Long-term effects of different diets on the central nervous system
Contents
Introduction
The food we consume does not only provide our body with the necessary energy for anabolic processes and to perform daily tasks, its nutritious factors have complex biochemical interactions with the molecular mechanisms in each cell, tissue and organ. The central nervous system is strongly affected by diet. The lack or abundance of certain nutrients can alter cognition, and cause or worsen neurological disorders. Most importantly, certain diets can improve brain health and prevent the development of neurological disorders.
We will discuss common diets such as the Standard American (Western) diet, the Mediterranean diet, the Plant-Based diet and the Pescatarian diet, as well as less widespread diets, such as the Ketogenic and Palaeolithic diets.
Standard American Diet
The standard American diet (SAD) is high in refined carbohydrates/sugars and animal-based products such as meats, dairy and eggs. It lacks in whole grains, fruits and vegetables while being high in saturated fat, processed/convenience foods and sodium (Grotto and Zied, 2010). The diet is also high in omega-6 fatty acids and low in omega-3 fatty acids. (Cordain et al, 2005). SADs are correlated to obesity, diabetes type II and hypercholesterolemia (Okręglicka, 2015).
The SAD has been linked to changes in appetite and behaviour. High-calorie foods induce behavioural changes due to the release of dopamine upon consumption. As dopamine is a “feel good” neurotransmitter this can cause addiction-like behaviour and affect the hippocampus and the hypothalamus which have roles in memory and food intake. Once these foods are not consumed, the individual may experience changes in their dopamine levels, receptors, mood and the dopamine-mediated reward system (Johnson and Kenny, 2010; Edward et al, 2011; Reichelt and Rank, 2017).
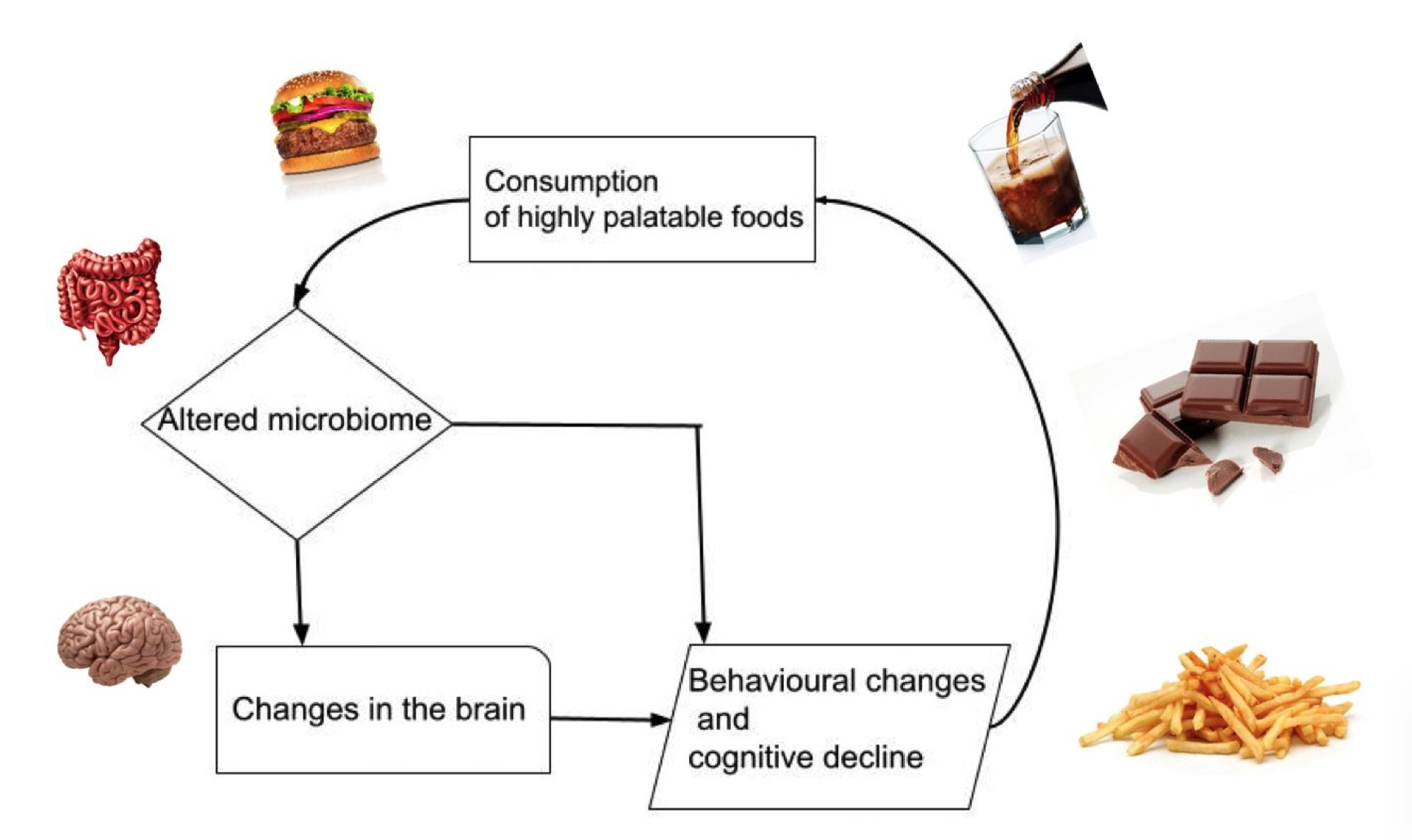
The disturbance of the hypothalamus due to the SAD causes increased appetite (Kanoski et al, 2010; Stevenson et al, 2020). This cycle repeats leading to decreased cognition and a further increase in food intake (Kanoski and Davidson, 2011), as shown in figure 1.
|
Figure 1 The Cycle of Overconsumption of Highly Palatable Foods and the Effects on the Brain. |
The high sugar and fat intake of the SAD also alters memory and contributes to the progression of some neurological disorders. People consuming the SAD are shown to have smaller hippocampi (Jacka et al, 2015), causing changes in memory (Ke et al, 2020). The negative effects on memory can occur even after short-term SAD consumption with participants fed a SAD performing worse in memory tasks compared to control groups (Francis and Stevenson, 2011).
SAD-associated conditions such as diabetes type II and hypercholesterolemia are linked with cognitive decline and brain disorders such as Alzheimer's disease (AD) (Xue-shan et al, 2016; Guo et al, 2016). Thus, AD may be improved with insulin treatment (Hölscher, 2020). Furthermore, lower consumption of high-fat dairy products and meat showed a lower risk of AD (Gu et al, 2010). As more countries adopt a more westernised diet they have seen increases in AD and dementia (Grant, 2014), showing the direct correlation between AD and the SAD.
