Circadian rhythm and its effects on food intake
Mégane Pernollet, Aurore Quéromain, and Ciara Reynolds
Contents
Intro
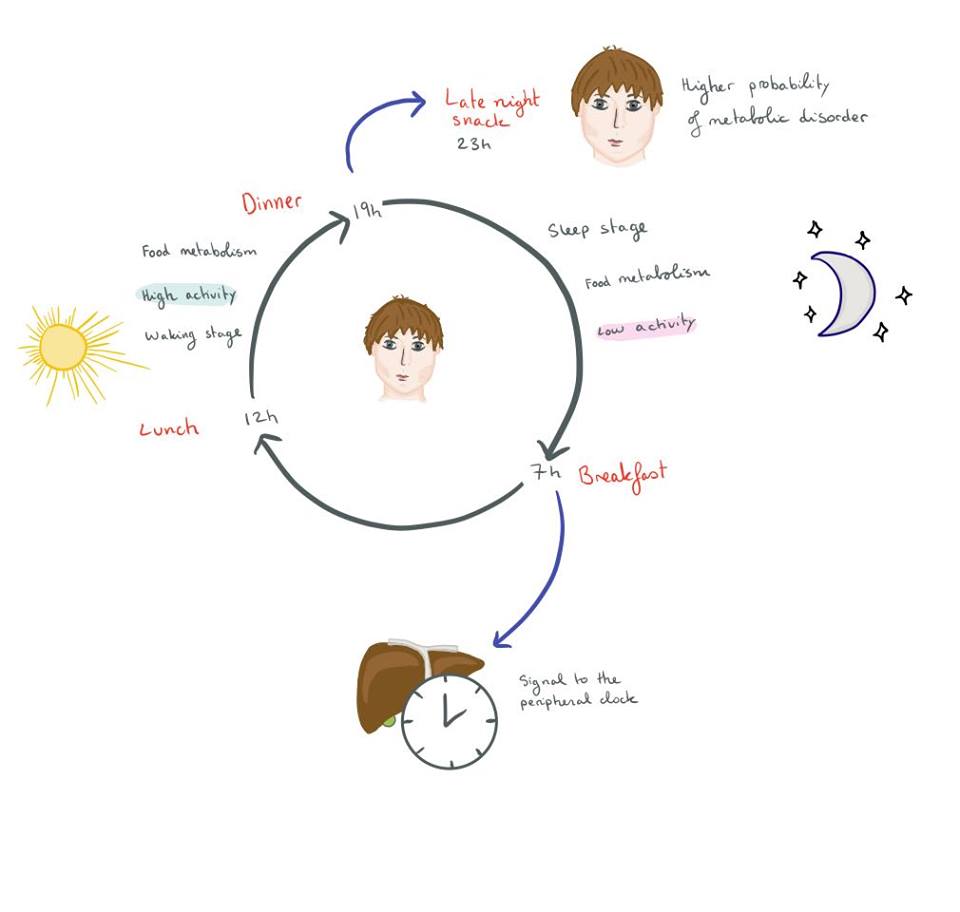
There is a complex relationship between circadian rhythms and the regulation of metabolism, especially the effects it has on food intake. Circadian rhythms are defined as any cycle which has a duration of 24 hours, with endogenous cues, but is influenced by exogenous zeitgebers (cues). This includes the sleep-wake cycle and its regulation by melatonin, influenced by fluctuations in daylight. A fundamental characteristic of living animals is the rhythmicity which is ensured by the circadian and sleep-wake cycles (Figure1). An essential role of the circadian rhythm is to organise the body’s metabolism with the day/night alteration (sleep-wake cycle).
The mechanism is as follows: photoreceptors in the retina respond to a change in the wavelength of light by sending impulses via the optic nerve towards the Supra-chiasmatic Nucleus (SCN). The SCN then stimulates melatonin release from the pineal gland. Melatonin’s influence is widespread, but an intricate communication with the hypothalamus must be noted. During early daylight hours when natural light is on the blue side of the spectrum, melatonin secretion is suppressed due to inhibition from the SCN on the pineal gland. Therefore when the wavelength of light is more on the reddish side such as in the evening, the SCN signals to the pineal gland to release melatonin.
|
Figure 1 Simplified circadian cycle |
Melatonin’s effects on the body are numerous and complex, but its main role is to set a biological rhythm in multiple different cells of organ systems as well as being a potent antioxidant, which is still under research.
