|
Size: 36855
Comment:
|
← Revision 91 as of 2018-04-29 21:24:38 ⇥
Size: 36854
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 116: | Line 116: |
| No specific breeds are known to be more susceptible to this disease. Prevalence of these diseases depends on the vectors which differ in species and number across the globe. | No specific breeds are known to be more susceptible to this disease. Prevalence of these diseases depend on the vectors which differ in species and number across the globe. |
Itt írjon a(z) BloodDisordersDog-ról/ről
Blood and Bleeding Disorders in Dogs
Contents
Blood and bleeding disorders can be classified into clotting, blood vessel and platelet disorders. These may be acquired, inherited genetically or arise due to mutations in somatic cells, either as a primary disease or as a secondary effect of another disease. The following disorders are discussed in relation to dogs, since they are more frequently encountered in small animal clinics, but are also observed in other species. They may manifest themselves differently and so are treated accordingly.
Clotting Disorders:
Haemophilia type A and B:
Haemophilia type A is a genetic disease, it is an X-linked recessive disorder and is therefore predominant in males (Snyder et al, 1999). It occurs due to the missing factor VIII and subsequent damage to the intrinsic coagulation pathway as well as the extrinsic pathway due to a defect in tissue factor and factor VII. Type B is found in dogs which have their intrinsic coagulation pathway blocked due to them lacking factor IX (Brinkhous et al, 1989). Although both of these can be seen to occur, type A is more frequently found in dogs.
Haemophilia shows abnormally prolonged blood clotting time as well as haemorrhages, which are painful and sometimes fatal. These may be brought out spontaneously or due to trauma, usually in joints such as the hip and the shoulder joints. This may lead to lameness in the puppy (Graham et al, 1949).
A series of tests were run to find a treatment of these two types of haemophilia by adding a sufficient amount of recombinant factor VIIa. It was concluded that in the case of type A, the plasma levels did increase when recombinant factor VIIa was added. It further increased in the case of type B when the same factor was added with comparable amounts to that of type A (Brinkhous et al, 1989). Addition of thromboplastin to the patient’s blood, total blood transfusions or plasma transfusions, for less severe cases, are seen to decrease the effect of haemophilia (Graham et al, 1949).
If untreated, these dogs will die before they reach maturity, only under the aforementioned treatment does the survival rate increase (Graham et al, 1949).
Both types of haemophilia are seen to affect a number of dogs. Less dogs are predisposed to haemophilia type B including Saint Bernard, Bichon Frise and German Shepherd. It is evident that purebreds have a higher, but not exclusive disposition to this disease due to its genetic origin (Dodds, 2011).
Fibrinogenemia:
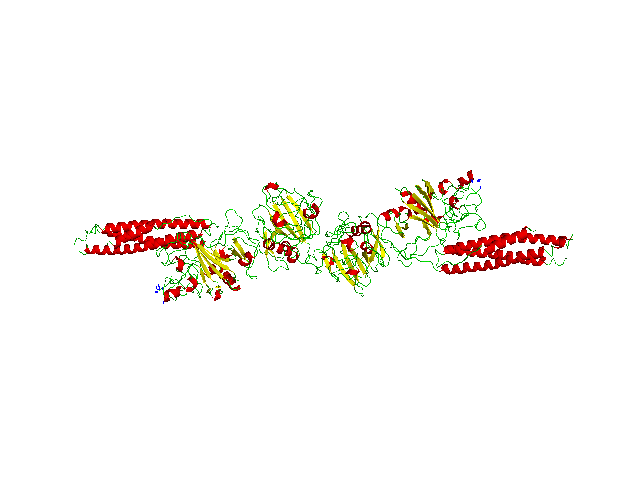
Fibrin (Figure 1) is an important coagulation factor that aids in clot formation and platelet aggregation. Its precursor is fibrinogen and it is transformed with the help of thrombin.
|
Fig.1 Fibrin Structure |
Three irregularities can be defined: Afibrinogenemia - meaning no fibrinogen is present, Hypofibrinogenemia - meaning decreased amount of fibrinogen and Dysfibrinogenemia – meaning the fibrinogen is corrupted. Although present, it occurs less frequently than haemophilia in dogs (Jolivet et al, 2017).
Afibrinogenemia is a genetic autosomal recessive disease. In this case, all fibrinogen is missing not due to lack of production or abnormality but because of an antibody reaction against fibrinogen (Wilkerson et al, 2005). Hypofibrinogenemia may also occur due to a defective liver, as it is the production site of fibrinogen (Jolivet et al, 2017). A/Dysfibrinogenemia is seen through gum bleeding and delayed blood clotting time. Studies show that after blood transfusion, fibrinogen is seen as a foreign material by such dogs’ immune system, causing an autoimmune reaction (Wilkerson et al, 2005).
