|
Size: 22108
Comment:
|
← Revision 60 as of 2018-04-27 16:02:26 ⇥
Size: 21517
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 11: | Line 11: |
| <<TableOfContents(Endocrine disruptors Brief introduction of specific endocrine disruptors . Methoxychlor (MXC) Genistein Bisphenol A (BPA) Vinclozolin DDE Effects on female fertility . Estrogenic endocrine disruptors . Methoxychlor (MXC) Genistein Bisphenol A (BPA) Anti-androgenic endocrine disruptors . Vinclozolin DDE Effects on male fertility . Estrogenic endocrine disruptors . BPA Anti-androgenic endocrine disruptors . Vinclozolin Clinical observations . Clinical observations: Companion animals Clinical observations: Farm animals Conclusion References)>> |
<<TableOfContents()>> |
Itt írjon a(z) Fertility_Alterations_Related_to_Endocrine_Disruptors-ról/ről
Fertility Alterations Related to Endocrine Disruptors
By M. F. Pettersen, N. A. Valen, M. K. Walthinsen
Introduction
The objective of this essay is to review the endocrine disruptors’ effects on the reproductive system, and whether it is a clinical concern in domestic animals. We will discuss the physiological effects in male and female animals, and dive deeper into the concerns related to different species. The ways of exposure and accumulative capacities of the endocrine disruptors will also be taken into consideration.
Table of Content
Contents
Endocrine Disruptors
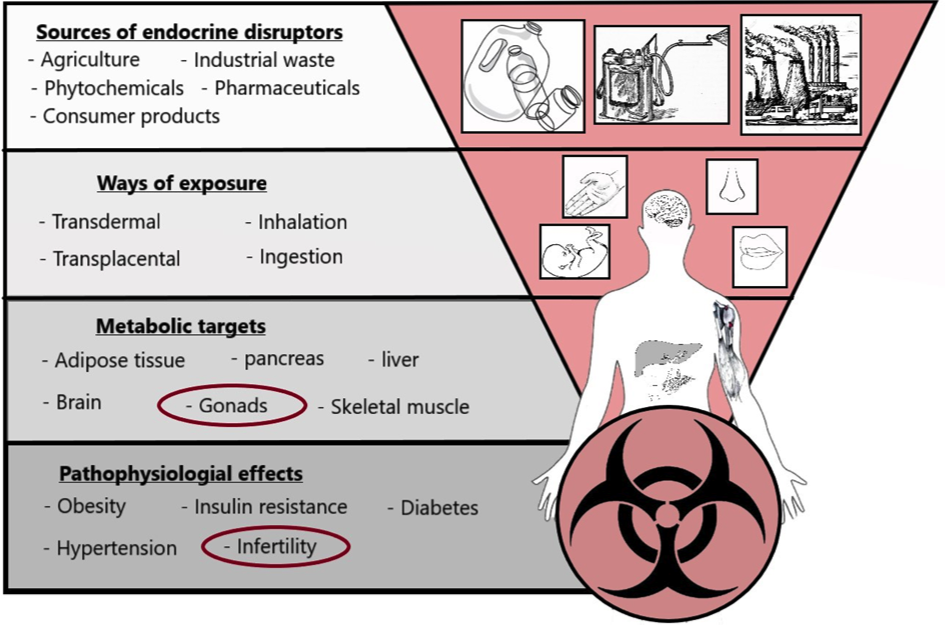
Endocrine disruptors (ED) are defined as substances found in our environment with the ability to alter the physiology of the mammalian or avian endocrine system. Both adult animals and developing organisms may experience harmful and long-lasting damage. Among these are developmental disorders, altered metabolic balance and reproductive problems. There is a broad spectre of ways of exposure, and they have an effect in really small doses. The endocrine disruptors may be incorporated orally, penetrate through skin or through mucous membranes. Offspring may receive disruptors through the placenta, with breast milk or into an egg. They are found in minerals (e.g. lead, mercury), micotoxins (e.g. zearalenon), agriculture (e.g. insecticides), industry (e.g. phthalates, BPA), among others (see Figure 1).