The sleep-wake cycle is defined by the awakening of an animal and the rhythm of their sleeping cycle. It usually revolves around a 24-hour clock but some research has shown that it could be shorter or longer depending on the individual (Garbazza et al., 2016). The molecular mechanisms by which hypothalamic neurons regulate the sleep-wake cycle with appetite and metabolism remain unclear (Starling, 2019).
Hormonal physiology
Traditionally food intake is regarded as being controlled by endogenous endocrine factors which are released in response to a meal. Today, research has shown that exogenous factors such as social meal times, and the role of certain neurotransmitters is undeniable. Previously, appetite was considered to be induced during a fasting period, when glucagon’s effects could no longer satisfy the metabolic needs of the organism. Today, many factors are taken into account when explaining food-intake regulation.
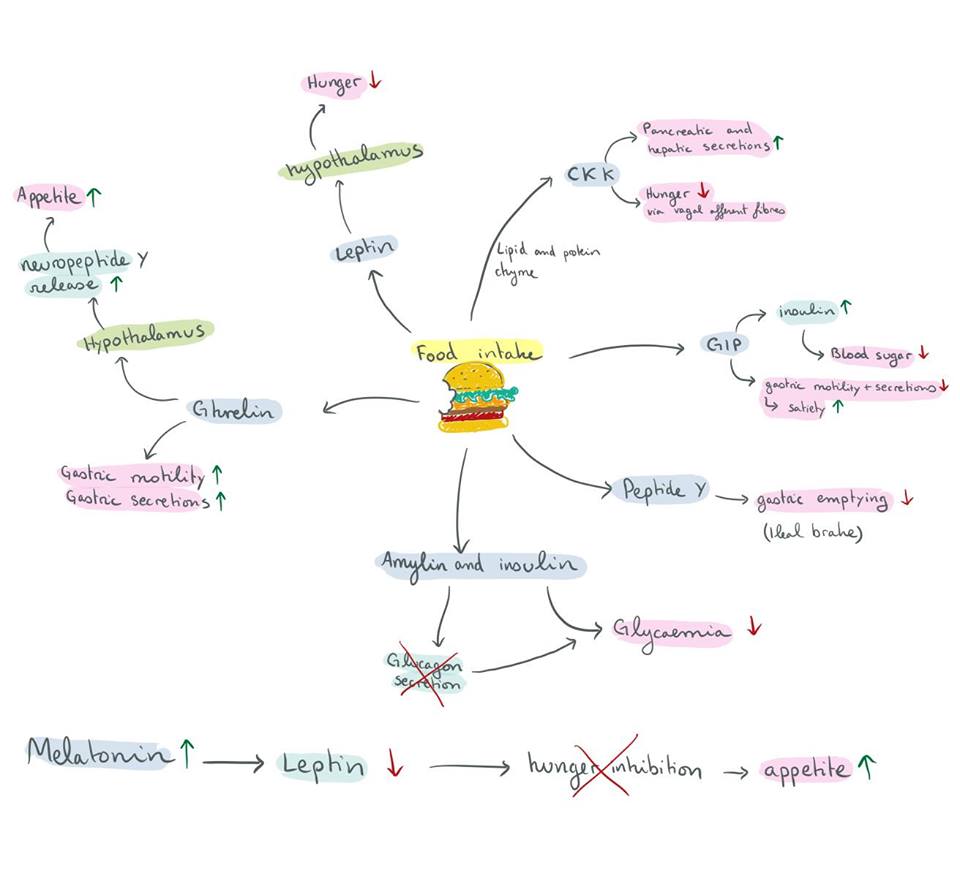
Firstly, the endocrine endogenous mechanisms are explained below (Figure 2). As with all conscious behaviours, the brain is the first to respond to the sensory input that food represents. Chemo and mechanoreceptors in the gastrointestinal tract also relay sensory input to the brain via the autonomic nervous system. Following a meal, Cholecystokinin (CKK) is released following the presence of (mainly) lipid and protein-filled chyme in the duodenum. Not only does it stimulate the digestion of proteins and lipids by increasing pancreatic and hepatic secretions, but it acts as a hunger suppressant via vagal afferent fibres that go to the nucleus tractus solitarius.
Glucagon-like peptide (GIP) is secreted by intestinal enteroendocrine cells in response to food intake. Its role is to stimulate the secretion of insulin (incretin), lowering blood sugar as a result. It also reduces gastric emptying, secretion, and motility, which promotes satiety.
Peptide - Y is released from endocrine cells in ileum and proximal segments of the large intestine mostly after a meal. It reduces gastric emptying (activates ileal brake) thus also promoting satiety.
