|
Size: 23266
Comment:
|
← Revision 11 as of 2017-05-05 10:01:19 ⇥
Size: 23236
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 8: | Line 8: |
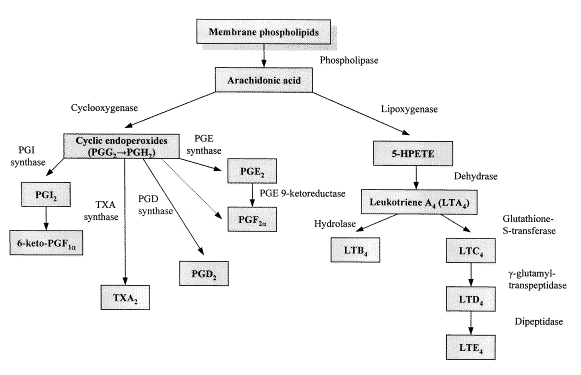
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:cox.png|COX}} <<BR>>'''Fig 1.'''<<BR>>''Prostaglandin Biosynthesis (From article: Role of cyclooxygenase-2 in cancer, Chetan S.Sonawane, Deepali M.Jagdale and Vilasrao J.Kadam.; 2011).'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:cox.png|COX}} <<BR>>'''Fig 1.'''<<BR>>''Prostaglandin Biosynthesis (From article: Role of cyclooxygenase-2 in cancer, Chetan S.Sonawane, Deepali M.Jagdale and Vilasrao J.Kadam.; 2011).'' || |
| Line 15: | Line 15: |
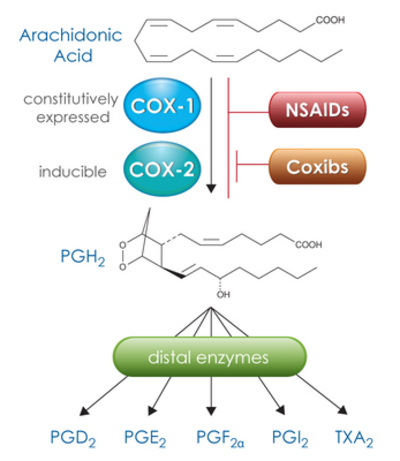
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:coxisoforms.png|isoforms}} <<BR>>'''Fig 2.'''<<BR>>''The products of arachidonic acid: COX-1 and COX-2.'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:coxisoforms.png|isoforms}} <<BR>>'''Fig 2.'''<<BR>>''The products of arachidonic acid: COX-1 and COX-2.'' || |
| Line 31: | Line 31: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:angiotensin.png|COX}} <<BR>>'''Fig 3.'''<<BR>>''Role of COX-2 in angiogenesis'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:angiotensin.png|COX}} <<BR>>'''Fig 3.'''<<BR>>''Role of COX-2 in angiogenesis'' || |
| Line 64: | Line 64: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:cancer.png|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''To a better future without cancer.'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:cancer.png|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''To a better future without cancer.'' || |
| Line 90: | Line 90: |
| 11. Serhan CN 2005 . Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. | 11. Serhan CN 2005 . Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids. |
| Line 128: | Line 128: |
| 30. Rainsford KD. Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions 1993. | 30. Rainsford KD 1993. Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions . |
| Line 130: | Line 130: |
| 31. Laufer S. Discovery and development of ML 3000. Inflammopharmacology 2001. | 31. Laufer S 2001. Discovery and development of ML 3000. Inflammopharmacology . |
| Line 132: | Line 132: |
| 32. Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut 1993. | 32. Hudson N, Balsitis M, Everitt S, Hawkey CJ 1993. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. |
| Line 134: | Line 134: |
| 33. Tries S, Neupert W, Laufer S. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. Inflamm Res 2002. | 33. Tries S, Neupert W, Laufer S 2002. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2. |
| Line 136: | Line 136: |
| 34. Wong S, Lee SJ, Frierson MR 3rd, Proch J, Miskowski TA, Rigby BS, et al. Antiarthritic profile of BF-389—a novel anti-inflammatory agent with low ulcerogenic liability. Agents Actions 1992. | 34. Wong S, Lee SJ, Frierson MR 3rd, Proch J, Miskowski TA, Rigby BS, et al 1992. Antiarthritic profile of BF-389—a novel anti-inflammatory agent with low ulcerogenic liability. Agents Actions . |
| Line 138: | Line 138: |
| 35. Higgs GA, Mugridge KG, Moncada S, Vane JR. Inhibition of tissue damage by the arachidonate lipoxygenase inhibitor BW755C. Proc Natl Acad Sci USA 1984;81: arachidonic acid-induced ear inflammation model. Inflamm Res 1998. | 35. Higgs GA, Mugridge KG, Moncada S, Vane JR 1998. Inhibition of tissue damage by the arachidonate lipoxygenase inhibitor BW755C. Proc Natl Acad Sci USA 1984;81: arachidonic acid-induced ear inflammation model. Inflamm Res . |
| Line 140: | Line 140: |
