|
Size: 12235
Comment:
|
Size: 12237
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 55: | Line 55: |
| . Some studies in vitroand in vivoshow that UV-filters present in sunscreens and cosmetics can induce developmental and reproductive defects, by endocrine. But other studies did not lead to this conclusion. | . Some studies in vitro and in vivo show that UV-filters present in sunscreens and cosmetics can induce developmental and reproductive defects, by endocrine. But other studies did not lead to this conclusion. |
Camphor: An Endocrine Disruptor
Introduction
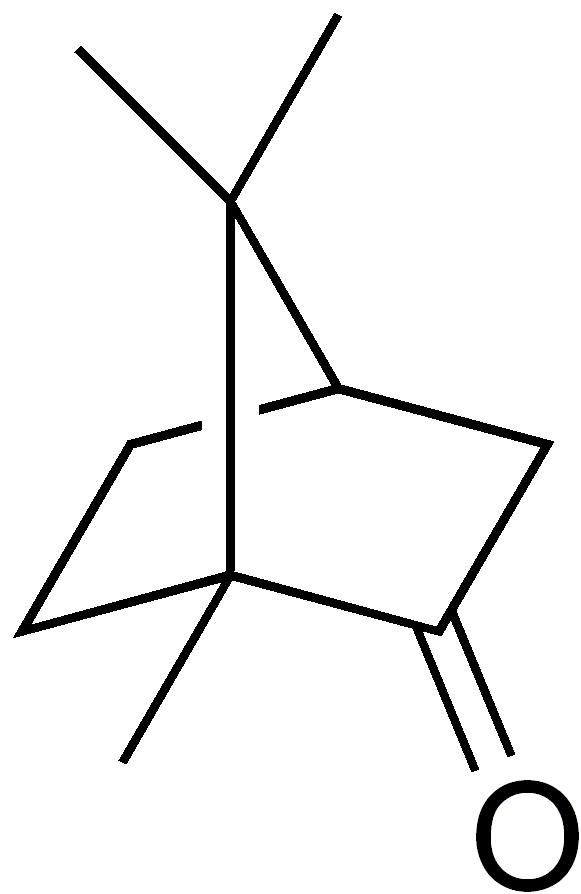
In vertebrates, response to environmental stimuli and maintenance of homeostasis is insured by two main systems: the nervous and the endocrine systems. While the nervous system uses fast electrochemical action potentials delivered by neurons as messengers, the endocrine system uses slower chemical messengers called hormones, circulating through the blood. Hormones are involved in the regulation of several physiological functions such as reproduction, metabolism and energy balance, growth and development, immune response and maintaining blood homeostasis. Their production, release and reabsorption is finely regulated but can be altered by molecules or substances, called endocrine disruptors. This alteration can have direct consequences on the individuals but also on their offspring [1]. Camphor (Figure 5) C10H16O, is a chemical compound used in sunscreens as a UV-blocker, that has recently been characterised as an endocrine disruptor (Figure 1,2,3,4) . Entering the organism through the skin, it has a weak oestrogenic effect, altering the sexual behaviour [2].
Importance of Camphor
- Camphor is widely used in sunscreens and other cosmetic products as a UV-filter.
- Human exposure to UV filters differs according to many factors, such as geographic location, season, lifestyle, gender or occupation. This results in a high individualization. For instance, in Australia, 56% of people apply sunscreen at least 5 days a week, and 27% up to 2 days a week [5].
- But frequent application of sunscreen could result in penetration of UV filters in the organism. Indeed, studies on healthy volunteers, show that UV-filters can detected in the blood plasma 2 hours after application, and in urine after 24 hours [4]. In the US, UV filters applied on the skin have been found in urine samples in 96% of cases. In Switzerland, 85% of breast milk samples tested contained traces of UV filters [3].