Amylin is secreted from the endocrine pancreatic islets. It is co-secreted with insulin from B-cells, supplementing its response to hyperglycemia and also inhibiting the release of Glucagon from A-cells in order to continue lowering blood glycaemia.
Ghrelin is released from enteroendocrine cells of the G.I.T. It’s role is to ‘prepare’ the digestive system for food intake by increasing gastric motility and secretions just before a meal. Not only does it have significant endocrine effects on the GIT, but it also widely impacts neural processes, particularly the hypothalamus (nucleus arcuatus). Ghrelin will cause the release of neuropeptide-Y which in turn stimulates appetite in the short term.
Leptin is synthesized by enterocytes and adipose tissues; its primary function is to regulate adipose tissue mass and hunger. It acts directly on leptin receptors of cells, and indirectly mediates the effects of other endocrine food-intake/metabolism regulators. Leptin’s action in the hypothalamus via leptin receptors inhibits hunger sensations; this exact mechanism is complex, and not completely understood. (Hopkins et al. 2016), (Klok, Jacobsdottir and Drent, 2007)
|
Figure 2 Hormonal effects |
Increased levels of melatonin cause a down-regulation of leptin, as demonstrated on rats which underwent pinealectomies or melatonin injections. Leptin’s normal signal informs the hypothalamus when adipose tissues are well-developed, inhibiting hunger due to sufficient storage reserves. High levels of melatonin, such as the ones seen in sleep-deprived individuals, were shown to reduce hunger-inhibiting signals. The over-eating which ensued was attributed to the cascade triggered by increased melatonin levels (Kus et al.,2004).
Pathology
Behavioral changes
Disturbed circadian rhythm has many negative effects on the metabolism of different organ systems, and on food intake behavior. On the behaviour side, humans who have their circadian rhythms disrupted (kept awake in light conditions for an entire night) show a marked preference for higher-fat foods than those that have maintained a normal light-dark and sleep-wake cycle. (Cain et al., 2015). This disrupted choice of diet can lead to obesity and related metabolic illnesses in those who frequently have disturbed circadian rhythms.
Food preference isn’t the only thing affected by circadian disruption, appetite is also modified. The suggested mechanism for this effect is: a reduction in serum leptin (which works as an appetite inhibitor) and an increase in serum ghrelin (which stimulates appetite). Low leptin levels mean that the individual receives a lower satiety signal, and is thus more likely to consume more food. (Cain et al., 2015)
In addition, a high-fat diet will disrupt the circadian rhythm’s ability to synchronise according to light cues. This creates a positive feedback mechanism where individuals will struggle to set themselves back into a ‘normal’ rhythm and promotes an eating behaviour associated with metabolic disease (Mendoza et al., 2008).
Digestive effects
In the digestive tract itself, it has been found that disrupted circadian rhythms cause increased permeability of the intestinal epithelial layer in mice, causing gut leakiness (Summa et al., 2013). This can lead to increased gut inflammation, as well as a change in the microbiome of the gut. This increased intestinal permeability also allows for the damage of other organs, such as liver cells, when harmful materials are able to leave the digestive tract. A circadian disruption can also lead to exacerbated bowel syndrome symptoms (Nokjov et al., 2010), an increased risk in developing colorectal cancer and gastrointestinal complaints commonly associated with jetlag (Preuss et al., 2008).
Summa’s et al., 2013 research provided evidence that a perturbed circadian clock contributes to intestinal epithelial cells being vulnerable to injury. Similarly, sleep deprivation, both acute and chronic, increases inflammation and pathology in DSS (Dextran Sodium Sulfate: induces colitis) treated mice (Tang et al., 2009). Together, these observations designate that circadian organisation is important for optimal gastrointestinal physiological function and highlight the relevance of disruption of circadian rhythms for pathologies within the digestive tract, in particular those involving intestinal epithelial barrier integrity (Summa et al., 2013).
Maintenance of epithelial barrier integrity is necessary for protection from proinflammatory intestinal luminal contents, such as bacteria endotoxins (i.e. lipopolysaccharide, LPS) (Turner, 2009). Turner, 2009 observed that the mucosal barrier is maintained by a complex network of integrating proteins, including tight junction, adherens junction, and desmosomes. Disruption of the barrier (i.e. gut leakiness) is associated with numerous diseases including metabolic syndrome, diabetes, cardiovascular disease, amyotrophic lateral sclerosis, Parkinson’s disease and alcoholic liver disease (Creely et al., 2007; Keshavarzian et al., 2009; Forsyth et al., 2011). Consequently, intestinal hyperpermeability is a clinical pathology and the development of gut leakiness may represent a biologically significant therapeutic target for numerous diseases; however, the factors contributing to the onset of intestinal permeability are poorly understood (Summa et al., 2013).