It was found that Saint Bernard dogs are predisposed to Hypofibrinogenemia while the Borzoi and Bichon Frise breeds show Dysfibrinogenemia (Wilkerson et al, 2005).
Diagnosis and differentiation of Hypofibrinogenemia and Dysfibrinogenemia is done by determination of fibrinogen by coagulometric and non-coagulometric methods. To diagnose primary Hypofibrinogenemia, any other diseases that can affect fibrinogen production, for example liver disease and chronic bleeding, have to be excluded (Jolivet et al, 2017).
No specific treatment is in use yet. Cryoprecipitate (contains fibrinogen, Von Willebrand factor, factor VIII, XIII and fibronectin) is used to manage bleeding, together with plasma transfusion and avoidance of trauma (Jolivet et al, 2017).
Disseminated Intravascular Coagulation (DIC):
Disseminated intravascular coagulation (DIC) disorder is an acquired blood clotting disorder. This results in the activation of coagulation causing thrombosis (Figure 2). Most frequently, fibrinolysis and haemorrhage also forms (Estrin et al, 2006). It can be split into overt (apparent DIC) and non-overt (hidden DIC) types (Ralph and Brainard, 2012). DIC is a secondary disease, meaning it is always caused as a by-product of another disorder or infection (Yu et al, 2015).
|
Fig.2 Thrombosis clogging a blood vessel |
Some of the symptoms seen to occur along with DIC are; Neoplasia, presence of bacteria and toxins in tissues (sepsis), pancreatitis, heatstroke, liver disease, gastric dilatation-volvulus and physical injury (Estrin et al, 2006).
Diagnosis can be difficult especially in cases of non-overt DIC. It takes into consideration pre-existing clinical conditions and abnormalities in thromboplastin activation time, prothrombin activation time, thrombin clot time, lower antithrombin, higher fibrinolysis markers and the occurrence of erythrocyte fragmentation. However, this does not surely identify non-overt cases of DIC. In recent studies, a method is being developed by which platelet activation is accounted for by measuring CD62P and the aggregation of platelets to leukocytes to measure sepsis in dogs which would indicate DIC (Yu et al, 2015).
From research gathered, there is no correlation between disseminated intravascular coagulation and breed of dog, gender or even age.
DIC is often discovered in the later stages of a disease. So, in most cases, the prognosis of the primary diseases which develops is a negative one and thus the DIC is often irreversible. The primary disorder itself is treated and so, the treatment is not specific to DIC. The mortality rate is 50-77% due to it increasing complications of primary disorders (Estrin et al, 2006).
Von Willebrand’s Disease:
Von Willebrand’s disease is a common inherited bleeding disorder caused by the lack or highly reduced concentration of von Willebrand's factor (vWF), a protein in the blood plasma which is essential to blood clotting and haemostasis in the body. All dogs, whether male or female, have 2 vWF genes, one obtained from the mother and the other from the father. It seems that in heterozygous carriers, although the vWF concentration is low, heamostasis occurs at a normal level (Venta et al, 2000). It has been found that even if only one of the genes consists of a mutant allele, the dog will be considered infected. If both genes are abnormal, the severity of the disease increases. There are three types of this disease. The most common form is Type 1 which is characterised by low vWF concentration of normal structure and is inherited in a recessive way in some breeds but in a dominant way with incomplete penetrance in others (Crespi et al, 2018). Type 2 is inherited recessively in dogs and results in low vWF concentration of abnormal structure and Type 3, the most severe form from a clinical point of view, is characterised by the absence or reduced amount of vWF (Venta et al, 2000).
Clinical signs of this disease include haematuria, epistaxis, gingival or genital mucosal bleeding, lameness, and prolonged bleeding from cut nails or wounds and excessive bleeding in the reproductive and intestinal tracts. Uncontrollable bleeding is sometimes observed after surgeries, infections, endocrine disorders and also after certain medications are administered. Concurrent hypothyroidism exacerbates the disease (Dodds, 1984). However, dogs may also have this disease without expressing the bleeding symptoms.
The diagnosis of this disease requires the veterinarian to obtain a blood sample from the patient to run laboratory diagnostics, particularly the vWF antigen assay which measures the amount of vWF in the plasma of the blood. Generally, a dog is considered to be at risk of the disease if the value of vWF in plasma is below 50% and severely affected if it is below 15%. It is also possible to perform a buccal mucosal screening test to determine if the dog exhibits unusual amounts of bleeding.
Unfortunately, there is no known way to induce or increase the production of the vWF so treatment very often involves the control of the bleeding using sutures and bandages. If severe bleeding occurs, the patient must undergo a blood transfusion via IV infusion of fresh whole blood or plasma, with topical use of haemostatic compounds. Care is taken to avoid giving medication that interferes with haemostasis (Dodds, 1984).