Physiological effects of Camphor and its derivatives
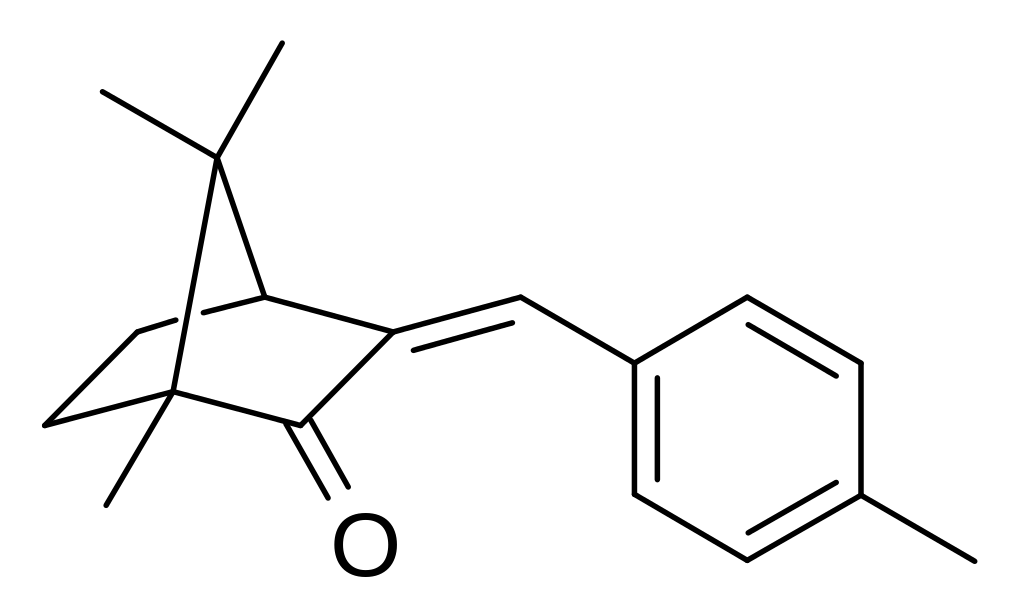
In sunscreen and other cosmetic products, 4-methylbenzylidene camphor (4-MBC), an organic camphor derivative, is used. Thus, its maximum amount has been limited at 4% by the Food and Drug Administration (FDA).Because of its highly lipophilic nature, 4-MBC is absorbed through the skin and can been found in internal tissues, including placenta [6]. Increased use of sunscreen is correlated with increased 4-methylbenzylidene camphor (4-MBC) absorption and increased risk of adverse effects. Once in the organism, 4-MBC exhibits a toxic activity as oestrogenic endocrine disruptor [6].
Comparison of dermal vs oral exposure of 4-MBC (Figure 6) indicates that dermal exposure results in a three-time-greater oestrogenic effect in rats [8].
- Studies on zebrafish embryos and cell cultures indicate that 4-MBC can also be neurotoxic. Indeed, in zebrafish embryos, 4-MBC exposure results in abnormal axial curvature and impaired motility. In neuronal mammalian cell culture (Neuro-2a), 4-MBC induces inhibition of cellular acetylcholinesterase (AChE) activity.
High dose of 4-MBC (up to 100 μM) in neuroblastoma cell lines (SH-SY5Y) decrease cell viability and induce apoptosis. However, such effects have not been observed in vivo [6].
- When ingested before mating, during pregnancy or during lactation, 4-MBC induce a region- and sex-dependant alteration in the expression of oestrogenic genes in the brain of both the mother and the offspring.
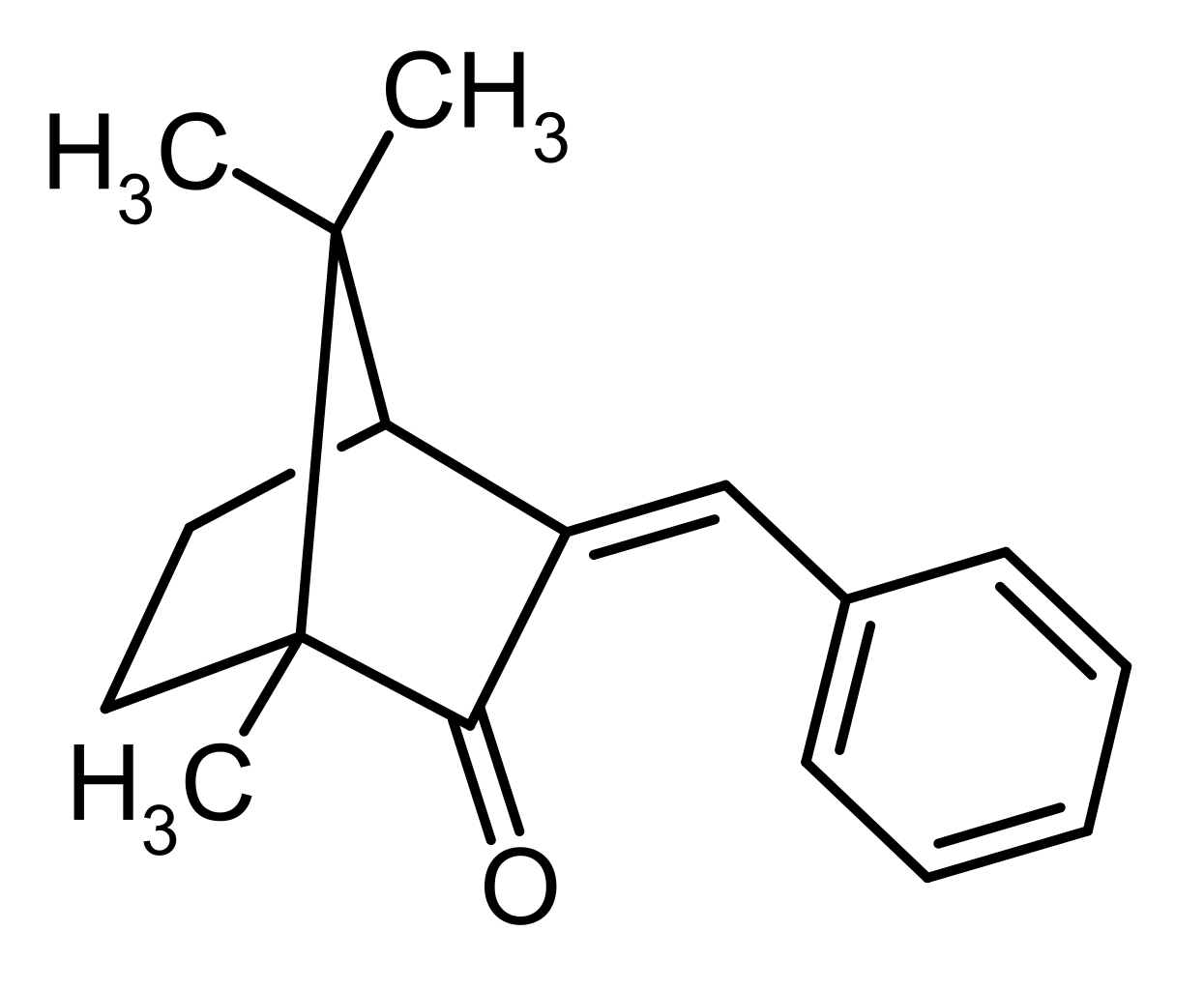
- 3-benzylidene camphor (3-BC) is a lipophilic compound (Figure 7) similar to 4-methylbenzilidene camphor (4-MBC). In the EU, it is used in sunscreens, with a maximal concentration of 2%.
- After 65 days of topical application on rats, 3-BC can be detected in different tissues, including the brain.