Several different types of evidence suggest a potential role for circadian clock genes in the regulation of intestinal barrier function. Firstly, many diseases associated with circadian disruption exhibit increased gut leakiness (Portaluppi et al., 2012). Moreover, the primary mechanism of DSS-induced colitis in rodents is impaired intestinal barrier integrity, and Summa’s et al., 2013 study showed that circadian disruption intensifies colitis in DSS-treated mice. Swanson et al., 2011 showed the implication of the circadian clock genes in the regulation of intestinal epithelial barrier integrity in vitro. However, direct in vivo evidence to support the hypothesis that disruption of the circadian homeostasis alters intestinal barrier function is lacking.
|
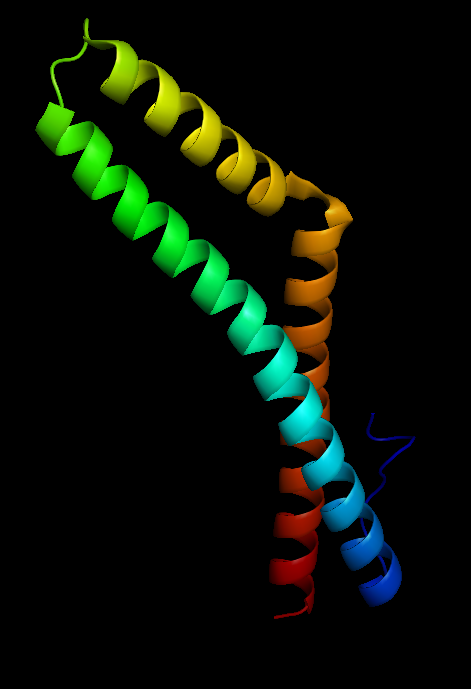
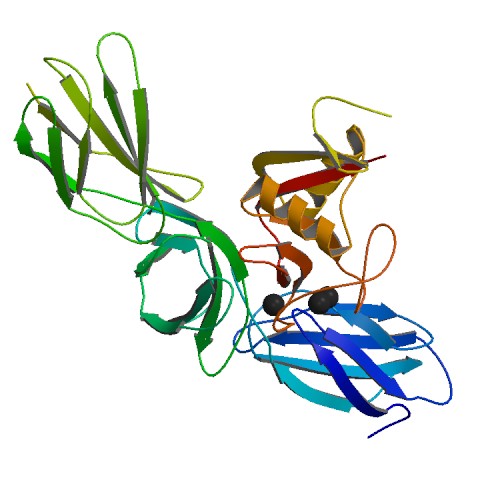
Figure 3 Occludin structure |
Occludin (Figure 3) is an enzyme that oxidises NADH and influences critical aspects of cell metabolism like glucose uptake, ATP production and gene expression. It was first discovered in tight junctions as an integral plasma protein membrane. It is now thought to be found in all body tissues. Its principle functions are to regulate the formation, maintenance and function of tight junctions. Furthermore, in human cells (Figure 4), manipulation of occludin has resulted in its capability to influence the expression of glucose transporters and the activation of transcription factors (Castro et al., 2018).
|
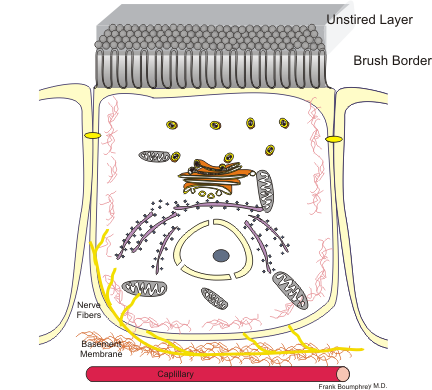
Figure 4 An enterocyte cell |
Skeletal effects
Disrupted circadian rhythms also have an impact on the skeletal system, specifically the joints. In a study by Kc et al. (2015) when mice were fed a high fat diet, those whose circadian rhythms were artificially shifted by using differently timed lighting than they were used to showed an increased progression of osteoarthritis over their non-shifted counterparts. There were also more pronounced signs of knee osteoarthritis in shifted mice fed a normal diet over their non-shifted counterparts.