| 36. Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjörnsson B, Kogner P "Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo". Cancer Research. 64 (20): 7210–5. (Oct 2004). | 36. Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjörnsson B, Kogner P 2004. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Research. 64 (20): 7210–5. |
| Line 142: | Line 142: |
| 37. Lau L, Hansford LM, Cheng LS, Hang M, Baruchel S, Kaplan DR, Irwin MS . "Cyclooxygenase inhibitors modulate the p53/HDM2 pathway and enhance chemotherapy-induced apoptosis in neuroblastoma". Oncogene. 26 (13): 1920–31. March 2007. | 37. Lau L, Hansford LM, Cheng LS, Hang M, Baruchel S, Kaplan DR, Irwin MS 2007. Cyclooxygenase inhibitors modulate the p53/HDM2 pathway and enhance chemotherapy-induced apoptosis in neuroblastoma. Oncogene. 26 (13): 1920–31. |
| Line 144: | Line 144: |
| 38. Blanke CD. A phase II trial of celecoxib, irinotecan, 5-fluorouracil and leucovorin in patients with unresectable or metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2002. | 38. Blanke CD 2002. A phase II trial of celecoxib, irinotecan, 5-fluorouracil and leucovorin in patients with unresectable or metastatic colorectal cancer. Proc Am Soc Clin Oncol. |
| Line 146: | Line 146: |
| 39. Subbaramaiah K. Microtubuleinterfering agents stimulate the transcription of cyclooxygenase-2. J Biol Chem. 2000. | 39. Subbaramaiah K 2000. Microtubuleinterfering agents stimulate the transcription of cyclooxygenase-2. J Biol Chem. |
| Line 148: | Line 148: |
| 40. Liu CH. Over-expression of cyclooxygenase (Cox)-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001. | 40. Liu CH 2001. Over-expression of cyclooxygenase (Cox)-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. |
| Line 150: | Line 150: |
| 41. Hwang DH. National Cancer Institute workshop on chemopreventive properties of nonsteroidal antiinflammatory drugs: role of COXdependent and independent mechanisms. Neoplasia. 2002. | 41. Hwang DH 2002. National Cancer Institute workshop on chemopreventive properties of nonsteroidal antiinflammatory drugs: role of COXdependent and independent mechanisms. Neoplasia. |
| Line 152: | Line 152: |
| 42. Zhang X. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med. 1999. | 42. Zhang X 1999. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med. |
THE ROLE OF NON-STEROIDAL ANTI-INFLAMMATORY DRUGS AS A POTENTIAL INHIBITOR OF CYCLOOXYGENASE IN THE THERAPY OF CANCER
Contents
- Cyclooxygenase (COX) is officially known as prostaglandin-endoperoxide synthase. The first step in prostaglandin synthesis (Figure 1) is the hydrolysis of phospholipids for production of free arachidonate and the enzyme that catalyzes this reaction is Phospholipase A2. The next step is a key reaction catalyzed by COX, in which the arachidonic acid produces the unstable prostaglandin PGG2 (rapidly converted to PGH2 by the peroxidase activity of COX), by using molecular oxygen. Then, PGH2 is converted to prostanoids, including thromboxane and prostaglandins, by specific isomerases and the derived products have their own range of biological activities.
Prostaglandin Biosynthesis:
|
Isoforms of COX:
Two different isoforms of COX have been discovered and are known as COX-1 and COX-2. They are encoded by 2 different genes1, and it has been postulated that while COX-1 is expressed in mammalian cells, particularly in endothelium, platelets and kidneys in physiological conditions, COX-2 is inducible in pathological conditions by inflammatory stimulation.2 3 COX-1, also called as prostaglandin G/H synthase 1, is an enzyme that in humans is encoded by the PTGS1 gene. 4 5 It is encoded by a gene which is involved in cell signaling, maintaining tissue homeostasis and regulates angiogenesis.