This disease infects a number of breeds however the Doberman Pinscher seems to be the most affected breed, where more than 70% of dogs are carriers, diagnosed at the age of four on average. It has been found that they have the mildest form and not all dogs show clinical signs. It is also been found that relatively low concentrations of vWF are found in 28% of Shetland Sheep dogs but it is Scottish Terriers, with the infected rate of 30%, and Chesapeake Bay Retrievers that posses the most severe form of this disease.
Blood Vessel Disorders:
Immune Mediated Haemolytic Anaemia (IMHA):
Immune Mediated Haemolytic Anaemia (IMHA) is amongst the most common types of anaemia (Bugress et al, 2000). It is usually diagnosed in middle aged female dogs, around the age of six. IMHA is especially prevalent in American Cocker Spaniels, English Springer Spaniels Poodles, Old English Sheepdogs, Irish Setters and Collies (Reimer et al, 1999). The disease is caused by the immune-mediated degradation of red blood cells resulting in a rapid decline of the total red blood cell count as a consequence of a type II immune response. IMHA can be classified into two types, Primary (idiopathic) IMHA, commonly known as Autoimmune Haemolytic Anaemia (AIHA) and Secondary disease (Bugress et al, 2000).
AIHA is caused by a spontaneous reaction taking place in the body. In a healthy dog, the immune system’s suppressor T cells prevent the attachment of the auto-antibodies to the antigens. In AIHA, an infected dog’s auto-antibodies bind to its own RBC surface antigens resulting in cell destruction. This is due to malfunctioning suppressor T cells and a surface glycoprotein called Glycophorin which binds the autoantibodies (Barker and Elson, 1995).
On the contrary, Secondary IMHA is a consequence of an underlying disease that activates the body’s immune response to foreign antigens that have attached to RBC or have caused disruption of the RBC membranes. Secondary IMHA has several infectious causes including bacterial, viral, rickettsial, parasitic and fungal infections. Other causes are neoplastic disorders, intrinsic red blood cell defects, venom and blood transfusions. Chemical substances found in onions, rye grass, castor beans, Vitamin K and Heparin may also be the cause. Moreover, studies show that drug administration, including antibiotics such as penicillin, may affect the likeliness of developing IMHA (Mccullough, 2003).
The initial symptoms of IMHA include lethargy, weakness and tachypnea (Archer et al, 2013). Upon clinical examination, observation of pale mucous membranes, tachycardia, splenomegaly, hepatomegaly, icterus, haemoglobinuria and cardiac murmurs are all signs of IMHA (Bugress et al, 2000).
In general, 60-75% of dogs suffer from Primary IMHA (Piek et al, 2008). Treating these dogs involves immunosuppressive treatment by administration of glucocorticoids. In some cases, IMHA can lead to Disseminated Intravascular Coagulation or primary thromboembolism. In such an encounter, anticoagulants heparin and warfarin are administered to prevent thrombus formation. Additional supportive care includes blood transfusions, oxytocin therapy and surgical procedures (Mccullough, 2003). Despite the treatment, studies reported a mortality rate of Primary IMHA ranging between 29%-70% particularly in the first 2 weeks of treatment (Piek et al, 2008). If the disease responsible for the secondary IMHA has not been identified, the dog is likely to be diagnosed as a primary IMHA patient and this has been shown to worsen the condition of the patient (Mccullough, 2013). As regards to prognosis, dogs suffering from IMHA generally have an average mortality of 41% (Reimer et al, 1999).