Cartilage, the important cushioning between joint surfaces, is formed by both collagen and proteoglycans (Figure 5); which are mucopolysaccharide bonded proteins found in connective tissue. Proteoglycans serve to fill in the gaps between an organism's cells and regulate the movement of molecules through the cell matrix.
|
Figure 5 Aggrecan, the major proteoglycan in cartilage, has 2316 amino acids |
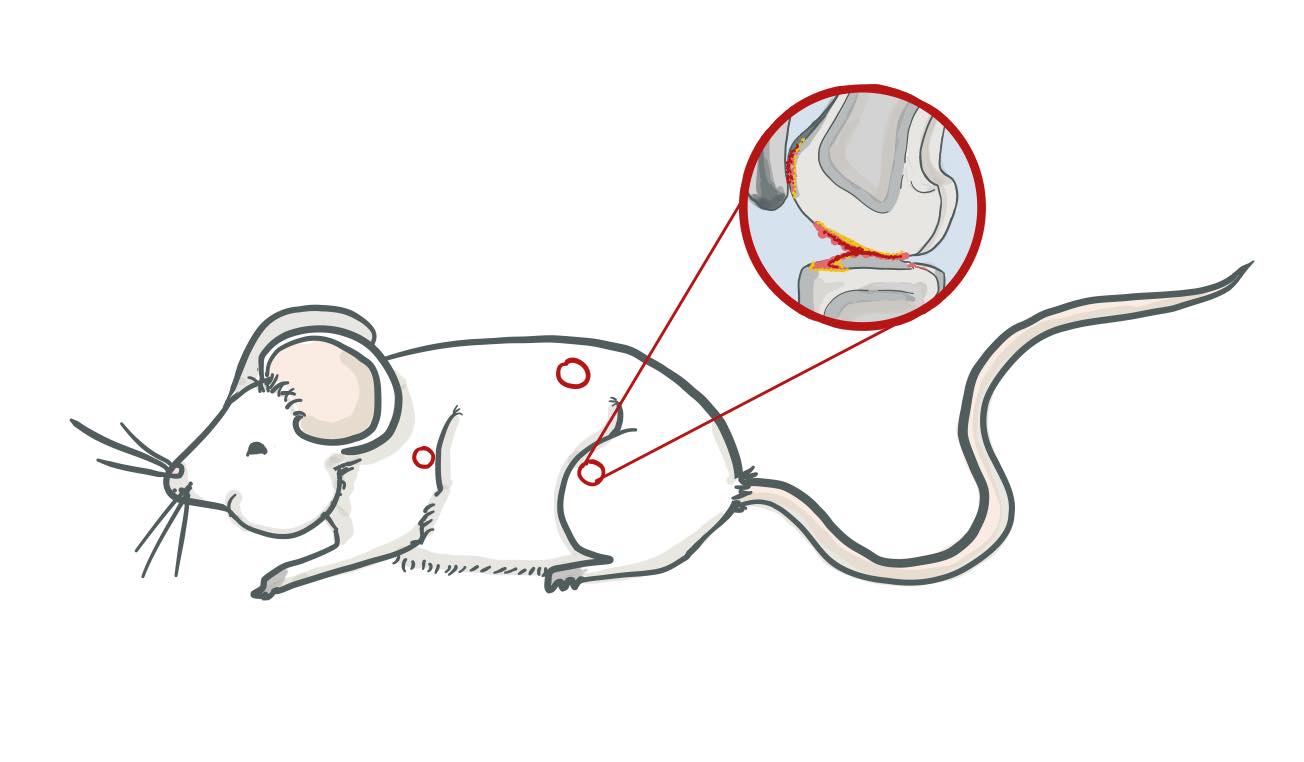
Both sets of mice with a shifted circadian rhythm had decreased proteoglycans in their joints, but those fed a high fat diet showed a much more pronounced proteoglycan decrease, as well as increased joint fibrillation (a splitting of the cartilage into many separate fibers). Neither set of non-shifted mice showed significant proteoglycan changes. When histopathological osteoarthritis grading was performed, with a higher score indicating more severe pathology, normal-diet non-shifted mice scored lower than normal-diet shifted mice, and high-fat shifted mice scored higher than normal-diet shifted mice. High-fat shifted mice showed proteoglycan loss and pathological changes in several joints, including the lumbar spine and glenohumeral joint; but non-shifted high-fat mice did not show changes in joints other than the knee (Figure 6).
|
Figure 6 Joint damage locations in high fat shifted mouse |
In addition to proteoglycan loss, shifted mice had significant disruptions in cartilage homeostasis. This was seen as a reduction in chondroprotective enzymes; as well as abnormally increased levels of chondrocyte hypertrophy markers and matrix degrading enzymes, which were even more elevated in the high fat shifted group. These changes cause a faster breakdown of joint cartilage, without sufficient growth of replacement cartilage.