|
Fig 1. Demonstration of how the EDCs can come into contact with and affect the mammalian body
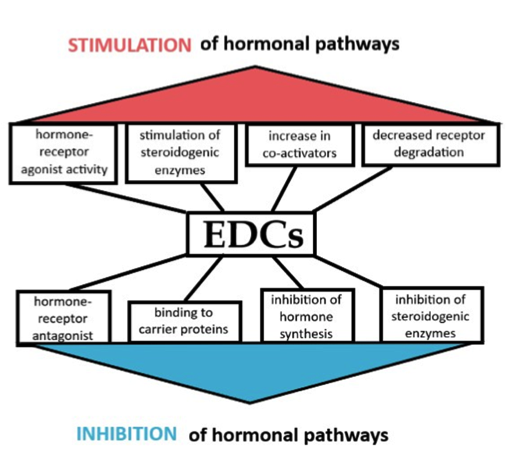
The endocrine disruptors may disturb the hypothalamic-pituitary-thyroid, hypothalamic-pituitary-gonad and hypothalamic-pituitary-adrenal axis in several ways. They may interfere with the synthesis, release, transport or degradation of hormones, receptors or signalling cascades of target cells, or the hypothalamus itself. The biochemical properties of mitochondria can be affected, as well as altered metabolic rate and gene-expression. In this article, we have chosen to focus on some of the most common endocrine disruptors eliciting a concerning impact on the male and female reproductive systems.
|
Fig 2. Effects of endocrine disruptors on hormonal pathways
Brief Description of Specific Endocrine Disruptors
Methoxychlor (MXC)
Methoxychlor (MXC) is an organochlorine pesticide. It is used as a replacement for DDT, another organochlorine known for its infamous, destructive impact on the environment. MXC metabolites possess estrogenic, anti-estrogenic and anti-androgenic properties (Gaido et al, 2000).
Genistein
Genistein is a flavonoid phytoestrogen found in several plants, including soy beans, medicinal plants, and coffee. It has been proven that genistein possesses estrogenic properties in several mammalian species. (Whitehead et al, 2002).
Bisphenol A (BPA)
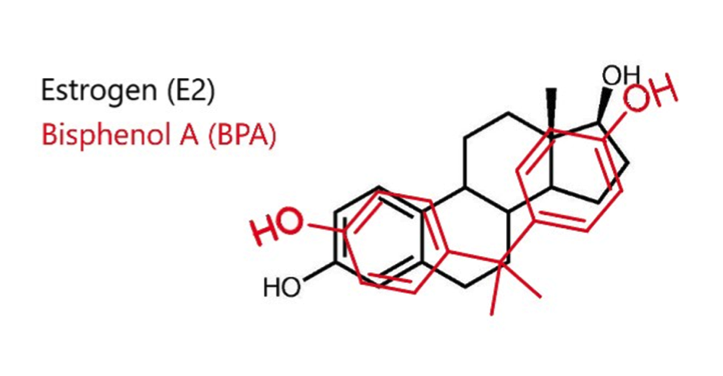
Bisphenol A (BPA) is a man-made monomer used to produce polycarbonate plastic and epoxy resins (Markey et al, 2002). Many studies have shown that BPA possess estrogenic properties, likely due to shared similarities in their chemical structure (indicated in Figure 3). It is to be found in i.e. plastic bottles, food containers and animal cages and has been spread to the environment through these common objects. Consequently, it proposes a huge potential health risk concerning, among several aspects, mammalian reproduction (Hunt et al, 2003).
|
Fig 3. Estrogen and Bisphenol A compared, demonstrating their structural similarities
Vinclozolin
Vinclozolin is a fungicide, commonly used to control diseases on fruits, berries and vegetables. In addition, it is used on the turf on golf courses. Vinclozolin has known anti-androgenic activity (Kelce et al, 1994).
DDE
Dichlorodiphenyldichloroethylene (DDE) is a common breakdown product from DDT. Due to the extensive utilization of DDT and DDE in society and agriculture during the 1900’s, they are still widely found in animal tissue samples. DDE is especially concerning as it is rarely excreted from the body once it’s consumed. The exception is breast milk, indicating that accumulation in infants commence almost directly post-partum. Less research is conducted on DDE’s impact on the reproductive system in mammals. However, it is proven to possess anti-androgenic effects (Andersen et al, 2002).