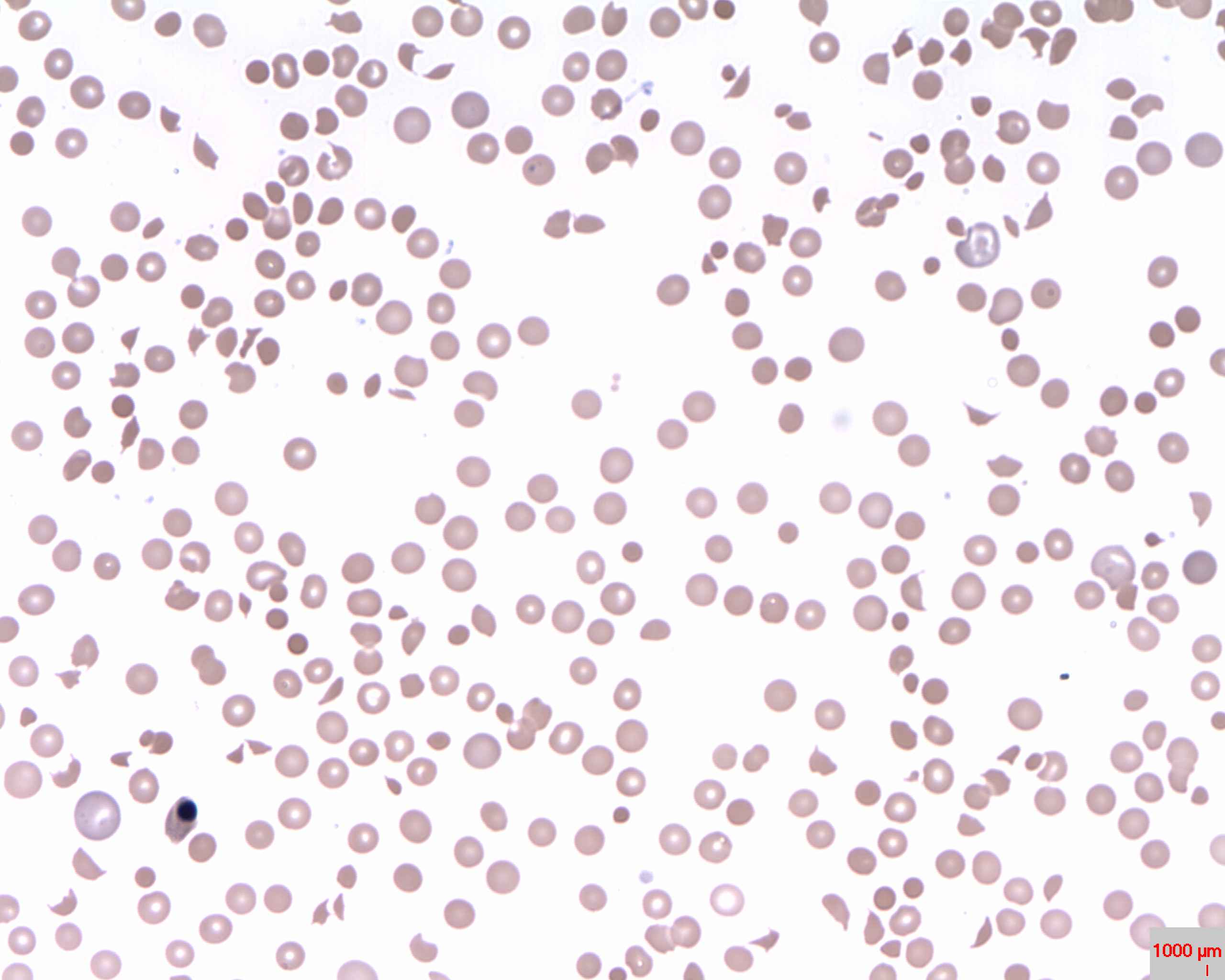
Diagnosing IMHA primarily involves blood testing. A result indicating a haematocrit value of less than 25% and a reticulocyte level higher than 1% is the expected characteristic of anaemia (Bennett et al, 1981). High levels of anti-erythrocyte antibodies, indicated during an auto-agglutination test, and a positive result of a direct Coombs test may confirm the presence of IMHA (Archer et al, 2013). Spherocytosis (Figure 3) is considered to be a determining factor of IMHA identified in 89% to 95% of dogs (Piek et al, 2008). Even though these tests indicate IMHA, they do not distinguish between primary and secondary IMHA. Therefore, diagnostic imaging is used to determine if there is the underlying disease. Further evidence can be shown through a urinalysis which confirms bilirubinuria and haemoglobinuria (Archer et al, 2013).
|
Fig.3 Histological slide of Spherocytes |
Leukopenia:
Leukopenia results from the destruction of haematopoietic differentiating leukocyte cells occurring in the bone marrow and to a lesser extent in other leukocyte producing organs, such as the thymus, lymph nodes and spleen. Neutrophils show the greatest decrease among leukocytes in white blood cell count (Goddard et al, 2008).
Neutrophils have an important role in the defence mechanisms against invading pathogenic agents (Woods et al, 1980). Neutropenia is a common type of leukocyte disorder in dogs. This disease is a result of decreased neutrophils in the blood. This is due, partly because the production by the bone marrow is reduced, but also because the utilisation of neutrophils increases and exceeds the ability of production. This disease can occur secondary to an immune-mediated process that decreases granulopoiesis or destroys circulating neutrophils (Brown and Rogers, 2001).