Attention is also significantly impaired in those individuals on the SAD. Consumption of a high-fat diet leads to increased simple reaction time and decreased attention in study participants (Edwards et al, 2011) and is associated with a higher prevalence of attention deficit hyperactivity disorder (Howard et al, 2011).
Other overall harmful effects of SAD on the CNS include neuroinflammation, brain tissue damage (Pistell et al, 2010) and which may explain why individuals on the SAD experienced prolonged recovery, after traumatic brain injuries (Wu et al, 2003).
Mediterranean diet
The Mediterranean diet (MD) is a traditional diet high in unprocessed plants (grains, vegetables, fruits, legumes, nuts, and seeds), moderate in seafood, and low in animal-based foods and discretionary foods. Extra virgin olive oil and red wine consumption are also characteristic (Radd-Vagenas et al, 2018).
MD has been suggested to reduce systemic inflammation and improve metabolism, which could improve symptoms of Alzheimer’s Disease. MD displays anti-inflammatory, antioxidant, and beneficial microbiome effects which can reduce cognitive decline and improve various neurological functions, such as memory and learning (Radd-Vagenas et al, 2018). Restoring glucose metabolism by reducing plasma glucose levels can reduce the risk for Alzheimer’s Disease and improve cognitive function during ageing (Huhn et al, 2015; Radd-Vagenas et al, 2018). Furthermore, MD can have favourable effects on lipid metabolism, including reducing plasma LDL, and VLDL, and increasing HDL levels. These effects can decrease the risk of strokes (Demarin et al, 2011).
Higher adherence to MD relates to larger grey matter volume, cortex thickness and total brain volume (Gu et al, 2015). Additionally, it decreases amyloidosis and tau pathology, both of which are involved in many neurodegenerative diseases (Ballarini et al, 2021). An improvement in Parkinson's patients was observed, where mean scores of executive function, language, attention, concentration, and memory were higher than in the control group (Paknahad et al, 2020).
The MD ensures the intake of a large range of vitamins and minerals through vegetables, beans, grains, and fruits that nourish the nervous system. Antioxidant properties can be found in phenols, vitamin C, vitamin E, and carotenoids, while vitamin C is especially neuroprotective by eliminating reactive oxygen species and controlling neuroinflammation (Gu et al, 2010; Siervo et al, 2011).
Omega-3 fatty acids (such as DHA) derived from fish have been correlated to improved learning and memory in rodents. It also affects neuronal differentiation and synaptogenesis in the brain. Fish and cereals may reduce the prevalence of Alzheimer’s Disease in the elderly (Panza et al, 2004). Red wine and fruits contain plant polyphenols which act as antioxidants leading to enhanced cognition and reduced risk of age-related cognitive decline. Further neuroprotective properties of polyphenols include decreased mitochondrial dysfunction, hyperglycaemia, and chronic inflammation (Huhn et al, 2015). Potent antioxidants (such as catalase) and oleocanthal (ibuprofen-like compound) can also be found in freshly produced extra virgin olive oil which reduce brain inflammation (Siervo et al, 2011). A French cohort consuming three to four glasses of red wine per day showed lower risks of Alzheimer’s disease compared to non-alcohol drinkers (Panza et al, 2004).
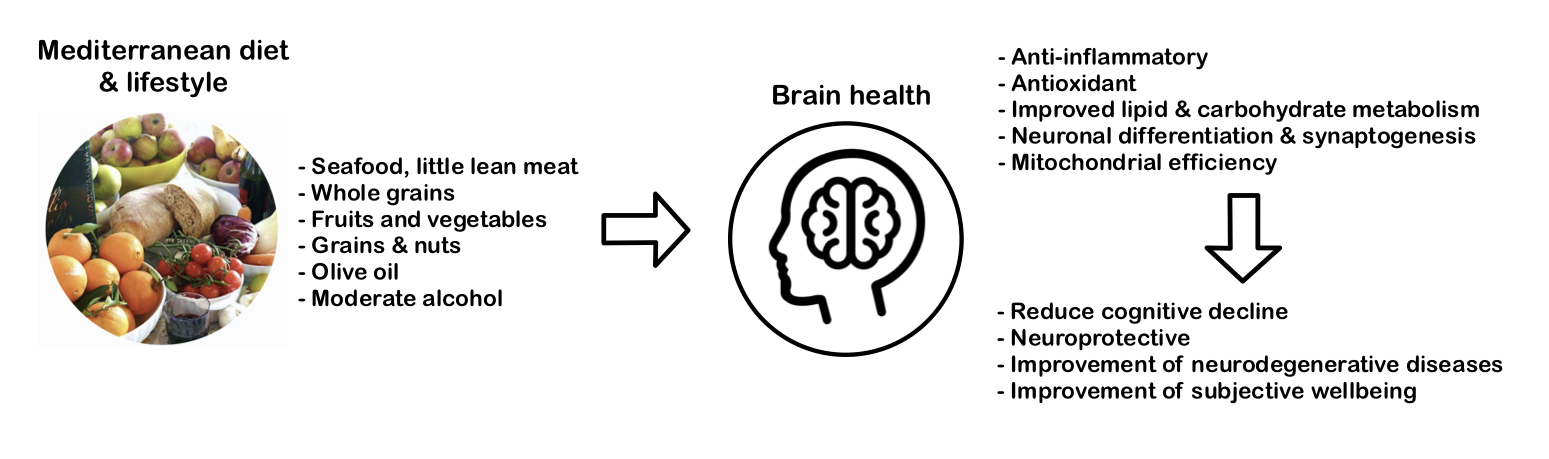
Stand-alone research found that adolescents adhering to MD had higher academic performance (maths, language, GPA) compared to the poor adherence group (Esteban-Cornejo, 2016). Another study with adolescents was conducted using the “Subjective happiness and health-related quality of life” (HRQOL) scores. They found that high adherence to MD was associated with higher scores in areas such as psychological well-being and better mood (Ferrer-Cascales et al, 2019). The figure 2 below demonstrates the composition and effects of MD on the brain.
|
Figure 2 The Components of a Mediterranean Diet, its Neurological Effects and Benefits. |
Most studies have pointed out that clinical trials suffer from inconsistent results due to differences in diet. Most results also rely on animal experiments instead of clinical observations (Féart et al, 2013; Huhn et al, 2015; Siervo et al, 2021). Crichton et al (2013) were one of the research groups that found no correlation between MD and higher cognitive function. A reason could be that MD is less of a diet and more of a culture that includes healthy habits, lifestyle, and social factors. This was shown by the association between MD and overall mental health in a community in Croatia (Andrade et al, 2020).