Effects on Female Fertility
Concerning endocrine disruptors’ alterations on the female reproductive system, they will execute their effect at the level of ovarian development and function, which can lead to reproductive abnormalities in adult life and, at worst, be transgenerational (Uzumcu et al, 2006). The primary ovarian function in an adult is to produce hormones needed for (1) folliculogenesis (ovarian development), (2) ovulation, and (3) the initiation and maintenance of mammalian pregnancy. The initial phase of folliculogenesis includes the formation of primordial follicles and follicular transition (conversion of follicle from primordial to primary) (McGee et al, 2000), and is regulated and influenced by steroid hormones, such as progesterone, androgens, and estrogens. This is significant because several environmental endocrine disruptors elicit their effect through estrogenic receptors found throughout the reproductive tract: ER-α and ER-β. ER-α is found in the uterus, whilst ER-β is present in both the fetal testis and ovary (Anway et al, 2006). In experiments conducted to investigate the role of endocrine disruptors’ effect on female fertility, oocytes, theca-, and granulosa cells have been utilized (Magnusson et al, 2015).
Estrogenic Endocrine Disruptors
Methoxychlor (MXC)
In vivo studies where rats were under chronic exposure of MXC, accelerated vaginal opening, early onset of the first estrus and reduced fertility were detected (Gray et al, 1989). By increasing the dosage of MXC, females showed continuous estrus. As a result, they were unable to get pregnant, even though they mated. In the same study, it was also proven that MXC exhibit transgenerational effects: the females off-springs (F1) suffered from irregular cycles with reduced fertility and had an earlier reproductive senescence than the control group. Within antral follicles, in vitro studies have shown that MXC induces atresia. This is mediated by mechanisms such as oxidative stress and ER-mediated pathways (Uzumcu et al, 2006). MXC also affects granulosa cells directly by inhibiting basal-, FSH-, and estradiol (E2)-stimulated progesterone secretion. However, no effect was detected on cAMP levels (Chedrese et al, 2001).
Genistein
In studies where genistein was administered to mice, accelerated vaginal opening and irregular estrus cycles were revealed. The highest dose of genistein given during the study resulted in infertility. It adversely affected the fertilizability and developmental capacity of fertilized oocytes to the blastocyst stage, resulting in a multi-oocyte follicle (MOF) phenotype (Nagao et al, 2001). Within rat ovarian follicles, genistein blocks LH-induced ovulation and testosterone production and decreases cAMP levels, yet it does not affect E2-synthesis. Genistein has shown similar properties to MXC concerning its effect on granulosa cells. However, it does not alter E2-accumulation (Uzumucu et al, 2006).
Bisphenol A (BPA)
Developmental exposure to BPA is connected to several abnormalities concerning female fertility. It induces early vaginal opening, early first – as well as irregular – estrus cycles, and MOF. This is directly related to reduced fertility and early reproductive senescence, as well as later instances of pathology. (Markey et al, 2002) In contrast to MXC and genistein, PBA stimulates basal and FSH-induced progesterone production. Nevertheless, it will inhibit FSH-stimulated E2-secretion in granulosa cells (Uzumucu et al, 2006).
Anti-Androgenic Endocrine Disruptors
Vinclozolin
Vinclozolin has been reported to induce changes on the normal pattern of sexual differentiation and sexual function in the adult. In addition, it possesses transgenerational effects. When given orally to 7-week-old female rats, it resulted in disturbances of development of the sex organs. In adult rats, a prolonged estrus cycle duration was indicated, being a result of alterations of the LH and E2:testosterone ratio in the serum(Shin et al, 2006). Analysis of in utero effects of vinclozolin exposed a masculinization of female embryos (Uzumucu et al, 2006).
DDE
DDE has been shown to alter the ovarian steroidogenesis (synthesis of steroid hormones). This may affect fertility negatively. In granulosa cells, DDE inhibits basal and FSH-induced progesterone secretion and cAMP production. Nevertheless, it will stimulate cell proliferation (Chedrese et al, 2001).
Effects on Male Fertility
When concerning males, endocrine disruptors usually affect the development and function of the testicles, as well as interfere with the balance, signaling and effect of sex hormones. These effects can all lead to lowered sperm count and quality, which we have seen rising instances of in the latest time period (Carlsen et al, 1992; Al-Hiyasat et al, 2002). However, the increased exposure of animals and humans to various endocrine disruptors over the last decades have also been linked to rising numbers of testicular cancers and physiological abnormalities such as cryptorchidism and hypospadias (Maffini et al, 2006). Several studies suggest the developing testicles in male fetuses could be especially vulnerable to estrogen mimics such as Bisphenol-A, also known as BPA, and Vinclozin, an androgen receptor antagonist (Schug et al, 2011; Anway et al, 2006; Manfo et al, 2014).