- In Danish women using sunscreen during pregnancy, 3-BC can be found in the placenta after birth, while it is not detected their children urine samples. Described as an oestrogenic disruptor, reports indicate that it can also affect the CNS. Rats treated pre- and postnatally with 3-BC show region- and sex-specific change of expression in genes involved in sexual behaviour : PR, oestrogen receptors (ERα,ERß) and steroid receptors coactivator-1 (SRC-1) [6].
- Some studies in vitro and in vivo show that UV-filters present in sunscreens and cosmetics can induce developmental and reproductive defects, by endocrine. But other studies did not lead to this conclusion.
- It is difficult to associate UV filters such as camphor with endocrine disruption in exposed subjects, but it remains a substance presenting a potential risk. Indeed, the majority of studies correlating camphor exposure to endocrine disruptive problems result from oral exposure.
- Because dermal exposure via cosmetic products is more frequent, more studies are necessary.
Disrupting Effects toward Oestrogen Receptor (ER)
Both 4-MBC and 3-BC present an oestrogen-like activity. Rat exposed to 3-BC during early development and postnatal life, show significant changes in expression of oestrogen receptors (ERs) and their associated target genes.
- During early development, 3-BC stimulates vitellogenin (VTG) production in juvenile fathead minnow, and in male rats, 3-BC leads to oestrogenic potency.
Finally, in Xenopus laevis frogs, 3-BC and 4-MBC exposure induce an negative sex ratio [7].
Disrupting Effects toward Androgen Receptor (AR)
No agonistic activation of ARs by 4-MBC and 3-BC has been shown. Nevertheless, recombinant yeast assays show that, depending on the concentration, 4-MBC and 3-BC could inactivate ARs. By study of luciferase activity, 4-MBC has been shown as a potential human AR antagonist.