Plant-Based (Vegetarian and Vegan) diet
The Plant-Based Diet (PBD) is a diet that excludes meat. Veganism, vegetarian, lacto-vegetarianism, and lacto-ovo-vegetarianism are versions of this diet. Fruit, vegetables, legumes, nuts, grains, and soy are typically dominant in this diet (McEvoy et al, 2012).
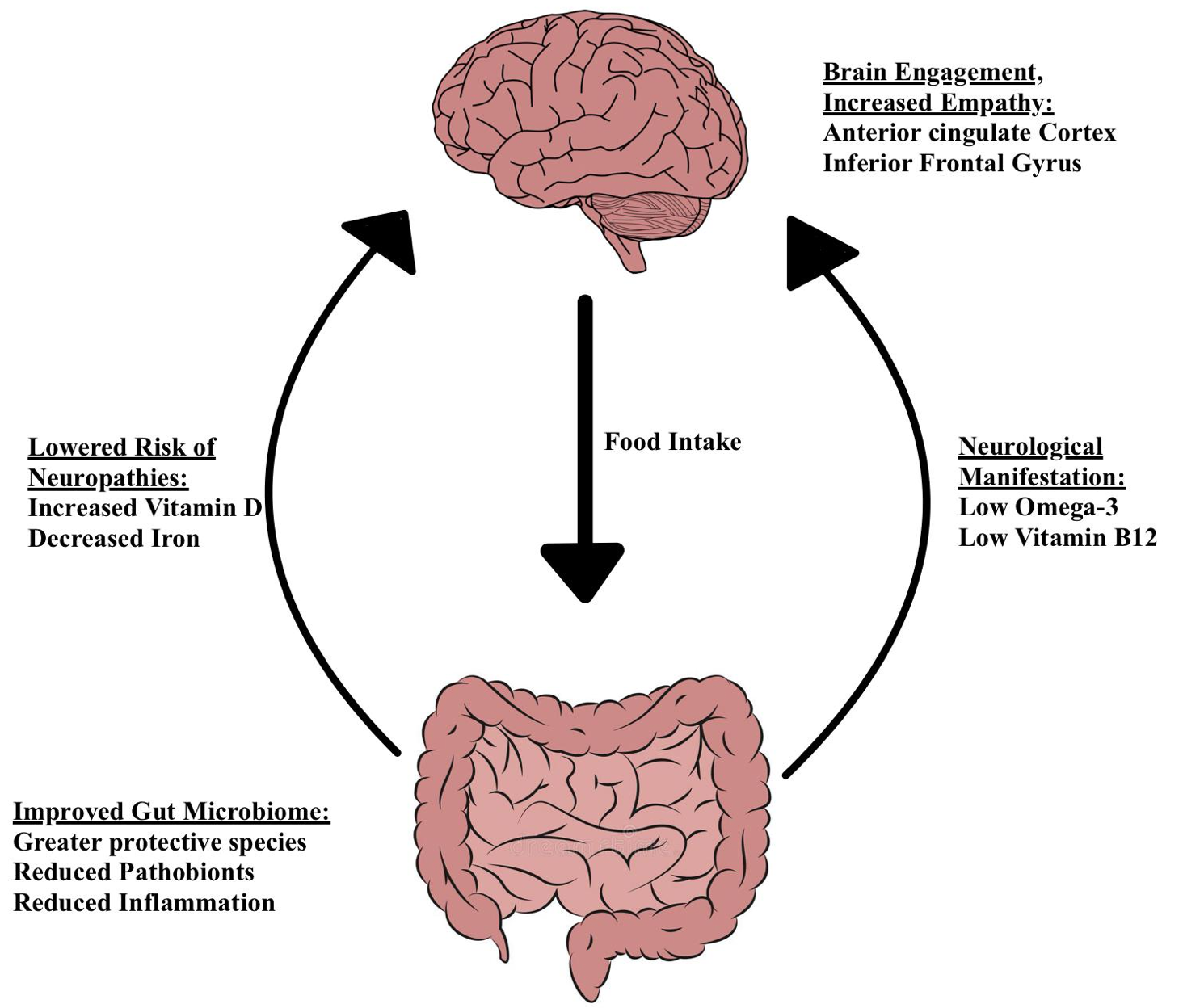
The structure and activity of bacteria in the human gut are influenced by long-term nutrition (David et al, 2013). PBD causes a major shift in the microbiota composition (Zimmer and colleagues, 2011). The gut profiles of PBD show fewer pathogens, more defensive species, and less inflammation (Yeh and Glick-Bauer, 2014). Changes in the gut-brain axis mediate pathogenesis and pathophysiology of psychiatric and neurologic pathologies (Martin et al, 2018), as shown in figure 3.
Individuals on a PBD are at high-risk for vitamin B12 deficiency. The degenerative abnormalities in the CNS are responsible for the neurological signs of vitamin B12 deficiency (Kapoor et al, 2017). PBD causes decreased iron reserves (McEvoy et al, 2012). This can be beneficial since increased iron concentrations have been linked to neuropathologies like Alzheimer's, Parkinson's, and multiple sclerosis (Ward et al, 2014).
Vitamin D deficiency is more common in strict PBD, especially those who live in northern latitudes (McEvoy et al, 2012). Vitamin D deficiency is linked to neurological illnesses (Moretti et al, 2018).
PBD may have fewer Omega-3 fatty acids (N-3) and more Omega-6 fatty acids (N-6) than meat-based diets (McEvoy et al, 2012). Low N-3 levels compared to N-6 levels are linked to a higher risk of suicide attempts and suicide (Liu et al., 2015) (Sublette et al, 2006). Sufficient N-3 can be obtained from plant sources, such as algae, however, most people consume an abundance of N-6 containing foods (Doughman et al, 2007). Thus, it is not necessarily the lack of N-3 that poses a threat to health, but rather the imbalance in the N-3:N-6 ratio (Liu et al, 2015).
Vegetarians and vegans, regardless of species, engage more empathy-related regions (anterior cingulate brain and inferior frontal gyrus) when seeing distressing scenarios (Filippi et al., 2010).
PBD can cause neuropathies, although these can be easily mitigated with the right supplementation. Those who followed a nutritious PBD had a lower incidence of total stroke (Baden et al, 2021). Medawar et al. (2019) conducted a systematic evaluation of clinical studies and found evidence supporting the health and disease benefits of a PBD. More research is needed to better understand mental and cognitive consequences of PBD.
|
Figure 3 The Neurological Effects of a Plant Based Diet on the Gut-Brain Axis. |
Pescatarian diet
The pescatarian diet resembles the vegetarian diet however, it incorporates seafood. Fish meat contains two polyunsaturated omega 3 fatty acid: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
EPA and DHA are essential components of membranes in the body, including membranes of human neurons (Valentine and Valentine, 2004). They, therefore, have an important role in neuronal function.
