|
Size: 16702
Comment:
|
Size: 16846
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 90: | Line 90: |
| ----- == Websites == * <<Anchor(12)>> 12. http://highline.huffingtonpost.com/miracleindustry/americas-most-admired-lawbreaker/chapter-7.html |
Gluten as an Endocrine Disruptor
Contents
General Overview
An endocrine disruptor such as gluten can cause significant interference to normal physiological processes, through invasion of hormone functioning. Gluten, a key component in wheat, can also be found in grains, such as barley, rye and oats. glutenin and gliadin are the main protein building blocks of wheat gluten. These molecules are of exogenous origin to our immune system and cause adverse effects in genetically susceptible people. Studies show the significant role gluten contributes to intestinal and extra-intestinal diseases. Effects from gluten can be instant or develop over a period of time depending on the quantity intake or patients sensitivity. Digestion of gluten occurs in the gastrointestinal tract. In coeliac disease patients, the elicited immune response to gluten is inflammation and destruction of villi and crypt cells of the small intestines. Evidence of exogenous opioid activity from gluten demonstrates gluten to be a versatile group of proteins with deleterious effects.
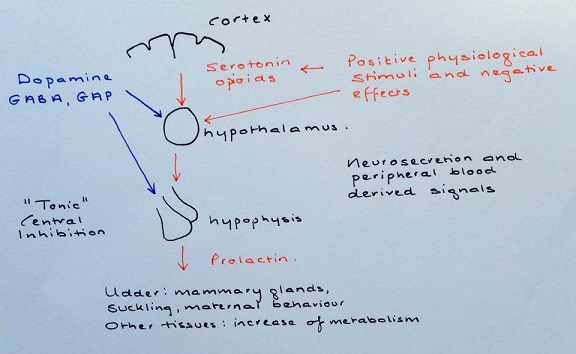
Prolactin and Gluten
Prolactin is a peptide hormone, which has a distinct relationship with the proteins contained in gluten. Prolactin’s primary roles include: reproduction, calcium metabolism, osmoregulation and maternal behaviour 11. Gluten acts as an endocrine disruptor in this case, by elevating the levels of prolactin in the body; causing significant physiological changes as a result. The primary reason for the increase in prolactin levels is said to be the increase in quantity of inflammatory cytokines 3. Prolactin plays a profound role in the immune response against gluten in a celiac patient’s intestines; through both innate and adaptive immunity. A correlation is significant between the level of prolactin and the number of CD4+ T and B lymphocytes 7.
It has been suggested that removing wheat or gluten from the diet can improve conception chances. The reason being; the disruption prolactin can cause to important reproductive hormones such as testosterone (sperm abnormalities, decrease in libido). Autoimmune diseases such as celiac disease have been found to be more commonly seen in females, mostly due to hormonal changes around time of sexual maturity and pregnancy.
|
Hyperprolactinaemia
Several recent studies have demonstrated a strong link between hyperprolactinaemia and coeliac disease, in patients consuming gluten in their diet 3,7,11. Normal hyperprolactinemia levels in a population should be between 0.4 – 3% on average 11. The quantity of prolactin produced in the body has shown positive correlation with degree of intestinal damage. Therefore it has been mentioned as a possible biomarker for autoimmune activity, which could hugely benefit immune/endocrinology studies in the future. Prolactin is unable to initiate an immune response alone but acts more as a modulator. Its modulatory roles include: interference with B cell tolerance induction, inhibiting apoptosis, enhancing antigen presentation, upregulating cytokine production and increasing antibody production 3.
|
Immune response: thymus
One way prolactin influences the immune response, is through the thymus. Epithelial cells of the thymus are known to have a high expression of prolactin receptors, which allows prolactin to directly influence the immune response 11. This reaction can then in turn induce interleukin-2 receptors on lymphocytes and also, growth factors for lymphocytes. Prolactin receptors can also be found on monocytes, macrophages, T and B lymphocytes, natural killer cells and granulocytes 11. Interestingly, hyperprolactinemia is more commonly observed in coeliac children/adolescents, than adult coeliac patients. This incorporates the fact that the thymus undergoes thymic involution with age.