Several factors contribute to neutropenia, including viruses, typically Canine parvovirus, fungal infections, including Histoplasma Capsulatum, Cryptococcus Neoformans and Ehrlichia, (Brown and Rogers, 2001) and bacterial infections such as sepsis and pneumonia. Other factors include injury, osteomyelitis, peritonitis and pyometra as they result in a high degree of inflammation. This process requires neutrophils, therefore it leads to a decreased level of neutrophils in the blood (Gommeren et al, 2006). Endotoxemia, which causes haemorrhages and kidney failure, may also be a leading cause of this disorder. Neutropenia could be the result of medication such as chemotherapy. Additionally, primary bone marrow disease and Immune-mediated diseases may also be a cause (Brown and Rogers, 2001). Severe neutropenia is accompanied by a decrease in monocytes and lymphocytes and so decreases the ability of skin, mucous membranes and the gastrointestinal tract to act as physical barriers. This increases susceptibility to further infections (Abrams-Ogg and Kruth, 2000). Patients with this disease show symptoms of fever, malaise and mucosal ulcers (Hammond et al, 1989).
Breeds susceptible to neutropenia include Grey Collies, Belgian Tervurens and Rottweilers, the latter suffering predominantly from Primary Immune Mediated Haemolytic Anaemia (IMHA). Susceptibility increases in unvaccinated puppies, due to the high prevalence of Parvovirus, and in intact females, as they have a higher risk for pyometra (Brown and Rogers, 2001).
Severe neutropenia is a curable condition. Antibiotics together with supportive care will result in the recovery of the patient. Granulocyte Colony Stimulating Factor (G-CSF) is a substance used to improve the functioning of neutrophils and increase the neutrophil counts in the blood (Brown and Rogers, 2001).
Platelet Disorders:
Thrombocytopenia:
Thrombocytopenia is a characteristic of many diseases (Lewis and Meyers, 1996). It develops due to a decrease in platelet count and is a major contributor to bleeding disorders in dogs (Botsch et al, 2009). Thrombocytopenia is most commonly seen in dogs suffering from Neoplasia. In addition, studies show that dogs with tumours, particularly lymphomas, are more likely to suffer from thrombocytopenia (Thomas and Rogers, 1999).
Thrombocytopenia most commonly results from the development of tumours from the spleen or bone marrow. The disease arises from intensive chemotherapy, which results in decreased platelet production by the bone marrow, an immune disease characterised by increased platelet destruction, Disseminated Intervascular Coagulation(DIC), causing increased platelet consumption, and tumour associated hemorrhages. Additionally, a sequestration of platelets results from splenomegaly and hepatomegaly as well as excessive blood loss (Thomas and Rogers, 1999). Neoplasia is a typical case of thrombocytopenia. A study showed that 72% of dogs with thrombocytopenia had Neoplasia (Grindem et al, 1994). Moreover, thrombocytopenia may have other causes such as miscellaneous disorders, and infectious inflammations caused by Ehrilichiosis, Babesiosis or gastrointestinal parasites. It has been proven that thrombocytopenia may also be caused by heparin treatment extending from 4-14 days after treatment (Botsch et al, 2009).
Thrombocytopenia usually develops in young dogs. Dogs with DIC and immune mediated thrombocytopenia seem to have a more severe form of this disease due to a very low platelet count (Grindem et al, 1994). Breeds most frequently affected are Cavalier King Charles Spaniel and Greyhounds (Botsch et al, 2009). Golden Retrievers with thrombocytopenia, as a result of neoplasia, are more susceptible than other dog breeds (Grindem et al, 1994). Immune-mediated thrombocytopenia is more likely to arise in female dogs (Botsch et al, 2009).
The characteristic symptoms of thrombocytopenia all arise from bleeding, which could either be internal, more specifically Haemopericardium, congested organs, Haemothorax and Haemoperitonium or external, from the prepuce and vulva. Moreover, canine patients showing signs of Epistaxis, Haematochezia, Haematuria and Haematemesis have a much lower platelet count. Other symptoms of thrombocytopenia include Oedema and Icterus (Botsch et al, 2009). Glucocorticoids are used as treatment in order to stabilise and increase the platelet count. The prognosis depends on diagnosis of the underlying disease causing the condition (Lewis and Meyers, 1996).
Tick borne Rickettsial Disorders:
Dogs and humans, amongst other species, are susceptible to tick-transmitted Ehrlichia which is composed of Ehrlichia canis, amongst others. Another genus classified in this order is Ricketssia which includes Rickettsia conorii and Rickettsia rickettsii, the latter being responsible for the Rocky Mountain Spotted Fever (Shaw, 2001).
Rocky Mountain Spotted Fever:
Once bitten, the tick must feed on the dog for 5-20 hours in order to transmit the disease and symptoms may arise anytime between 4-14 days after the tick bite. The clinical signs of this disease include poor appetite, depression, fever, abdominal pain, muscle or joint pain, coughing, vomiting, diarrhoea and swelling of the face and legs. They may also present nervous system abnormalities including head tilting, haemorrhages on the gums, retina and conjunctiva of the eyes. Thrombocytopenia and lymphocytopenia are also symptoms and they later develop into thrombocytosis and lymphocytosis respectively, weeks after the infection arises (Levin et al, 2014). However, since these symptoms are not specific, it helps to consider the region the dog lives in and history of possible tick exposure.