It is important to note that EPA and DHA may be synthesised from alpha-linolenic acid acquired from non-fish food sources. However, this is not very efficient and relying on this may lead to a deficiency of these fatty acids (Gerster, 1998).
DHA and EPA are essential in the development and function of the brain. Women who were supplemented with fish oil during pregnancy were shown to have children with better cognitive skills (Judge et al, 2007; Helland et al, 2003) and hand-eye coordination (Dunstan et al, 2006) when compared to control groups.
Due to DHA and EPA making up a large amount of the neuron membrane phospholipids, they have been linked with neurological and cognitive disorders such as Alzheimer’s disease (AD). Patients with AD were shown to have had lower levels of omega-3 fatty acids in the blood than those without the disease (Tully et al, 2003). Similarly, Gu et al (2010) found that diets high in DHA and EPA were inversely correlated to the risk of AD. Other studies that supplemented patients with fish oil as a treatment showed potentially promising results in mild cases of AD. Cases of moderate to severe AD did not show significant improvement when compared to the control group (Freund-Levi et al, 2006).
Studies in both rats (Naliwaiko et al, 2004) and humans (Mocking et al, 2016) used omega-3 fatty acids as a treatment for depression. The combination of EPA with antidepressants has been shown to aid in depression treatment. (Mocking et al, 2016).
Zanarini and Frankenburg (2003) found that EPA may be an effective treatment for borderline personality disorder while Montgomery and Richardson (2008) found it reduced depressive symptoms in bipolar patients however both studies contained a small sample size.
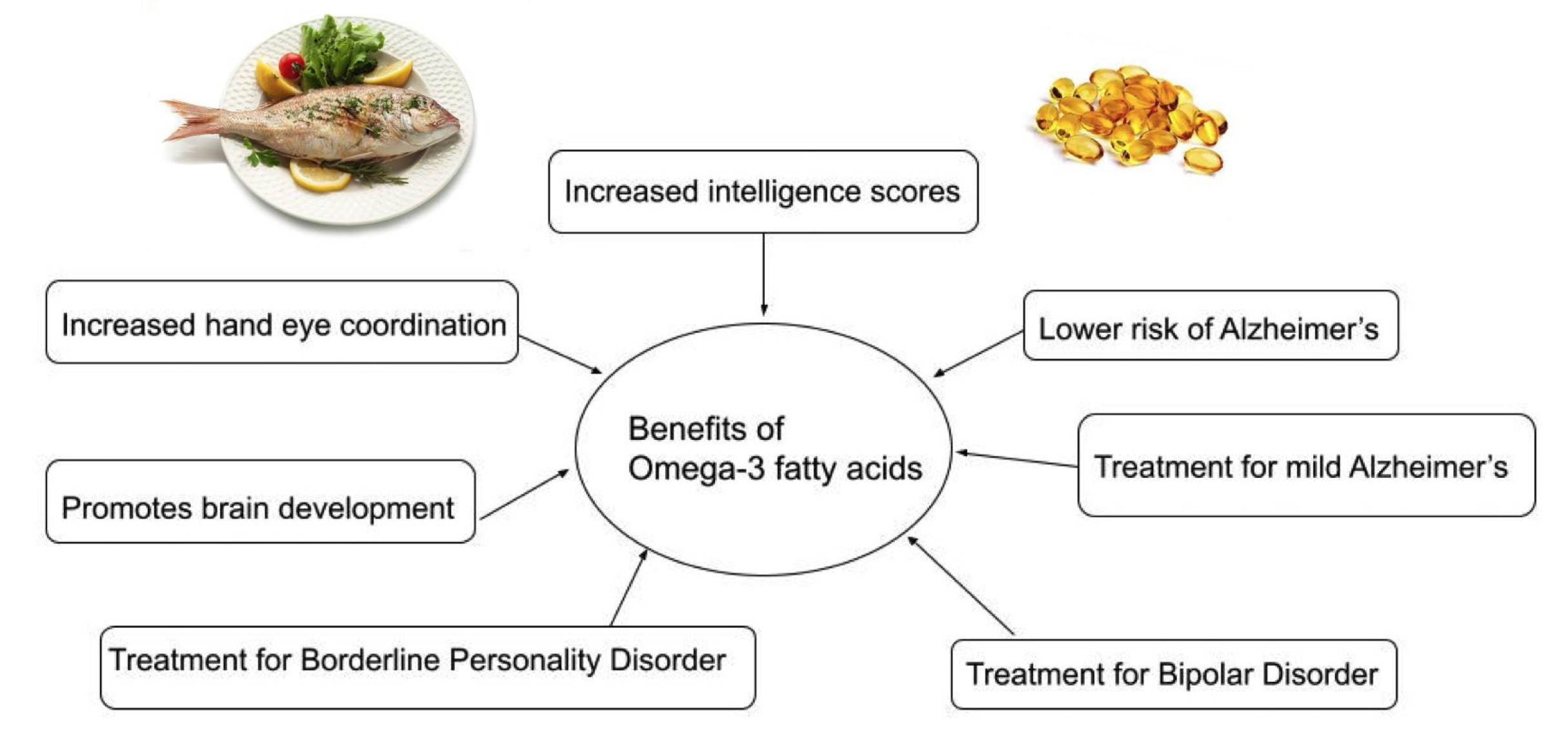
Due to the exclusion of meat in this diet, from a neurological view, the pescatarian diet has all the benefits of a vegetarian diet with additional benefits of higher amounts of EHA and DHA. The findings of the studies above are demonstrated in figure 4.
|
Figure 4 Benefits of Omega-3 Fatty Acids Found in Fish on the CNS. |
Keto diet
The ketogenic diet (KD), originally used by Wilder in 1921, is based on consuming a high amount of fats and a low percentage of proteins and carbohydrates (Hartman et al, 2007).