Estrogenic Endocrine Disruptors
BPA
Male rats who had ingested BPA have shown to give significantly lowered pregnancy rates when mated to healthy females, than males who were not exposed to the disruptor. The females who were mated to the BPA-exposed males had a significantly increased rate of fetus resorption during their pregnancies, when compared to the rats who were unexposed to BPA. (Al-Hiyasat et al, 2002). Exposure to BPA during puberty and adulthood affects both the synthesis of hormones such as LH, FSH, androgen and estrogen, and the receptors of these hormones, effectively altering sperm parameters. The oxidative stress in the testicles also increases by the inhibition of antioxidating enzymes, which can suggest that usage of antioxidants may help relieve fertility-disturbances caused by BPA (Manfo et al, 2014). Perinatal exposure to relatively low, environmentally relevant doses of BPA have also shown to cause both morphological and functional changes to the genital tracts of both males and females. These changes may affect the tissues negatively, making them more susceptible to disease, reduced fertility, and certain types of cancer, including mammary and prostate cancers (Maffini et al, 2006). Studies have found that the in-utero exposure to BPA was generally more detrimental to the experimental animals, producing effects such as feminization of males, atrophy of the testes and epididymis’s, enlarged prostate gland and alterations of sperm variables later in life. (Manfo et al, 2014)
Anti-Androgenic Endocrine Disruptors
Vinclozolin
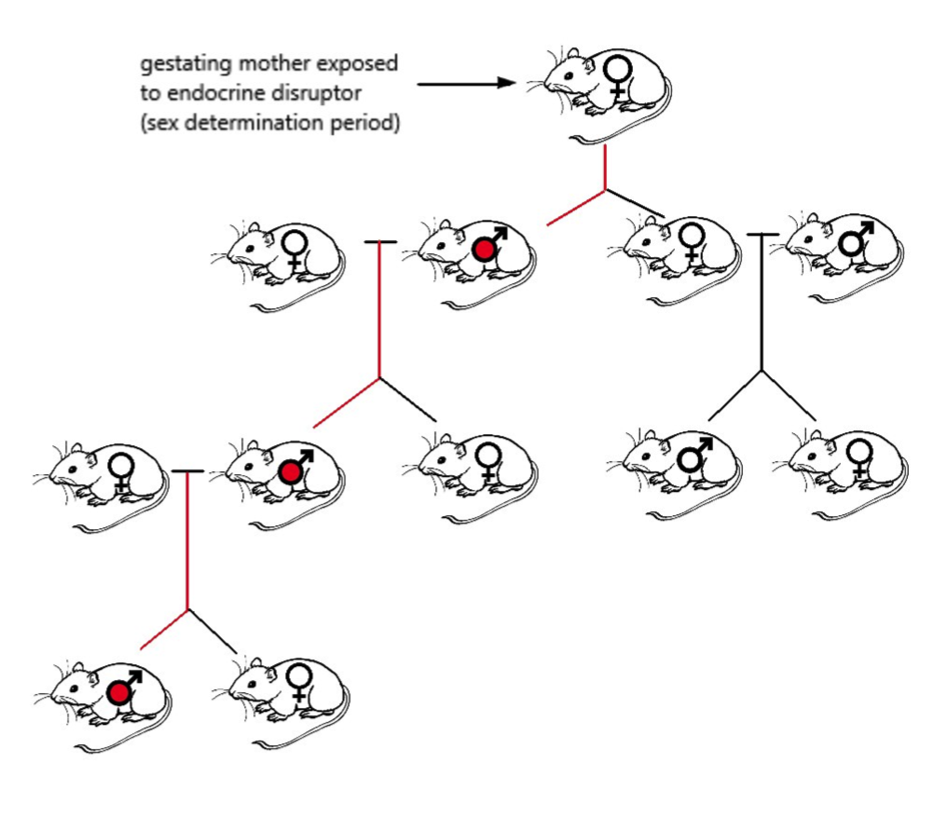
When pregnant rats (F0) where exposed to Vinclozolin, the male offspring (F1) were shown to have significantly lowered spermatogenetic capacity as well as lowered sperm count and motility in both the testicles and the epididymis. Causing further concern, these findings were seen not only in the F1-generation, but also in males of subsequent generations up until F4 (see Figure 4). This strongly suggests a transgenerational phenotype. In the subsequent generations, the male offspring also showed an increase in germ cell apoptosis. Various outcrosses to wild-type rats confirmed that these abnormalities were positively carried in the male lines whose progenitors had been exposed to Vinclozolin (Anway et al, 2006).