Gluten as an Exogenous Opoid
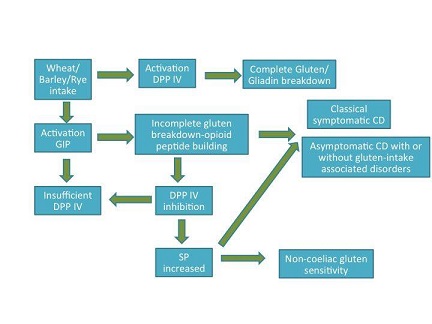
Gliadin, can additionally be degraded into a group of opioid-like polypeptides called exorphins. Opioid peptides are both of endogenous and exogenous origin. In the brain, endogenous opioid peptides attach to opioid receptors. This connection helps stimulate motivation, emotion, attachment behavior, response in stress and pain and control of food intake. Exogenous opioids or exorphins can be of food origin and have proven endogenous-opioid like activity. Gluten exorphins mask the gastrointestinal effects in patients suffering with coeliac disease (Pruimboom and de Punder (2015)). This phenomenon is termed asymptomatic coeliac disease (ACD). Pruimboom and de Punder (2015) reviewed that ACD affects patients suffering from other diseases such as diabetes mellitus type 1, psoriasis,depression, autism,schizophrenia and irritable bowel syndrome.
Competition for binding site
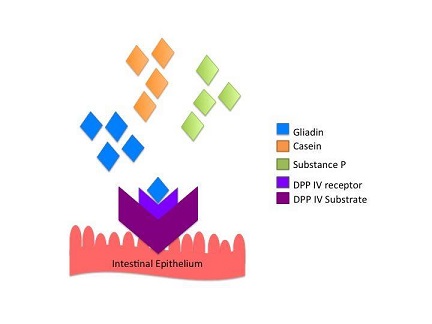
Opioid activity is stimulated upon enzymatic interaction. Dipeptidyl peptidase IV (DPP IV) is a major antigenic enzyme necessary for normal cellular metabolism. DPP IV is involved in the final breakdown reaction of gliadin into small digestible amino acids. These amino acids inhibit the presence of gluten epitopes that are notorious to elicit a pro-inflammatory immune response in genetically susceptible people. However, gluten is not the only source of exorphins in the diet. Other exorphins include, casein from dairy, vegetable proteins and other carbohydrate polypeptides. In particular, gliadin and casein display elevated substrate specificity for DDP IV compared to endogenous DPP IV substrates such as, substance P and glucagon-like peptide-1.
|
Outcome of Binding
Studies have shown that increase levels of gliadin in the body causes inactivation of DPP IV (Pruimboom and de Punder (2015). Therefore, an increase occurs in non-metabolized gliadin molecules with opioid activity. Inhibition of DPP IV leads to several intestinal and extra-intestinal effects. The neurotransmitter substance P is dependent on DPP IV activity in order to exert its effects. High levels of substance P in the gut not only causes intestinal pain and discomfort, it also induces adverse mood and depression (Pruimboom and de Punder (2015). A decreased level of serum DDP IV is an important indicator for diagnosing depression (Deng et el, 2013). Inhibition of DDP IV leads to high expression of the complement protein C1Q and intense C1Q expression has been detected in patients suffering from Alzheimer, autism and schizophrenia (Pruimboom and de Punder (2015).
|
Gluten and Type 1 Diabetes Mellitus
The pancreas is an endocrine organ which secretes insulin into the blood stream. A disease of the pancreas is type 1 diabetes mellitus(T1DM) whereby the proper production of Insulin is disrupted and therefore unable to carry out its function correctly. Although T1DM is a multifactorial disease, studies have shown that altering the diet of an individual genetically predisposed to or already suffering from T1DM can influence the development of the disease (Funda, 2011, Kort, 2011).
Gluten and Gut Permeability
Several studies have explored the theory of how decreasing Gluten intake in an individual’s diet can have a positive effect on the proliferation of Diabetes Mellitus, especially in those individuals already suffering from gluten sensitivities, intolerances or gluten sensitive chronic enteropathy disease such as coeliac disease(CD) (Hartmann, 2014, Marchese, 2012). Coeliac Disease is an important factor in the link between Gluten and T1DM. Since a symptom of CD is gut permeability, Gluten proteins are then able to pass through enterocytes and into the bloodstream where they incite the immune response system cells and also increase the pro inflammatory cytokine profile within the intestines (Funda, 2011, Sapone, 2011). It is this immune response which could lead to the attack on pancreatic beta cells, and cytokine production with insulin resistance, thus resulting in the onset of T1DM (Kort, 2011). Autoimmune Diseases like T1DM occur much more frequently in CD patients than in the general population (Marchese, 2012).
Protective Function of a Gluten Free Diet
There have been many studies linking permeability in the gut to diabetes and vice versa. It has been shown in these studies the role that Gluten plays in disrupting this relationship and the function of the pancreas in the body.