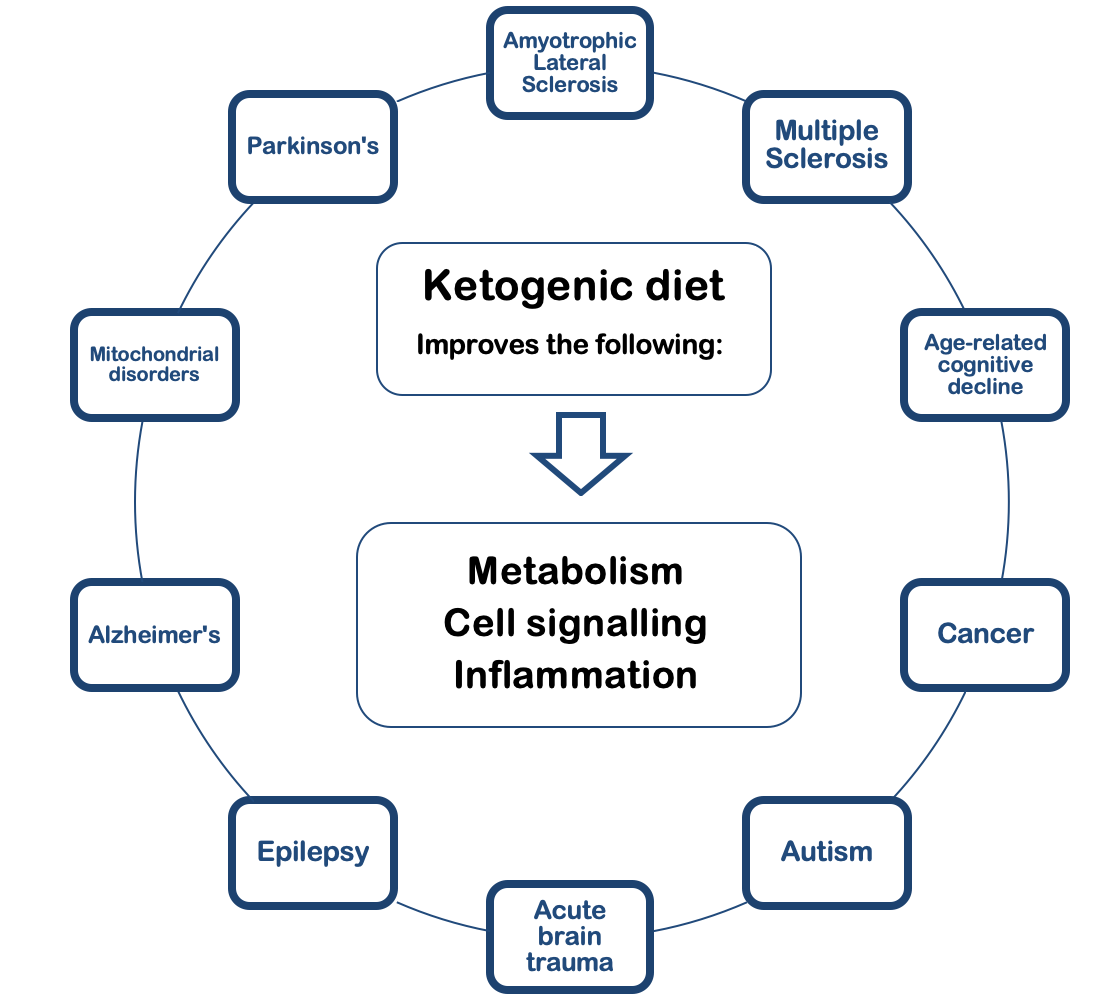
KD has been proven to be an effective therapy for epilepsy, although there is an incomplete understanding of the underlying mechanisms. It is speculated that any disease based on abnormal cellular respiration can be treated with KD. It not only affects metabolism but also cell signalling and inflammatory regulation (Rho and Stafstrom, 2012). The range of diseases potentially treatable with KD are listed in figure 5.
Fundamentally, KD improves mitochondrial functioning by replacing glucose with ketone bodies, leading to decreased reactive oxygen species and increased ATP levels. Due to its broad neuroprotective properties, it improves neurodegenerative illnesses such as Alzheimer’s Disease, Parkinson’s disease, mitochondrial disorders, GLUT1 deficiency syndrome, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis (Rusek et al, 2019; Sandu et al, 2019). This also applies to the hippocampus and prefrontal cortex dysfunctions present in general age-related cognitive decline (Hernandez et al, 2018).
Apart from improving energy homeostasis in diseases, KD may decrease damage during acute brain trauma such as strokes, and improve abnormal behaviours in neurodevelopmental disorders such as autism (Rho and Stafstrom, 2012).
KD can treat certain types of brain tumours and cancers since it deprives the rapidly dividing cancer cells of their primary energy source (glucose). KD is shown to reduce angiogenesis, inflammation, edema, and migration of the tumour itself, while enhancing standard cancer therapies (Vidali et al, 2015; Woolf et al, 2016).
|
Figure 5 The Positive Effects of Ketogenic Diet on Various Neurological Disorders. |
Most available studies are based on small sample sizes and short durations. KD has to be applied with caution and upon consultation with a medical professional since tolerance varies on a patient-to-patient basis. Healthy (epilepsy-free) individuals are discouraged from adopting this diet. It can worsen illnesses or create previously absent health problems, such as kidney failure, liver failure, hypoglycaemia, ketoacidosis, cardiomyopathy, and general malnutrition (Watanabe et al, 2020).
The modified Atkins diet was implemented in 2003 for epileptic children and adults as an alternative to KD, due to its difficult application. It is less restrictive than KD but shows similar efficacy in reducing seizures and better tolerability among patients (Kossoff, 2013). Furthermore, preliminary trials have already shown promising results for Alzheimer’s treatment (Brandt et al, 2019).