While metabolizing arachidonic acid (Figure 2) primarily to PGG2, also converts it to small amounts of a racemic mixture of 15-Hydroxyicosatetraenoic acids (15-HETEs). Even though the two stereoisomers have important biological activities, more importantly are further metabolized to a major class of anti-inflammatory agents, the lipoxins. Furthermore, COX-1 stimulates the production of the natural mucus lining of the inner stomach for protection and contributes to reduced acid secretion and pepsin content. 6 7 The major product of COX-1 is thromboxane A2 which induces platelet aggregation 8 9, thus inhibition of COX-1 which is committed by non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin is sufficient to explain why low dose aspirin is effective at reducing cardiac events.
|
COX-2, is also known as prostaglandin-endoperoxide synthase 2 and is encoded by the PTGS2 gene. It converts the arachidonic acid to prostaglandin endoperoxide H2. PGHs are targets for NSAIDs and PGHS-2 is a sequence homodimer. Each monomer of the enzyme has a peroxidase and a COX active site. 10 While metabolizing arachidonic acid, it also converts it to the racemic mixture of 15-HETEs like COX-1. Furthermore, aspirin-treated COX-2 metabolizes arachidonic acid to 15(R)-HETE which product can be further metabolized to epi-lipoxins, 11 which are anti-inflammatory agents.
COX-2 is naturally inhibited by Calcitirol that is the active form of Vitamin D 12 13. It’s unexpressed under normal conditions in most cells, but during inflammation the levels are elevated. Drug-candidates that selectively inhibit COX-2 were proved to substantially increase the risk for cardiovascular events like heart attack and stroke. Two different mechanisms may explain contradictory effects; low-dose aspirin protects against cardiovascular events by blocking COX-1 from forming Thromboxane A2, but on the other hand COX-2 forms prostacyclin which relaxes or unsticks platelets thus increasing risk of cardiovascular events due to clotting. 14 The expression of COX-2 is up regulated in many cancers. The overexpression along with increased angiogenesis and GLUT-1 expression is associated with gallbladder carcinomas. 15 Moreover, PGH2 (product of COX-2) is converted to PGE2, by prostaglandin E2 synthase, which can stimulate cancer progression thus stimulation of COX-2 may have benefit in the prevention and treatment of these types of cancer. 16
COX-2 seems to be related to cancers and abnormal growths in the intestinal tract. COX inhibitors have been shown to reduce the occurrence of cancers and pre-cancerous growth.
The growth of tumors depends on an increase in blood supply. Tumor cells ensure their own growth by secreting growth factors such as vascular endothelial growth factor that stimulates angiogenesis. Overexpression of COX-2 in colon cancer cells increases the production of vascular growth factors, the migration of endothelial cells and the formation of capillary-like networks in vitro17. These effects can be blocked by a selective inhibitor of COX-2, NS-398. The major role of COX-2 in angiogenesis (Figure 3) is thought to be induction of the synthesis of prostanoids, which then stimulate the expression of pro-angiogenic factors18. The effects of COX-2 are probably amplified via a feedback loop, because VEPF activates both phospholipase A2- mediated release of arachidonic acid and COX-2 expression, thereby enhancing PGI2 and PGE2 production. However, this mechanism does not operate in all types of cells, although COX-2 adjacent with VEGF and TGFβ in the parts of cancer specimens with higher microvascular density, showing that vessel sprouting is increased in the areas of tumors where COX-2 is expressed. Angiogenic stimuli even though is originated from various cell types, is common in pathogenesis of cancer, arthritis and ischemia.
Prostanoids bind to G-protein-coupled surface receptors and signaling via DP, IP, EP2 and EP4 receptors, leads to increased levels of cAMP. By contrast, activation of FP, TP and EP1 prostanoid receptors induce calcium mobilization19.
The TP receptor using specific agonists and antagonists has been shown to be involved in corneal and tumor angiogenesis20, 21.
|
The third isoform of COX
There is a third isoform of COX, which is known as COX-3. In 2002, the group of Daniel Simmons cloned a COX enzyme in dog brain which, unlike the other two forms of COX, was sensitive to inhibition with paracetamol. This enzyme was a variant of COX-1 and differs on the retention of intron 1 of the COX-1 gene, which in COX-3 contains an additional nucleotide. This variant is elicited by alternative splitting of COX-1 gene 22.