Taken together these findings show that a shifted circadian rhythm speeds joint degradation and when coupled with a secondary stressor, such as a high fat diet, the effects can be even more profound. The high fat diet as a stressor is particularly important due to the increased preference of high fat foods in animals with a disrupted circadian rhythm, as both conditions are likely to appear together.
Diabetes
The pathophysiology of type 2 diabetes mellitus (T2DM) is complex (Figure 7), but the relevance of the role of the circadian rhythm is clear.
|
Figure 7 Overview of diabetes |
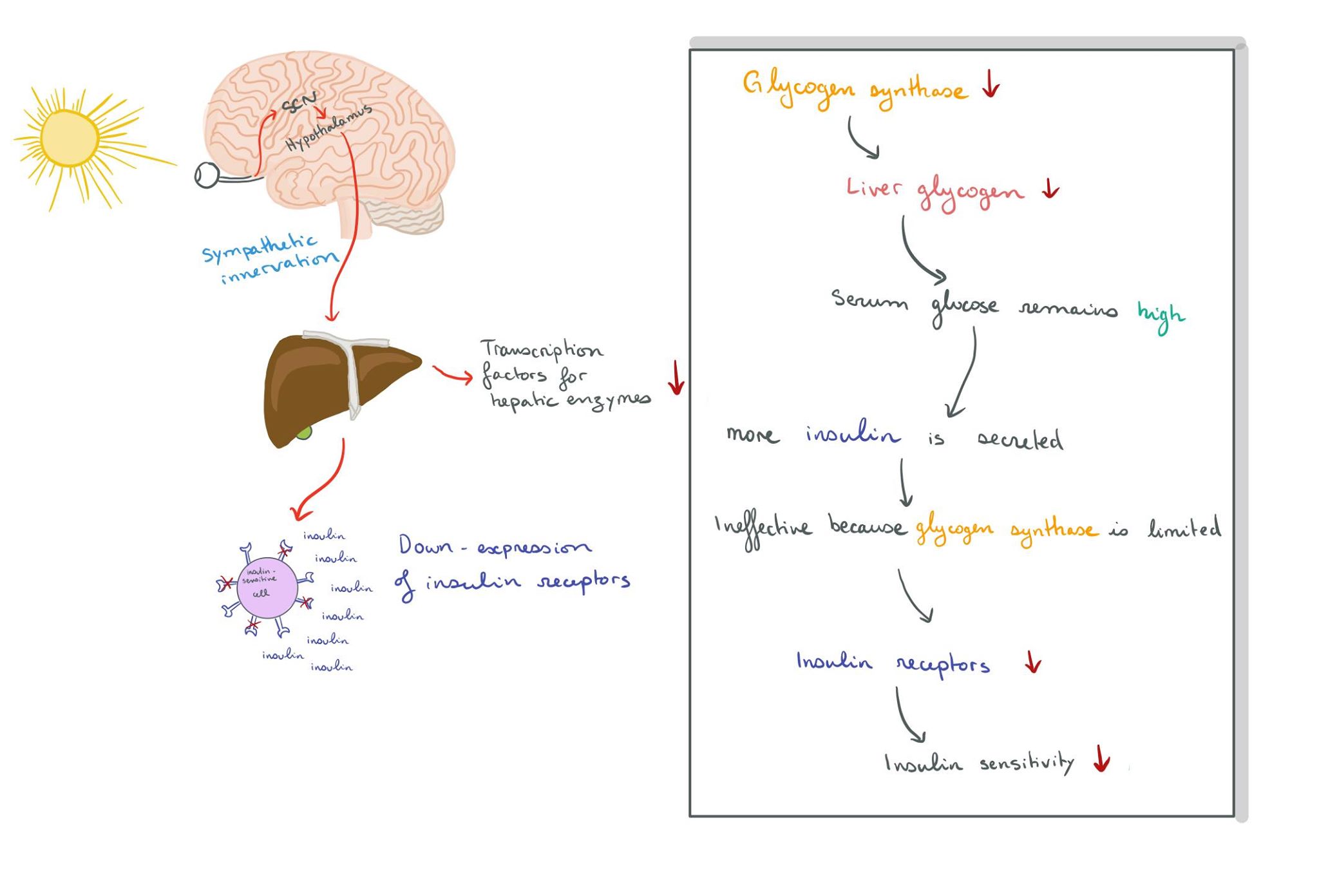
Firstly, the hepatic expression of glycogen synthase shows rhythmic fluctuations under influence from the SCN via melatonin receptors. Glycogen synthase is a rate-limiting enzyme of hepatic glycogenesis and as a result, low levels of glycogen synthase result in less liver glycogen formed. This means that blood glucose cannot be reduced as effectively, resulting in increased pancreatic insulin secretion. High levels of insulin lead to a down-regulation of insulin receptors on insulin-sensitive cells, causing insulin insensitivity and impaired gluconeogenesis (Kalsbeek et al.,2014). Therefore, higher levels of melatonin seen in sleep-deprived individuals reduce hepatic enzymatic transcription factors, resulting in high glycaemia and reducing insulin sensitivity.