Palaeolithic diet
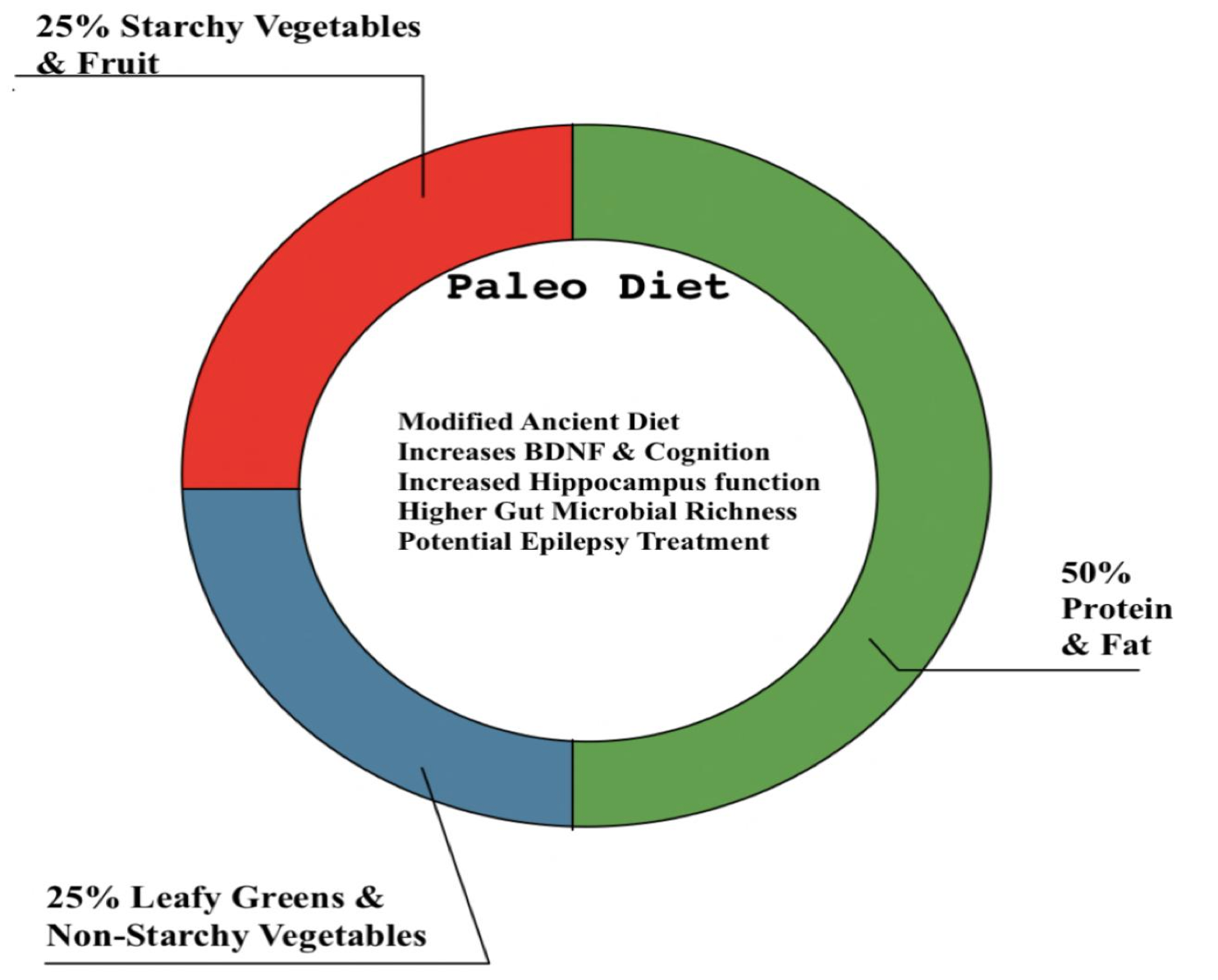
The palaeolithic diet (PD) resembles the diet of a prehistoric hunter-gatherer and limits the food that became available with farming, such as dairy products, convenience foods, sweets, grains, and alcohol (Zopf et al, 2018). The components and effects of PD are summarised in figure 6.
|
Figure 6 The Composition and Neurological Benefits of a Palaeolithic Diet. |
PD shows short-term improvement in patients with metabolic syndrome by increasing levels of BDNF (brain-derived neurotrophic factor) and cognition (Gyorkos et al, 2019). An increase in BDNF was also strongly linked to an increase in functional brain response in the right anterior hippocampus (Stomby et al, 2017).
When compared to microbiota from urban Italians on a Mediterranean diet, the gut microbiota of present PD groups has higher microbial richness and biodiversity (Zopf et al, 2018; Barone et al, 2019).
The palaeolithic ketogenic diet was shown to be effective, safe, and practical for treating paediatric absence epilepsy. However, it has yet to be clinically validated in the treatment of epilepsy (Clemens et al, 2013).
Bibliography
Andrade, V.; Jorge, R.; García-Conesa, M.-T.; Philippou, E.; Massaro, M.; Chervenkov, M.; Pinto, P. (2020): Mediterranean Diet Adherence and Subjective Well-Being in a Sample of Portuguese Adults. Nutrients 12: (12), 3837
Baden, M. Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J. E.; Rimm, E. B.; Willett, W. C.; Hu, F. B.; Rexrode, K. M. (2021): Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 96 (15)
Ballarini, T.; Melo van Lent, D.; Brunner, J.; Schröder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; Duzel, E.; Ertl-Wagner, B.; Fliessbach, K.; Freiesleben, S. D.; Frommann, I.; Glanz, W.; Hauser, D.; Haynes, J. D.; Heneka, M. T.; Janowitz, D. (2021): Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology 96: (24) 2920-2932
Barone, M.; Turroni, S.; Rampelli, S.; Soverini, M.; D’Amico, F.; Biagi, E.; Brigidi, P.; Troiani, E.; Candela, M. (2019): Gut microbiome response to a modern Paleolithic diet in a western lifestyle context. PLOS ONE 14
Brandt, J.; Buchholz, A.; Henry-Barron, B.; Vizthum, D.; Avramopoulos, D.; Cervenka, M. (2019): Preliminary Report on the Feasibility and Efficacy of the Modified Atkins Diet for Treatment of Mild Cognitive Impairment and Early Alzheimer’s Disease. Journal of Alzheimer’s Disease 1: (13)
Clemens, Z.; Kelemen, A.; Fogarasi, A.; Tóth, C. (2013): Childhood absence epilepsy successfully treated with the palaeolithic ketogenic diet. Neurology and Therapy 2: 71–76
Crichton, G. E.; Bryan, J.; Hodgson, J. M.; Murphy, K. J. (2013): Mediterranean diet adherence and self-reported psychological functioning in an Australian sample. Appetite 70: 53-59.