COX-3 is considered to play a key role in the biosynthesis of prostanoids known to be important mediators in pain and fever. NSAIDs, such as diclofenacor ibuprofen, are also potent inhibitors of COX-3 (in cultured cells), but being highly polar, they are unlikely to reach the brain. Moreover, aminopyrine and antipyrine have been shown to act centrally to cause their antipyretic and analgesic effects in mice.
COX inhibitors
The discovery of COX took place in 1991, by being cloned by Dr. Dan Simmons at Brigham Young University. Before its discovery, DuP-697 was developed by the DuPont Company, which was potent in many anti-inflammatory assays but did not have the ulcerogenic effects of NSAIDs. Since the confirmation of COX-2 existence became the building block for synthesis of COX-2 inhibitors. The first COX-2 inhibitors that appeared in market were celecoxib (December 1998) and rofecoxib (May 1999) and were based on DuP-69723.
The main COX inhibitors are the non-steroidal anti-inflammatory drugs (NSAIDs), but are not selective and inhibit all types of COX. The inhibition results in prostaglandin and thromboxane synthesis, has the effect of reduced inflammation, antipyretic, antithrombotic and analgesic effects. Previous studies proved that, when inflammation is occurred, the affected organ creates an enormous capacity to generate prostaglandins. In 1991, during the investigation of the expression of early-response genes in fibroblasts transformed with Rous sarcoma virus, a novel mRNA transcript that was similar to the seminal COX enzyme was identified. Another group discovered a novel cDNA species encoding a protein with similar structure to COX-1 while studying phorbol-ester-induced genes in Swiss 3T3 cells. The same laboratory proved that this gene truly expressed a novel COX enzyme. The two enzymes were renamed COX-1 and COX-224. As a result, scientists started emphasizing on selective COX-2 inhibitors. Because COX-2 is usually specific to inflamed tissue, there is much less gastric irritation associated with COX-2 inhibitors, with a decreased risk of peptic ulceration. Naturally COX inhibition is accomplished by culinary mushrooms (inhibit COX-1 and COX-2)25, a variety of flavonoids (inhibit COX-2)26, fish oils which provide alternative fatty acids to arachidonic acid that can be turned to anti-inflammatory prostacyclins instead of pro-inflammatory prostaglandins27, calcitriol (vitamin D)28 and others. Caution should be checked in combining low dose aspirin with COX-2 inhibitors due to potential increased damage to the gastric mucosa. COX-2 is upregulated when COX-1 is suppressed with aspirin, which is thought to be important in stimulating mucosal defense mechanisms and lessening the erosion by aspirin29.
One of the keys in developing COX-2 selective drugs is the larger active site of COX-2, which makes it possible to make molecules too big to fit into the COX-1 active site, but still able to fit into the COX-2. The larger active site is partly due to a polar hydrophilic side-pocket, which allows interactions with Arg513, which is replacement for His513 of COX-1. The central ring of the coxibs decides the orientation of the aromatic rings and, thus, the binding to COX enzyme although usually has no electrostatic interactions with any of the amino acid residues. The high lipophilicity of the active site does require low polarity of the central scaffold of the coxibs.
In recent years, it has been clarified that prostaglandin synthesis is only a part of the arachidonic acid pathway, this precursor being a substrate that gives rise to many other lipid mediators such as the leucotrienes and lipoxins. Leucotrienes themselves have a major role in the development and persistence of the inflammatory process thus they have complementary effects with prostaglandins, whereas the production of lipoxins counteracts the inflammatory action of leucotrienes. Furthermore, taking under consideration the roles of LTB and cysteinyl LTs (against which neither selective nor non-selective NSAIDs are effective) in the inflammatory process, dual inhibition of the COX and 5-LOX pathways could produce a wider spectrum of anti-inflammatory effects. It can be expected that blocking both pathways would limit the potential for COX-1/COX-2 inhibitors, NSAIDs, to produce an excess of LTs in a shunt process30-33.
In the past few decades, several compounds have been developed to block both COX and 5-LOX, but their use abandoned owing to liver toxocity34. Prototype experimental dual inhibitors (i.e. BW755C) have proved effective in preventing the production of both prostaglandins and leucotrienes and the consequent inhibition of migration and activation of inflammatory cells into inflamed sites. Importantly, the inhibition of migration of inflammatory cells towards the affected sites has translated into a reduction of tissue damaging or necrosis in a model of tissue damage and foreign body rejection35.