Basic blood test will show abnormalities including low number of platelets, anaemia and if the disease is in its early stages, a low number of white blood cells, which then elevate in later stages. It may also result in abnormal electrolyte, liver and kidney values. Laboratory diagnostics to determine IgG antibody titres may be performed to confirm the presence of this disease (Levin, 2014) as well as the use of PCR and isolation of the DNA of the infectious agent.
This disease is usually treated with the appropriate antibiotics along with supportive treatment and care if other symptoms arise during the course of the illness. In extremely severe cases, where this disease is left untreated for long periods of time, the dog may start to show signs of organ failure and so may need to be hospitalised and given IV fluids. Mortality from this disease is possible if left untreated or even if treated incorrectly. It is advised to take measures to reduce tick exposure and prevent the infectious agents from reaching the dog.
No specific breeds are known to be more susceptible to this disease. Prevalence of these diseases depend on the vectors which differ in species and number across the globe.
Ehrlichiosis:
Ehrlichiosis in a Rickettsia infection caused by the bacteria from the genus Ehrlichia, predominantly Ehrlichia canis spread by Rhipicephalus sangineus, the brown dog tick (Figure 4) and Ehrlichia lewinii spread by the Ambloyomma americanum, known commonly as the Lone star tick (Figure 5). In these cases, when the infection coming from the tick bite reaches the dog’s bloodstream, it is found in lymphocytes and monocytes. A new strain has also been discovered to accommodate granulocytes, more particularly neutrophils and is called Canine Granulocytic Ehrlichia (CGE) (Anderson et al, 1992). This disease is due to platelet dysfunction. In fact, infected dogs are unable to perform aggregation of platelets and hence, results in bleeding.
|
Fig.4 Rhipicephalus sangineus |
|
Fig.5 Ambloyomma americanum |
Ehrlichiosis is known to present itself in three stages. The Acute stage show symptoms of lethargy and loss of appetite, enlarged lymph nodes as well as limb oedema, 1-3 weeks after the tick bite. The Subclinical stage goes on for months or even years as no clinical signs are visible. Finally, the Chronic stage results in severe symptoms including fever, extreme weight loss, inflammation of the lungs which causes trouble breathing and abnormal bleeding. It also is characterised by lack of coordination, seizures, head tilting, paralysis, joint pain and kidney failure. Leukopenia and thrombocytopenia are also known symptoms. In very severe cases, this disease may be fatal (Skotarczak, 2015).
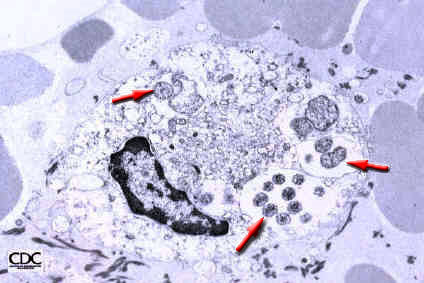
This disease requires immediate and accurate diagnosis for the prognosis to be a favourable one. It may be diagnosed by performing a blood smear and investigating it under an electron microscope. Infected cells will have inclusions called morulae (Figure 6).
|
Fig.6 Morulae |
As regards to treatment and breed disposition, this disease is similar to Rocky Mountain Spotted Fever. However, it is important to note that although the clinical signs are treated, dogs remain infected throughout their lifetime.
References:
Articles:
Abrams-Ogg, A. C., & Kruth, S. A. (2000). Infections Associated with Neutropenia in the Dog and Cat. Antimicrobial Therapy in Veterinary Practice, 3rd ed. Ames, IA, Blackwell Publishing, 471-489.
Anderson, B. E., Greene, C. E., Jones, D. C., & Dawson, J. E. (1992). Ehrlichia ewingii sp. nov., The Etiologic Agent of Canine Granulocytic Ehrlichiosis. International Journal of Systematic and Evolutionary Microbiology, 42(2), 299-302.
Archer, T., Mackin, A., & IMHA, P. O. (2013). Diagnosis of Immune-mediated Hemolytic Anemia. Today’s Veterinary Practice, 7(8), 32-36.
Barker, R. N., & Elson, C. J. (1995). Red Blood Cell Glycophorins as B and T-cell Antigens in Canine Autoimmune Haemolytic Anaemia. Veterinary Immunology and Immunopathology, 47(3-4), 225-238.