|
Fig 4. Exposure of gestating female rat causing changes in several generations of male progeny
Clinical Observations
Clinical Observations: Companion Animals
There are few studies regarding endocrine disruptors and reproductive health published about domestic animals, when comparing to humans and wildlife. Studies on commercial dog foods have shown presence of phytoestrogens in amounts that could have biological effects when ingested long term (Cerundolo et al, 2004). The phytoestrogens originate from the soybeans and soybean fractions, a common ingredient in dog food. Ingestion of phytoestrogens may have both beneficial and damaging health effects (Setchell, 1998). Human studies have shown that health benefits include a lowered risk of heart disease and breast cancer, but many phytoestrogens are also considered endocrine disruptors, having potential to cause negative health effects as well (Patisaul et al., 2010). In bitches for example, the phytoestrogen zearalenone (ZEA) has proven adverse effects on the reproductive organs (Magnusson et al, 2015).
Clinical Observations: Farm Animals
Endocrine disrupting substances originating from environmental pollution are biomagnified in the food chain, and therefore predictably seen in species of higher trophic ladder (Magnusson et al, 2015). Ruminants, being herbivorous, are in contrast less likely to be exposed to high concentrations of the substances. A route of exposure for herbivorous animals may be through grazing or drinking water. Studies in the Netherlands on dairy cattle drinking water contaminated with sewage outflows have shown reduced reproductive performance (Sweeney, 2002). Phytoestrogens have proven to cause adverse health effects in farm animals too. Pigs eating feedstuff contaminated with mycotoxins, such as zearalenone (ZEA), can develop symptoms such as impairment of ovulation, abortions, and decreased number of live embryos (Tiemann et al, 2007). In sheep, the so-called sweet-clover disease can result in prolapsed uterus and embryonic death. The reason is the binding of formononetin and genistein to the estrogen receptors, modulating estrogenic enzymes (Magnusson et al, 2015).
Conclusion
As we have discussed and reviewed in this essay, it is certain that endocrine disruptors indeed possess hazardous effects on both female and male reproductive health. The trend of declining reproductive quality is evident, while the usage of endocrine disrupting chemicals is continuously increasing. As discussed, this can pose an escalating threat to the fertility in all animals – including humans – and consecutive generations. Fortunately, the awareness of harmful EDCs is growing, and researchers are currently conducting studies that may well be the basis for future regulations regarding utilization and development of potential endocrine disruptors.
References
Al-Hiyasat A.S.; Darmani H.; Elbetieha A.M. (2002): Effects of bisphenol A on adult mouse fertility. Online article. URL: https://onlinelibrary.wiley.com/doi/full/10.1034/j.1600-0722.2002.11201.x Accessed: 04/09/18
Andersen H.R.; Vingaard A.M.; Rasmussen T.H.; Gjermandsen I.M.; Bonefeld-Jorgensen E.C. (2002): Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/11884232 Accessed: 04/07/18
Anway M.D.; Memon M.A.; Uzumcu M.; Skinner M.K. (2006): Transgenerational Effect of the Endocrine Disruptor Vinclozolin on Male Spermatogenesis. Online article. URL: https://onlinelibrary.wiley.com/doi/epdf/10.2164/jandrol.106.000349 Accessed: 04/09/18
Anway M.D.; Skinner M.K. (2006): Endocrinology. Volume 147. Issue 6. Pages s43-s49. URL: https://academic.oup.com/endo/article/147/6/s43/2878343 Accessed: 03/21/18
Carlsen E.; Giwercman A.; Keiding N.; Skakkebæk E.N. (1992): Evidence for decreasing quality of semen during past 50 years. Online article. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1883354/pdf/bmj00091-0019.pdf Accessed: 04/08/18
Cerundolo R.; Court M.H.; Michel K.E. (2004): Identification and concentration of soy phytoestrogens in commercial dog foods. Online article. URL: https://avmajournals.avma.org/doi/abs/10.2460/ajvr.2004.65.592 Accessed: 04/06/18