Gluten Free Diet
The method to prevent gluten acting as an endocrine disruptor (with regards to prolactin in coeliac disease cases), is simply to cut gluten out of the diet. Within a six month period of a gluten free diet, it is hypothesized to reduce or even completely alleviate the symptoms once shown before. Symptoms such as lesions in the gut lining of coeliac patients consuming gluten can normalize after it is excluded from the diet (Lauret et al, 2013).
In recent study carried out by Hartmann, 2014, on Pregnant non obese diabetic mice, whereby half of the mice where fed a Gluten Free Diet and the other half, the control species were fed a Gluten Containing Diet. When the offspring were born the results of their health where compared and it was found that not only did the GFD decrease incidence of diabetes from 64% to 15%. It was also shown that the GFD increase presence of forkhead box P3 Regulatory T-cells, the anti inflammatory cytokine profile and tight junction related genes in the gut, also the pro inflammatory cytokine profile was decreased. On the contrary the GCD promoted proinflammatory cytokines IFN-y and tumour necrosis factor-a in the small intestine. This study also suggested that gluten affects the micro flora of the intestines and that these two in conjunction could have a combined affect on the proliferation on T1DM. It is also evident that a GFD ingested by a mother genetically prone to T1DM could protect her offspring from the development of T1DM even if they are weaned to a GSD. This protection comes from the shift in gut biota while the immune system is developing in the fetus and young offspring. It is therefore hypothesized that early life interventions in intestinal environment can effect onset of T1DM. A similar study was carried out by Funda, 2011, whereby the same dietary modifications were made on mice again as a model organism. This time it was the balance of the inflammatory cytokine profile and growth of gut biota in response to a Gluten Free or Gluten Standard Diet. Again, the mice fed on a GCD developed an immune profile which contributed to a higher type 1 diabetes incidence associated with gluten intake. In a study carried out on Sicilian Children in by Marchese, 2013 it was found that those with T1DM and CD fed on a GFD were able to decrease the severity of the hypoglycaemic episodes.
References
1. Antvorskov, J.C., Fundova, P., Buschard, K. and Funda, D.P., 2013. Dietary gluten alters the balance of pro‐inflammatory and anti‐inflammatory cytokines in T cells of BALB/c mice. Immunology, 138(1), pp.23-33.
2. De Kort, S., Keszthelyi, D. and Masclee, A.A.M., 2011. Leaky gut and diabetes mellitus: what is the link?. Obesity Reviews, 12(6), pp.449-458.
* 3. Delvecchio, M., Faienza, M.F., Lonero, A., Rutigliano, V., Francavilla, R. and Cavallo, L., 2014. Prolactin may be increased in newly diagnosed celiac children and adolescents and decreases after 6 months of gluten-free diet.Hormone Research in Paediatrics, 81(5), pp.309-313.
4. Deng, J., Lamb, J.R., Mckeown, A.P., Miller, S., Muglia, P., Guest, P.C., Bahn, S., Domenici, E.H. and Rahmoune, H., 2013. Identification of altered dipeptidyl-peptidase activities as potential biomarkers for unipolar depression. Journal of affective disorders, 151(2), pp.667-672.
5. Hansen, C.H.F., Krych, Ł., Buschard, K., Metzdorff, S.B., Nellemann, C., Hansen, L.H., Nielsen, D.S., Frøkiær, H., Skov, S. and Hansen, A.K., 2014. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes, 63(8), pp.2821-2832.
6. Jackson, J.R., Eaton, W.W., Cascella, N.G., Fasano, A. and Kelly, D.L., 2012. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatric Quarterly, 83(1), pp.91-102.
* 7. Lauret, E. and Rodrigo, L., 2013. Celiac disease and autoimmune-associated conditions. BioMed research international, 2013.
8. Marchese, A., Lovati, E., Biagi, F. and Corazza, G.R., 2013. Coeliac disease and type 1 diabetes mellitus: epidemiology, clinical implications and effects of gluten-free diet. Endocrine, pp.1-2.
* 9. Pruimboom, L. and de Punder, K., 2015. The opioid effects of gluten exorphins: asymptomatic celiac disease. Journal of Health, Population and Nutrition, 33(1), p.1.
10. Sapone, A., Lammers, K.M., Casolaro, V., Cammarota, M., Giuliano, M.T., De Rosa, M., Stefanile, R., Mazzarella, G., Tolone, C., Russo, M.I. and Esposito, P., 2011. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC medicine, 9(1), p.23.
* 11. Shelly, S., Boaz, M. and Orbach, H., 2012. Prolactin and autoimmunity. Autoimmunity reviews, 11(6), pp.A465-A470.
Websites
* 12. http://highline.huffingtonpost.com/miracleindustry/americas-most-admired-lawbreaker/chapter-7.html