Bennett, D., Finnett, S. L., Nash, A. S., & Kirkham, D. (1981). Primary Autoimmune Haemolytic Anaemia in the Dog. The Veterinary Record, 109(8), 150-153.
Botsch, V., Küchenhoff, H., Hartmann, K., & Hirschberger, J. (2009). Retrospective Study of 871 Dogs with Thrombocytopenia. The Veterinary Record, 164(21), 647-651.
Brinkhous, K. M., Hedner, U., Garris, J. B., Diness, V., & Read, M. S. (1989). Effect of Recombinant Factor VIIa on the Hemostatic Defect in Dogs with Hemophilia A, Hemophilia B, and Von Willebrand Disease. Proceedings of the National Academy of Sciences, 86(4), 1382-1386.
Brown, R. M., & Rogers, K. S. (2001). Neutropenia in Dogs and Cats. Compendium on Continuing Education for the Practicing Veterinarian. North American Edition, 23(6), 534-543.
Burgess, K., Moore, A., Rand, W., & Cotter, S. M. (2000). Treatment of Immune-mediated Hemolytic Anemia in Dogs with Cyclophosphamide. Journal of Veterinary Internal Medicine, 14(4), 456-462.
- Crespi JA, Barrientos LS, Giovambattista G (2018). Von Willebrand Disease Type 1 in Doberman Pinscher Dogs: Genotyping and Prevalence of the Mutation in the Buenos Aires Region, Argentina. Journal of Veterinary Diagnostic Investigation, 30(2), 310-314.
- Dodds, W. J. (1984). Von Willebrand's Disease in Dogs. Modern Veterinary Practice, 65(9), 681-686.
Estrin, M. A., Wehausen, C. E., Lessen, C. R., & Lee, J. A. (2006). Disseminated Intravascular Coagulation in Cats. Journal of Veterinary Internal Medicine, 20(6), 1334-1339.
Goddard, A., Leisewitz, A. L., Christopher, M. M., Duncan, N. M., & Becker, P. J. (2008). Prognostic Usefulness of Blood Leukocyte Changes in Canine Parvoviral Enteritis. Journal of Veterinary Internal Medicine, 22(2), 309-316.
Gommeren, K., Duchateau, L., Paepe, D., Vanholen, L., Vandenberghe, A., & Daminet, S. (2006). Investigation of Physiologic Leukopenia in Belgian Tervuren Dogs. Journal of Veterinary Internal Medicine, 20(6), 1340-1343.
Graham, J. B., Buckwalter, J. A., Hartley, L. J., & Brinkhous, K. M. (1949). Canine Hemophilia: Observations on the Course, the Clotting Anomaly, and the Effect of Blood Transfusions. Journal of Experimental Medicine, 90(2), 97-111.
Grindem, C. B., Breitschwerdt, E. B., Corbett, W. T., Page, R. L., & Jans, H. E. (1994). Thrombocytopenia Associated with Neoplasia in Dogs. Journal of Veterinary Internal Medicine, 8(6), 400-405.
Hammond IV, W. P., Price, T. H., Souza, L. M., & Dale, D. C. (1989). Treatment of Cyclic Neutropenia with Granulocyte Colony-Stimulating Factor. New England Journal of Medicine, 320(20), 1306-1311.
Jolivet, F., Diquélou, A., Trumel, C., Privat, S., & Dossin, O. (2017). Fibrinogen Deficiency in a Dog - A Case Report. BioMed Central Veterinary Research, 13(1), 183.
Levin, M. L., Killmaster, L. F., Zemtsova, G. E., Ritter, J. M., & Langham, G. (2014). Clinical Presentation, Convalescence, and Relapse of Rocky Mountain Spotted Fever in Dogs Experimentally Infected via Tick Bite. Public Library of Science (PLOS) One, 9(12), e115105.
Lewis, D. C., & Meyers, K. M. (1996). Canine Idiopathic Thrombocytopenic Purpura. Journal of Veterinary Internal Medicine, 10(4), 207-218.
- Mccullough, S. (2003). Immune-mediated Hemolytic Anemia: Understanding the Nemesis. Veterinary Clinics: Small Animal Practice, 33(6), 1295-1315.
Piek, C. J., Junius, G., Dekker, A., Schrauwen, E., Slappendel, R. J., & Teske, E. (2008). Idiopathic Immunemediated Hemolytic Anemia: Treatment Outcome and Prognostic Factors in 149 Dogs. Journal of Veterinary Internal Medicine, 22(2), 366-373.
Ralph, A. G., & Brainard, B. M. (2012). Update on Disseminated Intravascular Coagulation: When to Consider it, When to Expect it, When to Treat it. Topics in Companion Animal Medicine, 27(2), 65-72.