Chedrese P.J.; Feyles F. (2001): The diverse mechanism of action of dichlorodiphenyldichloroethylene (DDE) and methoxychlor in ovarian cells in vitro. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/11738522 Accessed: 04/06/18
Gaido K.W.; Maness S.C.; McDonnell D.P.; Dehal S.S.; Kupfer D.; Safe S. (2000): Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/10999957 Accessed: 04/06/18
Gray L.E.; Ostby J.; Ferrell J. et al. (1989): A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Online Article. URL: https://www.ncbi.nlm.nih.gov/pubmed/2925022 Accessed: 04/06/18
Kelce W.R.; Monosson E.; Gamcsik M.P.; Laws S.C.; Gray L.E. (1994): Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/8209380 Accessed: 04/07/18
Maffini M.V.; Rubin B.S.; Sonnenschein C.; Soto A.M.; (2006): Endocrine disruptors and reproductive health: The case of bisphenol-A. Online article. URL: https://www.sciencedirect.com/science/article/pii/S0303720706002292 Accessed: 04/08/18
Magnusson U.; Persson S. (2015): Endocrine Disruptors in Domestic Animal Reproduction: A Clinical issue?. Online article. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4584497/ Accessed: 03/18/18
Manfo F.P.; Jubendrass R.; Nantia E.A.; Moundipa P.F.; Mathur P.P. (2014): Adverse effects of bisphenol A on male reproductive function. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/24162092 Accessed: 04/09/18
Markey C.M.; Rubin B.S.; Soto A.M.; Sonnenschein C. (2002): Endocrine disruptors: from Wingspread to environmental developmental biology. URL: https://www.ncbi.nlm.nih.gov/pubmed/12650721 Accessed: 04/06/18
McGee E.A.; Hsueh A.J.W. (2000): Endocrine Reviews. Volume 21. Issue 2. Pages 200-214. URL: https://academic.oup.com/edrv/article/21/2/200/2423956 Accessed: 04/06/18
Nagao T.; Yoshimura S.; Saito Y.; Nakagomi M.; Usumi K.; Ono H. (2001): Reproductive effects in male and female rats of neonatal exposure to genistein. URL: https://www.researchgate.net/profile/Makoto_Ema2/publication/10695389_Higher_susceptibility_of_newborn_than_young_rats_to_3-methylphenol/links/0deec522bf144bfe97000000/Higher-susceptibility-of-newborn-than-young-rats-to-3-methylphenol.pdf#page=190 Accessed: 04/06/18
Patisaul H.B.; Jefferson W. (2010): The pros and cons of phytoestrogens. Online article. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3074428/?_escaped_fragment_=po=0.175439 Accessed: 03/26/18
Schug T.T.; Janesick A.; Blumberg B.; Heindel J.J. (2011): Endocrine disrupting chemicals and disease susceptibility. Online article. URL: https://www.sciencedirect.com/science/article/pii/S096007601100166X Accessed: 04/08/18
Setchell K.D.R. (1998): Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Printed in USA. American Society for Clinical Nutrition. Page 1333S-1341S.
Shin J.H.; Moon H.U.; Kim T.S. et al. (2006): Repeated 28-day oral toxicity study of vinclozolin in rats based on the draft protocol for the “Enhanced OECD Test Guideline No. 407” to detect endocrine effects. URL: https://link.springer.com/article/10.1007/s00204-006-0116-y Accessed: 04/06/18
Sweeney T. (2002): Domestic Animal Endocrinology 23. Is exposure to endocrine disrupting compounds during fetal/post-natal development affecting the reproductive potential of farm animals?. Elsevier Science Inc. Page 203-209.
Tiemann U.; Dänicke S. (2007): In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs. Online article. URL: https://hal.archives-ouvertes.fr/hal-00577519/document Accessed: 04/02/18
Uzumcu M.; Zachow R. (2006): Developmental Exposure to Environmental Endocrine Disruptors: Consequences within the Ovary and on Female Reproductive Function. Online article. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1950429/ Accessed: 04/06/18
Whitehead S.A.; Cross J.E.; Burden C.; Lacey M. (2002): Acute and chronic effects of genistein, tyrphostin and lavendustin A on steroid synthesis in luteinized human granulosa cells. Online article. URL: https://www.ncbi.nlm.nih.gov/pubmed/11870108 Accessed: 04/07/18
Figures
Figure 1: Marit Fausa Pettersen
Figure 2: Marit Fausa Pettersen
Figure 3: Marit Fausa Pettersen
Figure 4: Marit Fausa Pettersen