David, L. A.; Maurice, C. F.; Carmody, R. N.; Gootenberg, D. B.; Button, J. E.; Wolfe, B. E.; Ling, A. V.; Devlin, A. S.; Varma, Y.; Fischbach, M. A.; Biddinger, S. B.; Dutton, R. J.; Turnbaugh, P. J. (2013): Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563
Demarin, V.; Lisak, M.; Morović, S. (2011): Mediterranean Diet in Healthy Lifestyle and Prevention of Stroke. Acta clinica Croatica 50: (1)
Doughman, S. D.; Krupanidhi, S.; Sanjeevi, C. B. (2007): Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Current Diabetes Reviews 3: (3):198-203
Dunstan, J. A.; Simmer, K.; Dixon, G.; Prescott S. L. (2006): Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 93: (1) 45-50
Edwards, L. M.; Murray, A. J.; Holloway, C. J.; Carter, E. E.; Kemp, G. J.; Codreanu, I. (2011): Short-term consumption of a high-fat diet impairs whole-body efficiency and cognitive function in sedentary men. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 25: (3) 1088–1096
Esteban-Cornejo, I.; Izquierdo-Gomez, R.; Gómez-Martínez, S. Padilla-Moledo, C.; Castro-Piñero, J.; Marcos, A.; Veiga, O. L. (2016): Adherence to the Mediterranean diet and academic performance in youth: the UP&DOWN study. European Journal of Nutrition 55: 1133–1140
Féart, C.; Samieri, C.; Allès, B.; Barberger-Gateau, P. (2013): Potential benefits of adherence to the Mediterranean diet on cognitive health. Proceedings of the Nutrition Society 72: (1) 140-152
Ferrer-Cascales, R.; Albaladejo-Blázquez, N.; Ruiz-Robledillo, N.; Clement-Carbonell, V.; Sánchez-SanSegundo, M.; Zaragoza-Martí, A. (2019): Higher Adherence to the Mediterranean Diet is Related to More Subjective Happiness in Adolescents: The Role of Health-Related Quality of Life. Nutrients 11: (3) 698
Filippi, M.; Riccitelli, G.; Falini, A.; Di Salle, F.; Vuilleumier, P.; Comi, G.; Rocca, M. A. (2010): The brain functional networks associated with human and animal suffering differ among omnivores, vegetarians and vegans. PLoS ONE 5
Francis, H. M. and Stevenson, R. J. (2011): Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behavioral Neuroscience, 125: (6) 943–955
Freund-Levi, Y.; Eriksdotter-Jonhagen, M.; Cederholm, T.; Basun, H.; Faxen-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L. O.; Palmblad, J. (2006): Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Archives of Neurology 63: 1402–1408
Gerster, H. (1998): Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? International Journal for Vitamin and Nutrition Research 68: (3) 159-73
Glick-Bauer, M. and Yeh, M-C. (2014): The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 6: 4822–4838
Grant, W. B. (2014): Trends in Diet and Alzheimer’s Disease During the Nutrition Transition in Japan and Developing Countries, Journal of Alzheimer's Disease 38: (3) 611 – 620
Grotto, D. and Zied, E. (2010): The Standard American Diet and Its Relationship to the Health Status of Americans. Nutrition in Clinical Practice 25: 603-612
Gu, Y.; Nieves, J. W.; Stern, Y.; Luchsinger, J. A.; Scarmeas, N. (2010): Food combination and Alzheimer disease risk: a protective diet. Archives of Neurology 67: (6) 699-706
Gu, Y.; Brickman, A. M.; Stern, Y.; Habeck, C. G.; Raylighi, Q. R.; Luchsinger, J. A.; Manly, J. J.; Schupf, N.; Mayeux, R.; Scarmeas, N. (2015): Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 85: (20) 1744-1751
Gu, Y.; Luchsinger, J. A.; Stern, Y.; Scarmeas, N. (2010): Mediterranean Diet, Inflammatory and Metabolic Biomarkers, and Risk of Alzheimer’s Disease. Journal of Alzheimer's Disease 22: (2) 483-492
Guo, C.; Zhang, S.; Li, J. Y; Ding, C.; Yang, Z. H.; Chai, R.; Wang, X.; Wang, Z. Y. (2016): Chronic hyperglycemia induced via the heterozygous knockout of Pdx1 worsens neuropathological lesion in an Alzheimer mouse model. Scientific Reports 12: (6)
Gyorkos, A.; Baker, M. H.; Miutz, L. N.; Lown, D. A.; Jones, M. A.; Houghton-Rahrig, L. D. (2019): Carbohydrate-restricted diet and exercise increase brain-derived neurotrophic factor and cognitive function: A randomized crossover trial. Cureus.
Hartman, A. L.; Gasior, M.; Vining, E. P. G.; Rogawski, M. A. (2007): The Neuropharmacology of the Ketogenic Diet. Pediatric Neurology 36: (5) 281-292
Helland, I. B.; Smith, L.; Saarem, K.; Saugstad, O. D.; Drevon, C. A. (2003): Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 111: (1) 39-44
Hernandez, A. R.; Hernandez, C. M.; Campos, K.; Truckenbrod, L.; Federico, Q.; Moon, B.; McQuail, J. A.; Maurer, A. P.; Bizon, J. L.; Burke, S. N. (2018): A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus. Frontiers in Aging Neuroscience 10: (391)
Hölscher, C. (2020): Brain insulin resistance: role in neurodegenerative disease and potential for targeting. Expert Opinion on Investigational Drugs 29: (4) 333-348
Howard, A. L.; Robinson, M.; Smith, G. J.; Ambrosini, G. L.; Piek, J. P.; Oddy, W. H. (2011): ADHD is associated with a ‘‘Western’’ dietary pattern in adolescents. Journal of Attention Disorder 15: (5) 403–411
Huhn, S.; Kharabian Masouleh, S.; Stumvoll, M.; Villringer, A.; Witte, A. V. (2015): Components of a Mediterranean diet and their impact on cognitive functions in aging. Frontiers in Aging Neuroscience 7: (132)
Jacka, F. N.; Cherbuin, N.; Anstey, K. J.; Sachdev, P.; Butterworth, P. (2015): Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Medicine 8: (13) 215
Johnson, P. M.; Kenny, P. J. (2010): Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience 13: (5): 635-641
Judge, M. P.; Harel, O.; Lammi-Keefe, C. J. (2007): Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. The American Journal of Clinical Nutrition 85: (6) 1572-7
Kanoski, S. E., Davidson, T. L. (2011): Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiology & Behavior 103: (1): 59-68
Kanoski, S. E.; Zhang, Y.; Zheng, W.; Davidson, T. L. (2010): The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. Journal of Alzheimer's Disease, 21: (1): 207-219
Kapoor, A.; Baig, M.; Tunio, S. A.; Memon, A. S.; Karmani, H. (2017): Neuropsychiatric and neurological problems among vitamin B12 deficient young vegetarians. Neurosciences 22: 228–232
Ke, X.; Fu, Q.; Sterrett, J.; Hillard, C. J.; Lane, R. H.; Majnik, A. (2020): Adverse maternal environment and western diet impairs cognitive function and alters hippocampal glucocorticoid receptor promoter methylation in male mice. Physiological Reports 8: (8)
