). For example in winter flounder, levels of AFP secretion increase during the winter season before the onset of freezing levels in the water (Fletcher et al., 2001). Interestingly, winter flounder produces significantly more AFPs than its close relative the yellow flounder. Research has not yet been able to show what causes the difference between the winter and yellow flounders AFP secretions; however, as AFPs appear to be a relatively new evolutionary adaptation, environment may be an important factor in the production of AFPs (Fletcher et al., 2001). In the case of winter versus yellow flounder the winter flounder live in much shallower and colder water, which corresponds to their higher AFP production as compared to yellow flounder. Similarly to the winter flounder, shorthorn sculpin, ocean pout and juvenile cod AFPs on a season cycle. Shorthorn sculpin and juvenile cod produce AFPs prior to the onset of winter (Fletcher et al., 2001). Whereas ocean pout produce AFPs all year round but at a higher rate during the winter (Fletcher et al., 2001). Adult cod on the other hand only produces AFPs in response to freezing (Fletcher et al., 2001).
AFP dependent teleosts can also be classified based on the type of AFP they produce. The types of AFPs are classified by structure. Type 1 AFP, produced by flounders and sculpins, was the first type of AFP to have its structure determined (Fletcher et al., 2001). It is classified as an alanine rich, single, long, amphipathic, alpha helix (Ewart et al., 1999). Type 1 is most effective at depressing freezing temperatures than the other types and due to its high alanine content and internal salt bridges makes it extremely stable (Fletcher et al., 2001). Type 2, produced by sea ravens, herring and smelt is a cysteine rich globular protein, with many beta sheets (Ewart et al., 1999). Unfortunately there is not much known about the binding site of Type 2, as it is not easily reproduced. Type 3 is found in eelpouts and contains both alpha helices and beta sheets; it is similar to type 1 in its hydrophobic binding sites (Ewart et al., 1999; Fletcher et al., 2001). Lastly there is Type 4 a helix bundle produced by longhorn sculpins (Fletcher et al., 2001).
The distribution of AFP types among species of teleosts follows no taxonomic relationship, as seen in figure 4 (Fletcher et al., 2001). AFP types appear to be randomly distributed across branches of teleosts as opposed to being confined to one branch. Fletcher et al. (2001), suggest that the perplexing distribution of AFPs could be a result of the relatively new evolutionary need for AFPs combined with the variety of binding properties of ice.
|
In arthropods the AFP structures are rich in Threonine and Cysteine and consist of a repeating sequence (Ewart et al., 1999). A large number of Coleoptera, beetles, have been identified to have AFPs compared to Diptera, Hymenoptera and Lepidoptera (Dunam et al., 2004). However, half of the insects described in the world belong to Coleoptera therefore it is not accurate to say that beetles are more dependent on AFPs than other species.
Arthropods, especially those inhabiting the Arctic, are exposed to extreme overwintering conditions. Dunam et al. (2004), show that compared to fish and plants, arthropods have a greater degree of thermal hysteresis activity. Artic species of arthropods are especially interesting since their overwintering mechanisms can be classified as either freeze avoiding or freeze tolerant. AFPs are mostly present in freeze avoiding arthropods, since AFPs alone are not sufficient in freeze tolerant species that can survive in overwintering conditions up to -70C (Dunam et al., 2004).
Freeze avoiding arthropods depend on AFPs in both larval and mature stages. The freeze avoiding species rely on AFPs to prevent freezing from external ice across the cuticle as well as to prevent ice nucleation in the gut and the hemolymph (Dunam et al., 2004). In the presence of glycerol AFPs, freeze avoiding species are able to completely block ice nucleation by inhibiting ice nucleating bacteria and hemolymph proteins (Dunam et al., 2004).
On the other hand, freeze tolerant species do not rely as heavily on AFPs. Unfortunately the function of AFPs in freeze tolerant species is not yet known. While the inhibition of recrystallization is seen in freeze tolerant species, Dunam et al. (2004) suggest that there are likely additional AFP functions that are yet to be discovered.
References
Celik, Y., Drori, R., Pertaya- Brauna, N. et al.. (2013). Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. PNAS, 110: (4).
DeLuca, C., Comley, R & Davies, P. (1998). Antifreeze Proteins Bind Independently to ice. Biophysical Journal, 74: (3) 1502-1508.143
Dolev, M.B., Liu, J.J., Qin, Y. et al.. 143 Ice shaping in solutions of ice-binding proteins – Melting vs growing morphologies. Cryobiology, 67: (3) 438-439.
Drori, R., Celik, Y., Davies, P. & Braslavsky, I. (2014). Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. Journal of Royal Society Interface, 11: 98.
Dunam, J.G., Bennett, V., Sformo, T., Hochstrasser, R., & Barnes, B.M. (2004). Antifreeze proteins in Alaskan insects and spiders. Journal of Insect Physiology. 50, 259-266
Ewart, K., Lin, Q., & Hew, C. (1999). Structure, function and evolution of antifreeze proteins. Cellular and Molecular Life Sciences , 55, 271-283.
Fletcher, G., Hew, C. L., & Davies, P. (2001). Antifreeze Proteins of Teleost Fishes. Annual Review of Physiology , 63, 359-390.
Garnham, C.P., Campbell, R.L., Davies, P.. (2011). Anchored clathrate waters bind antifreeze proteins to ice. Proceedings of the National Academy of Sciences, 108: (18) 7363-7357.
Lee, J.H., Park, A.K., Do, H., et al.. (2012). Structural basis for antifreeze activity of ice-binding protein from arctic yeast. Journal of Biological Chemistry, 287: (14) 11460-11468.
Sun, T., Lin, F.H., Campbell, R.L., Allingham, J.S., & Davies, P.. (2014). An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters. Science, 343: (6172) 795-798
Yang, D.S., Hon, W.C., Bubanko, S., et al.. (1998). Identification of the Ice-Binding Surface on a Type III Antifreeze Protein with a “Flatness Function” Algorithm. Biophysical Journal, 74: (5) 2142-2151.
Figures
Figure 1 - PDB ID: 1EZG
- Liou, Y.C., Tocilj, A., Davies, P.L., Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein.
Figure 2 - PDB ID: 4KE2
- Sun, T., Lin, F.H., Campbell, R.L., Allingham, J.S., Davies, P.L. An antifreeze protein folds with an interior network of more than 400 semi-clathrate waters.
Figure 3 - Made by Sophie Flint.
Figure 4 - Fletcher, G., Hew, C. L., & Davies, P. (2001). Antifreeze Proteins of Teleost Fishes. Annual Review of Physiology , 63, 359-390.