Reimer, M. E., Troy, G. C., & Warnick, L. D. (1999). Immune-mediated Hemolytic Anemia: 70 Cases (1988-1996). Journal of the American Animal Hospital Association, 35(5), 384-391.
Shaw, S. E., Day, M. J., Birtles, R. J., & Breitschwerdt, E. B. (2001). Tick-borne Infectious Diseases of Dogs. Trends in Parasitology, 17(2), 74-80.
- Skotarczak, B. (2003). Canine Ehrlichiosis. Annals of Agricultural and Environmental Medicine, 10(2), 137-142.
Snyder, R. O., Miao, C., Meuse, L., Tubb, J., Donahue, B. A., Lin, H. F., ... & Read, M. S. (1999). Correction of Hemophilia B in Canine and Murine Models Using Recombinant Adeno-associated Viral Vectors. Nature Medicine, 5(1), 64.
Thomas, J. S., & Rogers, K. S. (1999). Platelet Aggregation and Adenosine Triphosphate Secretion in Dogs with Untreated Multicentric Lymphoma. Journal of Veterinary Internal Medicine, 13(4), 319-322.
Venta, P. J., Li, J., Yuzbasiyan-Gurkan, V., Brewer, G. J., & Schall, W. D. (2000). Mutation Causing Von Willebrand's Disease in Scottish Terriers. Journal of Veterinary Internal Medicine, 14(1), 10-19.
Wilkerson, M. J., Johnson, G. S., Stockham, S., & Riley, L. (2005). Afibrinogenemia and a Circulating Antibody Against Fibrinogen in a Bichon Frise Dog. Veterinary Clinical Pathology, 34(2), 148-155.
Woods, C. B., Pollock, R. V. H., & Carmichael, L. E. (1980). Canine Parvoviral Enteritis. Journal of the American Animal Hospital Association, 16(2), 171-179.
Yu, D., Noh, D., & Park, J. (2015). Flow Cytometric Evaluation of Disseminated Intravascular Coagulation in a Canine Endotoxemia Model. Canadian Journal of Veterinary Research, 79(1), 52-57.
Others:
Bleeding Disorders of Dogs. MSD Manual, Veterinary Manual.https://www.msdvetmanual.com/dog-owners/blood-disorders-of-dogs/bleeding-disorders-of-dogs
Cheryl Yuill: Rocky Mountain Spotted Fever in Dogs, Infectious Diseases, Parasites. VCA Hospitals https://vcahospitals.com/know-your-pet/rocky-mountain-spotted-fever-in-dogs
Cornell University College of Veterinary Medicine, Animal Health Diagnostic Center (2014): Canine Von Willebrand Disease https://ahdc.vet.cornell.edu/sects/coag/clinical/vonwill/
Ernest Ward: Von Willebrand’s Disease in Dogs. Medical Conditions. VCA Hospitals https://vcahospitals.com/know-your-pet/von-willebrands-disease-in-dogs
Heart and Blood: Blood Disorders. Dog Health Handbook https://www.dog-health-handbook.com/canine-blood-disorders.html
Rickettsial Infection in Dogs. PetMD https://www.petmd.com/dog/conditions/infectious-parasitic/c_dg_ehrlichiosis?page=show
W. Jean Dodds, D.V.M, The Humane Society Veterinary Medical Association (2011) - Guide To Congenital And Heritable Disorders In Dogs https://www.hsvma.org/assets/pdfs/guide-to-congenital-and-heritable-disorders.pdf
Figures:
Figure 1: Amolinski (2008) Fibrin + ligand. Wikimedia commons https://commons.wikimedia.org/wiki/File:Fibrin_+_ligand.png
Figure 2: Zyjacklin (2014) Thrombosis formation. Wikimedia commons https://commons.wikimedia.org/wiki/File:Thrombosis_formation.gif
Figure 3: Prof. Osaro Erhabor (2014) Spherocytes. Wikimedia commons https://commons.wikimedia.org/wiki/File:Spherocytes.jpg
Figure 4: Daktaridudu (2016) Rhipicephalus-sanguineus-female-male. Wikimedia commons https://commons.wikimedia.org/wiki/File:Rhipicephalus-sanguineus-female-male.jpg
Figure 5: James Gathany, Dr. Amanda Loftis, Dr. William Nicholson, Dr. Will Reeves, Dr. Chris Paddock (2006) Amblyomma americanum tick 2. Wikimedia commons https://commons.wikimedia.org/wiki/File:Amblyomma_americanum_tick_2.jpg
Figure 6: Lamiot (2009) EhrlichiosisAndMorulaeUS CDC. Wikimedia commons https://commons.wikimedia.org/wiki/File:EhrlichiosisAndMorulaeUS_CDC.jpg
Written by Attard Vancell Ashley, Pace Moore Katherine and Zerafa Victoria