Kossoff, E. H.; Cervenka, M. C.; Henry, B. J.; Haney, C. A.; Turner Z. (2013): A decade of the modified Atkins diet (2003–2013): Results, insights, and future directions, Epilepsy & Behavior 29: (3) 437-442
Liu, J. J.; Green, P.; Mann, J.; Rapoport, S. I.; Sublette, M. E. (2015): Pathways of polyunsaturated fatty acid utilization: Implications for brain function in Neuropsychiatric Health and disease. Brain Research 1597: 220–246
Martin, C. R.; Osadchiy, V.; Kalani, A.; Mayer, E. A. (2018): The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology 6: 133–148
McEvoy, C. T.; Temple, N.; Woodside, J. V. (2012): Vegetarian diets, low-meat diets and Health: A Review. Public Health Nutrition 15: 2287–2294
Medawar, E.; Huhn, S.; Villringer, A.; Witte, A. V. (2019): The effects of plant-based diets on the body and the brain: A systematic review. Translational Psychiatry 9
Mocking, R. J. ; Harmsen, I. ; Assies, J. ; Koeter, M. W. ; Ruhé, H. G. ; Schene, A. H. (2016): Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Translational Psychiatry 15: (3) 756
Montgomery, P. and Richardson, A. J. (2008): Omega-3 fatty acids for bipolar disorder. The Cochrane Database of Systematic Reviews 16: (2)
Moretti, R.; Morelli, M. E.; Caruso, P. (2018): Vitamin D in neurological diseases: A rationale for a pathogenic impact. International Journal of Molecular Sciences 19: 2245
Naliwaiko, K.; Araújo, R. L.; da Fonseca, R. V.; Castilho, J. C.; Andreatini, R.; Bellissimo, M. I.; Oliveira, B. H.; Martins, E. F.; Curi, R.; Fernandes, L. C.; Ferraz, A. C. (2004): Effects of fish oil on the central nervous system: a new potential antidepressant? Nutritional Neuroscience 7: (2) 91-9
Okręglicka, K. (2015): Health effects of changes in the structure of dietary macronutrients intake in western societies. Roczniki Państwowego Zakładu Higieny 66: (2) 97-105
Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. (2020): The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complementary Therapies in Medicine 50
Panza, F.; Solfrizzi, V.; Colacicco, A.; D'Introno, A.; Capurso, C.; Torres, F., Del Parigi, A.; Capurso, S.; Capurso, A. (2004). Mediterranean diet and cognitive decline. Public Health Nutrition 7: (7) 959-963
Radd-Vagenas, S.; Duffy, S. L.; Naismith, S. L.; Brew, B. J.; Flood, V. M.; Fiatarone Singh, M. A. (2018): Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. The American Journal of Clinical Nutrition 107: (3) 389–404
Reichelt, A. C.; Rank, M. M. (2017): The impact of junk foods on the adolescent brain. Birth Defects Research 109: (20) 1649–1658
Rho, J.; Stafstrom, C. (2012): The Ketogenic Diet as a Treatment Paradigm for Diverse Neurological Disorders. Frontiers in Pharmacology 3: (59)
Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S. J. (2019): Ketogenic Diet in Alzheimer’s Disease. International Journal of Molecular Sciences 20: (16) 3892
Sandu, C.; Burloiu, C. M.; Barca, D. G.; Magureanu, S. A.; Craiu, D. C. (2019): Ketogenic Diet in Patients with GLUT1 Deficiency Syndrome. Maedica 14: (2) 93–97
Siervo, M.; Shannon, O. M.; Llewellyn, D. J.; C. M. S., Blossom; Fontana, L. (2021): Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radical Biology and Medicine 176: 105-117
Stevenson, R. J.; Francis, H. M.; Attuquayefio ,T.; Gupta, D.; Yeomans, M. R. ; Oaten, M. J.; Davidson, T. (2020): Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. Royal Society Open Science 7: (19)
Stomby, A.; Otten, J.; Ryberg, M.; Nyberg, L.; Olsson, T.; Boraxbekk, C-J. (2017): A palaeolithic diet with and without combined aerobic and resistance exercise increases functional brain responses and hippocampal volume in subjects with type 2 diabetes. Frontiers in Aging Neuroscience 9
Sublette, M. E.; Hibbeln, J. R.; Galfalvy, H.; Oquendo, M. A.; Mann, J. J. (2006): Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. American Journal of Psychiatry 163: 1100–1102
Tully, A. M.; Roche, H. M.; Doyle, R.; Fallon, C.; Bruce, I.; Lawlor, B.; Coakley, D.; Gibney, M. J. (2003): Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. British Journal of Nutrition 89: (4) 483-489
Valentine, R. C.; Valentine, D. L. (2004): Omega-3 fatty acids in cellular membranes: a unified concept. Progress in Lipid Research 43: (5) 383-402
Vidali, S.; Aminzadeh, S.; Lambert, B.; Rutherford, T.; Sperl, W.; Kofler, B.; Feichtinger, R. G. (2015): Mitochondria: The ketogenic diet—A metabolism-based therapy. The International Journal of Biochemistry & Cell Biology 63: 55-59
Ward, R. J.; Zucca, F. A.; Duyn, J. H.; Crichton, R. R.; Zecca, L. (2014): The role of iron in Brain Ageing and Neurodegenerative Disorders. The Lancet Neurology 13: 1045–1060
Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C. (2020): Scientific evidence underlying contraindications to the ketogenic diet: An update. Obesity Reviews 21: (10)
Woolf, E. C.; Syed, N.; Scheck, A. C. (2016): Tumor Metabolism, the Ketogenic Diet, and β-Hydroxybutyrate: Novel Approaches to Adjuvant Brain Tumor Therapy. Frontiers in Molecular Neuroscience 9: (122)
Wu, A.; Molteni, R.; Ying, Z.; Gomez-Pinilla, F. (2003): A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience 119: (2) 365-75
Xue-Shan, Z.; Juan, P.; Qi, W.; Zhong, R.; Li-Hong, P.; Zhi-Han, T.; Zhi-Sheng, J.; Cordain, L.; Eaton, S. B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B. A.; O'Keefe, J. H.; Brand-Miller, J. (2005): Origins and evolution of the Western diet: health implications for the 21st century. The American Journal of Clinical Nutrition 81: (2): 341-354
Xue-Shan, Z.; Juan, P.; Qi, W.; Zhong, R.; Li-Hong, P.; Zhi-Han, T.; Zhi-Sheng, J.; Gui-Xue, W.; Lu-Shan, L. (2016): Imbalanced cholesterol metabolism in Alzheimer's disease. Clinica Chimica Acta 1: (456) 107-114
Zanarini, M. C; Frankenburg, F. R. (2003): omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. The American Journal of Psychiatry 160: (1) 167-169
Zimmer, J.; Lange, B.; Frick, J-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. (2011): A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. European Journal of Clinical Nutrition 66: 53–60
Zopf, Y.; Reljic, D.; Dieterich, W. (2018): Dietary effects on microbiota—new trends with gluten-free or paleo diet. Medical Sciences 6: 92