Furthermore, sympathetic innervation of the liver was shown to be important in generating rhythmic plasma glucose concentrations. Interactions of the SCN and hypothalamus affect signaling to internal organs, demonstrating that light cues to the SCN can affect other organ systems. Experiments with neurotransmitters released by the SCN showed an increase in plasma glucose concentration, as well as increased glucagon secretion (which stimulates gluconeogenesis). All of the above are signals which promote insulin secretion in order to reduce blood glucose.
The series of experiments concluded that the biological clock was directly able to affect blood glucose concentration via the SCN and its sympathetic hepatic influence on gluconeogenesis, glucose uptake, insulin sensitivity and release.
This hepatic rhythmicity in blood glucose concentration directly affects pancreatic release of glucagon and insulin release. As a result, the pancreas also shows circadian rhythm following hepatic glucose cues. This intricate interaction between the biological clock and blood glucose concentration illustrates how circadian disruptors can easily affect glucose metabolism, leading to disorders such as T2DM.
Conclusion
This research has demonstrated that the effect of food intake is intrinsically linked to the circadian rhythm. It was observed that hormonal regulation has an important role in the food uptake process which then influences the circadian rhythm. A disrupted circadian rhythm can cause serious implications in metabolic processes all over the body. The authors investigated the importance of the circadian rhythm on eating behaviours, digestive physiology, the effect it has on the skeletal system, and diabetes. Eating behaviours were shown to be affected by the circadian rhythm as melatonin has a clear role in the uptake of food via appetite regulation. Disrupted gut physiology leading to gut leakiness due to a deregulated circadian rhythm affecting the intestinal barrier was observed. For the skeletal system, it was found that a shifted circadian rhythm increases joint degradation speed and when coupled with a second stressor like a high-fat diet, the effects can be more pronounced. In this case of blood glucose concentration, the hepatic rhythmicity directly affects the pancreatic release of glucagon and insulin release. Overall, it is clear that the circadian rhythm has an essential role in the regulation of metabolic processes. Therefore, the importance of having a regulated circadian rhythm should not be overlooked when understanding metabolism and possible disorders.
Bibliography
Cain, S.W., Filtness, A.J., Phillips, C.L., Anderson, C. (2015): Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work. Environ. Health 41, 288–293. https://doi.org/10.5271/sjweh.3486
Castro, V., Skowronska, M., Lombardi, J., He, J., Seth, N., Velichkovska, M., Toborek, M. (2018): Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J. Cereb. Blood Flow Metab. 38, 317–332. https://doi.org/10.1177/0271678X17720816
Creely, S.J., McTernan, P.G., Kusminski, C.M., Fisher, ff. M., Da Silva, N.F., Khanolkar, M., Evans, M., Harte, A.L., Kumar, S. (2007): Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 292, E740–E747. https://doi.org/10.1152/ajpendo.00302.2006
Forsyth, C.B., Shannon, K.M., Kordower, J.H., Voigt, R.M., Shaikh, M., Jaglin, J.A., Estes, J.D., Dodiya, H.B., Keshavarzian, A. (2011): Increased Intestinal Permeability Correlates with Sigmoid Mucosa alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. PLoS ONE 6, e28032. https://doi.org/10.1371/journal.pone.0028032
Garbazza, C., Bromundt, V., Eckert, A., Brunner, D.P., Meier, F., Hackethal, S., Cajochen, C. (2016): Non-24-Hour Sleep-Wake Disorder Revisited – A Case Study. Front. Neurol. 7. https://doi.org/10.3389/fneur.2016.00017
Hopkins, M., Blundell, J., Halford, J., King, N., Finlayson, G. (2000): The Regulation of Food Intake in Humans, in: Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., Korbonits, M., McLachlan, R., Morley, J.E., New, M., Perreault, L., Purnell, J., Rebar, R., Singer, F., Trence, D.L., Vinik, A., Wilson, D.P. (Eds.), Endotext. MDText.com, Inc., South Dartmouth (MA).