- Both 4-MBC and 3-BC decrease testosterone formation and inhibit androgen metabolism in HEK-293 cells. [7]
Disrupting Effects toward Progesterone Receptor (PR)
In males, 4-MBC exposure during development induce an increased level of progesterone receptors (PR) mRNA. This increase was not observed in females.
- In 4-MBC treated females, PR expression in the ventromedial hypothalamic area is decreased, but not in males [6].
- At different developmental stages, 4-MBC disturbs the expression of membrane-associate PR.
- At very low doses of 4-MBC, expression level of PR protein is reversibly down-regulated and both 4-MBC and 3-BC act as antagonists of PR [7].
Conclusion
- Camphor and in particular two of its derivatives, 4-MBC and 3-BC, are used in sunscreen and other cosmetic products as UV-filters. Their application on the skin seems to lead to an absorption followed by an accumulation in the organism.
- Their main effects appear to be disruption of the endocrine system, mainly through an oestrogen-like effect.
- Consequences of this disruption are mainly changes in the sexual behaviour, but the mechanism of their action remains not well known. More studies are necessary to conclude of the risk of camphor use as an UV-filter.
- Release and spreading of products containing camphor derivatives in nature, for example by disposal of sunscreens, represent a real risk of affecting other living organisms. This could also lead to disturbing the equilibrium of various ecosystems, by modifying the sex ratio or fertility of animals.
- Endocrine disruptors in general represent a real risk for human health but also for the environment. Camphor is one example of those substances able to alter the endocrine system, and it is widely used in products that are meant to protect health in the first place.
- With increased use of sunscreen due to health campaigns, more studies have to be conduct to insure a minimal risk of camphor use as a UV-filter.
References
Text References
Text Books
[1] Mozo, D. R. (2012) Endocrine Disrupters-Solutions to new challenges. Instituto Sindical de Trabajo, Ambiente y Salud
Websites
[2] National Center for Biotechnology Information. PubChem Database. 4-Methylbenzylidene camphor, CID=6440721, https://pubchem.ncbi.nlm.nih.gov/compound/6440721
Scientific Articles
[3] Krause, M.; Klit, A.; Blomberg, M. J.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N. E.; Drzewiecki, K. T. (2012): Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. International Journal of Andrology 35: (3) 424-236
[4] Janjua, N. R.; Kongshoj, B.; Andersson, A. M.; Wulf, H. C. (2008): Sunscreens in human plasma and urine after repeated whole-body topical application. The Journal of the European Academy of Dermatology and Venereology 22: (4) 456-461
[5] Neale, R.; Williams, G.; Green, A. (2002): Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Archives of Dermatology 138: 1319-1325
[6] Ruszkiewicza, J. A.; Pinkasa, A.; Ferrera, B.; Peresa T. V.; Tsatsakisb, A.; Aschnera, M. (2017): Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicology Reports 4: 245-259
[7] Wang, J.; Pan, L.; Wu, S.; Lu, L.; Xu, J.; Zhu, Y.; Guo, M.; Zhuang, S. (2016): Recent Advances on Endocrine Disrupting Effects of UV Filters. International Journal of Environmental Research 13: 782-793
[8] Søeborg, T.; Ganderup, N. C.; Kristensen, J. H.; Bjerregaard, P.; Pedersen, K. L.; Bollen, P.; Hansen, S. H.; Halling-Sørensen, B. (2006): Distribution of the UV filter 3-benzylidene camphor in rat following topical application. Journal of Chromatography. 834: 117–121.
Figure References
Figure 1: https://commons.wikimedia.org/wiki/Cinnamomum_camphora#/media/File:Cinnamomum_camphora_001.JPG See wiki commons licensing
Figure 2: https://commons.wikimedia.org/wiki/Cinnamomum_camphora#/media/File:Cinnamomum_camphora4.jpg See wiki commons licensing
Figure 3: https://commons.wikimedia.org/wiki/Cinnamomum_camphora#/media/File:Cinnamomum_camphora6.jpg See wiki commons licensing
Figure 4: https://commons.wikimedia.org/wiki/Cinnamomum_camphora#/media/File:Cinnamomum_camphora2.jpg See wiki commons licensing
Figure 5: https://commons.wikimedia.org/wiki/File:Camphor_structure.png See wiki commons licensing
Figure 6: https://commons.wikimedia.org/wiki/File:4-Methylbenzylidene_camphor.svg See wiki commons licensing
Figure 7: https://commons.wikimedia.org/wiki/File:3-Benzylidene_camphor_structure.svg See wiki commons licensing