Small tumors of the sympathetic nervous system appear to have expressed abnormal levels of COX-236 which lead to an adverse effect on the tumor suppressor protein p.53 that is found in the cytosol. When cellular DNA is damaged, the protein is transported to the nucleus where it promotes its mediated apoptosis37. Two of the metabolites of COX-2, prostaglandin A2 and A1, when present in high qualities bind to p.53 in cytosol and inhibit its ability to cross into the nucleus. Coxibs (i.e. Celebrex), by selectively inhibiting the overexpressed COX-2, allow p.53 to work properly. Functional p.53 allows DNA damaged neuroblastoma cells to commit suicide through apoptosis, halting tumor growth.
COX-2 upregulation has also been linked to the phosphorylation and activation of E3 ubiquitin ligase HDM2, a protein that mediates p.53 ligation and tagged destruction, through ubiquitination37, but the mechanism for this neuroblastoma hyperactivity is unknown. In vitro, the use of COX-2 inhibitors lowers the level of active HDM2 found in neuroblastoma cells. The exact process is unknown, but this mediated reduction in active HDM2 concentration level restores the cellular p53 levels.
COX-2 role in the treatment of cancer
Even though many studies are under progress, it is too early to know the exact role of selective COX-2 inhibitors in the therapy of cancer. Selective COX-2 inhibitors are being evaluated intensively in conjuction with chemotherapy and radiotherapy in patients with cancers and there are several trials to evaluate the inhibitors. A Phase II trial is evaluating treatment with celecoxib plus irinotecan, 5-flurouracil and leucovorin in patients with measurable, incurable, colorectal cancer38.
Selective COX-2 inhibitors are also being evaluated in patients with non-small-cell lung cancer. It is observed that paclitaxel induces COX-2 and stimulates prostaglandin biosynthesis in cell culture, and postulated that this might reduce the efficacy of paclitaxel39.
Although significant progress has been made in defining the connection between COX-2 and carcinogenesis, many questions remain. First, it is important to establish whether selective COX-2 inhibitors are effective in either preventing or treating cancer, and the numerous clinical trials that are under way should provide crucial information. Moreover, another issue concerns the potential use of selective COX-inhibitors to reduce chemotherapy related side-effects.
Genetic studies, using either transgenic or knockout technology, have firmly established the link between COX-2 and tumorigenesis40. However, whether inhibition of COX-2 is the sole reason for the anti-tumorigenic effects of pharmacological inhibitors of COX-2 is less certain41. For example, high concentrations of either NSAIDs or selective inhibitors suppress the growth of cells in culture that do not express COX-242. Therefore, as well as assessing efficacy, the safety of selective COX-2 inhibitors needs to be monitor carefully in cancer treatment studies. Nowadays, major emphasis has been placed on evaluating the role of selective COX-2 inhibitors in preventing cancer. We should focus, however, that there is growing interest in finding out whether these agents are also useful in treating cancer. In most preclinical studies, the growth rate of tumors was reduced rather than causing tumor regression. As a result, selective COX-2 inhibitors will be most beneficial when administered in combination with standard therapy (Figure 4).
|
REFERENCES
1. Smith WL, Garavito RM, DeWitt 1996. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem.
2. Jouzeau JY, Terlain B, Abid A, Nedelec E, Netter P 1997. Cyclo-oxygenase isoenzymes. How recent findings affect thinking about nonsteroidal anti-inflammatory drugs.
3. Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P 1990. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes.
4. Yokoyama C, Tanabe T 1989. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochemical and Biophysical Research Communications. 165 (2): 888–94.
5. Jump up ^ Funk CD, Funk LB, Kennedy ME, Pong AS, Fitzgerald GA 1991. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment.
6. Laine L, Takeuchi K, Tarnawski A 2008. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology.
7. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, eds 2008.
8. Parker KL, Brunton LL, Lazo JS 2006. Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill Medical Publishing Division. p. 1126.
9. Weitz JI 2008. Chapter 112. Antiplatelet, Anticoagulant, and Fibrinolytic Drugs. In Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison's Principles of Internal Medicine (17th ed.). New York: McGraw-Hill Medical.
10. O'Banion MK 1999. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 13.
11. Serhan CN 2005 . Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids.
12. Wang Q1, He Y, Shen Y, Zhang Q, Chen D, Zuo C, Qin J, Wang H, Wang J, Yu Y 2014. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 289 (17): 11681–11694.
13. Kassi E1, Adamopoulos C, Basdra EK, Papavassiliou AG 2013.Role of Vitamin D in Atherosclerosis. AHA. 128 (23): 2517–2531.