Kalsbeek, A., la Fleur, S., Fliers, E. (2014): Circadian control of glucose metabolism. Mol. Metab. 3, 372–383. https://doi.org/10.1016/j.molmet.2014.03.002
Kc, R., Li, X., Forsyth, C.B., Voigt, R.M., Summa, K.C., Vitaterna, M.H., Tryniszewska, B., Keshavarzian, A., Turek, F.W., Meng, Q.-J., Im, H.-J. (2015): Osteoarthritis-like pathologic changes in the knee joint induced by environmental disruption of circadian rhythms is potentiated by a high-fat diet. Sci. Rep. 5, 1–7. https://doi.org/10.1038/srep16896
Keshavarzian, A., Farhadi, A., Forsyth, C.B., Rangan, J., Jakate, S., Shaikh, M., Banan, A., Fields, J.Z. (2009): Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 50, 538–547. https://doi.org/10.1016/j.jhep.2008.10.028
Klok, M.D., Jakobsdottir, S., Drent, M.L. (2007): The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 8, 21–34. https://doi.org/10.1111/j.1467-789X.2006.00270.x
Kus, I., Sarsilmaz, M., Colakoglu, N., Kukne, A., Ozen, O.A., Yilmaz, B., Kelestimur, H. (2004): Pinealectomy increases and exogenous melatonin decreases leptin production in rat anterior pituitary cells: an immunohistochemical study. Physiol. Res. 53, 403–408.
Mendoza, J., Pévet, P., Challet, E. (2008): High-fat feeding alters the clock synchronization to light. J. Physiol. 586, 5901–5910. https://doi.org/10.1113/jphysiol.2008.159566
Nojkov, B., Rubenstein, J.H., Chey, W.D., Hoogerwerf, W.A. (2010): The Impact of Rotating Shift Work on the Prevalence of Irritable Bowel Syndrome in Nurses. Am. J. Gastroenterol. 105, 842. https://doi.org/10.1038/ajg.2010.48
Portaluppi, F., Tiseo, R., Smolensky, M.H., Hermida, R.C., Ayala, D.E., Fabbian, F. (2012): Circadian rhythms and cardiovascular health. Sleep Med. Rev. 16, 151–166. https://doi.org/10.1016/j.smrv.2011.04.003
Preuss, F., Tang, Y., Laposky, A.D., Arble, D., Keshavarzian, A., Turek, F.W. (2008): Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 295, R2034–R2040. https://doi.org/10.1152/ajpregu.00118.2008
Starling, S. (2019): Neuronal clock coordinates appetite. Nat. Rev. Endocrinol. 15, 253–253. https://doi.org/10.1038/s41574-019-0196-4
Summa, K.C., Voigt, R.M., Forsyth, C.B., Shaikh, M., Cavanaugh, K., Tang, Y., Vitaterna, M.H., Song, S., Turek, F.W., Keshavarzian, A. (2013): Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PLoS ONE 8. https://doi.org/10.1371/journal.pone.0067102
Swanson, G., Forsyth, C.B., Tang, Y., Shaikh, M., Zhang, L., Turek, F.W., Keshavarzian, A. (2011): Role of Intestinal Circadian Genes in Alcohol-Induced Gut Leakiness. Alcohol. Clin. Exp. Res. 35, 1305–1314. https://doi.org/10.1111/j.1530-0277.2011.01466.x
Tang, Y., Preuss, F., Turek, F.W., Jakate, S., Keshavarzian, A. (2009): Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 10, 597–603. https://doi.org/10.1016/j.sleep.2008.12.009
Turner, J.R. (2009): Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809. https://doi.org/10.1038/nri2653
Figure Sources
Cell_enterocyte.png (432×390) [WWW Document], n.d. URL https://upload.wikimedia.org/wikipedia/commons/e/ef/Cell_enterocyte.png (accessed 4.25.20).
Occludin.png (471×689) [WWW Document], n.d. URL https://upload.wikimedia.org/wikipedia/commons/4/43/Occludin.png (accessed 4.25.20).
PBB_Protein_ACAN_image.jpg (500×500) [WWW Document], n.d. URL https://upload.wikimedia.org/wikipedia/en/4/4b/PBB_Protein_ACAN_image.jpg (accessed 4.25.20).