14. Ruan, C. H.; So, S. P.; Ruan, K. H 2011. Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: Mechanisms of COX-2 inhibitor risk to heart disease. Life Sciences. 88 (1–2): 24–30.
15. Legan M 2010. Cyclooxygenase-2, p53 and glucose transporter-1 as predictors of malignancy in the development of gallbladder carcinomas. Bosn J Basic Med Sci. 10 (3): 192–6.
16. Menter DG, Schilsky RL, DuBois RN 2010. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin. Cancer Res. (5): 1384–90.
17. Tsujii M, Kawano S and Tsuji S 1998. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell;. 93:705–716.
18. Tsujii, M 1998, et al Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705–716, Williams, C.S. et al. (2000) Host cyclooxygenase-2 modulates carcinoma growth. J. Clin. Invest. 105, 1589–1594.
19. Narumiya, S. 1999 et al Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79, 1193–1226.
20. Daniel, T.O. 1999 et al Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 59, 4574–4577.
21. 7 Pradono, P 2002. et al Gene transfer of thromboxane A2 synthase and prostaglandin I2 synthase antithetically altered tumor angiogenesis and tumor growth. Cancer Res. 62, 63–66.
22. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL 2002. From the cover: COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA.
23. Flower, R. J 2001.The development of COX2 inhibitors. Nature Reviews Drug Discovery. 2 (3): 179–91, 2003 Dannhardt, G; Kiefer, W. "Cyclooxygenase inhibitors--current status and future prospects". European journal of medicinal chemistry. 36 (2): 109–26.
24. Flower, R. J. 2003. The development of COX2 inhibitors. Nature Reviews Drug Discovery. 2 (3): 179–91.
25. Zhang Y, Mills GL, Nair MG 2002. Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. Journal of Agricultural and Food Chemistry. 50 .
26. O'Leary KA, de Pascual-Teresa S, de Pascual-Tereasa S, Needs PW, Bao YP, O'Brien NM, Williamson G 2004. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutation Research. 551.
27. Cleland LG, James MJ, Proudman SM 2006. Fish oil: what the prescriber needs to know. Arthritis Research & Therapy.
28. Moreno J, Krishnan AV, Peehl DM, Feldman D 2006. Mechanisms of vitamin D-mediated growth inhibition in prostate cancer cells: inhibition of the prostaglandin pathway. Anticancer Research. 26 (4A): 2525–30.
29. Wallace JL 2008. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself?. Physiological Reviews. 88 (4): 1547–65.
30. Rainsford KD 1993. Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions .
31. Laufer S 2001. Discovery and development of ML 3000. Inflammopharmacology .
32. Hudson N, Balsitis M, Everitt S, Hawkey CJ 1993. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs.
33. Tries S, Neupert W, Laufer S 2002. The mechanism of action of the new antiinflammatory compound ML3000: inhibition of 5-LOX and COX-1/2.
34. Wong S, Lee SJ, Frierson MR 3rd, Proch J, Miskowski TA, Rigby BS, et al 1992. Antiarthritic profile of BF-389—a novel anti-inflammatory agent with low ulcerogenic liability. Agents Actions .
35. Higgs GA, Mugridge KG, Moncada S, Vane JR 1998. Inhibition of tissue damage by the arachidonate lipoxygenase inhibitor BW755C. Proc Natl Acad Sci USA 1984;81: arachidonic acid-induced ear inflammation model. Inflamm Res .
36. Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, Sveinbjörnsson B, Kogner P 2004. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Research. 64 (20): 7210–5.
37. Lau L, Hansford LM, Cheng LS, Hang M, Baruchel S, Kaplan DR, Irwin MS 2007. Cyclooxygenase inhibitors modulate the p53/HDM2 pathway and enhance chemotherapy-induced apoptosis in neuroblastoma. Oncogene. 26 (13): 1920–31.
38. Blanke CD 2002. A phase II trial of celecoxib, irinotecan, 5-fluorouracil and leucovorin in patients with unresectable or metastatic colorectal cancer. Proc Am Soc Clin Oncol.
39. Subbaramaiah K 2000. Microtubuleinterfering agents stimulate the transcription of cyclooxygenase-2. J Biol Chem.
40. Liu CH 2001. Over-expression of cyclooxygenase (Cox)-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem.
41. Hwang DH 2002. National Cancer Institute workshop on chemopreventive properties of nonsteroidal antiinflammatory drugs: role of COXdependent and independent mechanisms. Neoplasia.
42. Zhang X 1999. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med.