Department of Physiology, University of Veterinary Medicine
The Physiological Effects and Medicinal Use of Hyperbaric Oxygen Therapy
- By: Se Eun Choi, Paulette Stewart and Jana-Liza Tabacchi
Supervisor: Bárány Zoltán; Budapest, Hungary 2018
Contents
Abstract
Hyperbaric oxygen therapy (HBOT) is a medical treatment in which patients are administered 100% oxygen at higher than normal atmospheric pressure (sea level is 1 atm) while in an enclosed chamber, usually 2-3 atm. HBOT has been used since 1662 but was applied safely in the 1930s after being developed further by the U.S military (Gill and Bell, 2004). HBOT was mainly used to treat decompression sickness in scuba divers but now is being utilized as therapy for various conditions in human and animal medicine. The therapeutic effects of HBOT can be explained by the physiology of hyperoxic conditions and with various gas laws; Boyle’s Law, the Ideal Gas Law, Charles’ Law and Henry’s Law (Jones and Wyatt, 2018). In this paper, three different applications of HBOT in veterinary medicine will be examined according to the physiological mechanisms.
Introduction
The relationship between atmospheric pressure and respiration
When pressure increases, the surrounding molecules are squeezed together. This means the density of the gas molecules have increased and therefore more air can be breathed in during every breath. Due to evolved mammalian anatomy, we possess the ability to resist environmental pressure changes, to a certain degree. This is the reason why lung volume does not change under higher atmospheric pressure, as long as the normal breathing cycle is continued. Hence, more O2 molecules are able to occupy the lungs and then taken up by alveolar diffusion. 1atm is the pressure experienced at sea level. In the hyperbaric oxygen chamber pressure can be increased, for example raising the atm to 2 atm. This changes the density of the air molecules and allows the lungs to take up nearly twice as much air. As haemoglobin's oxygen carrying level is not rate limiting, higher atmospheric pressure stands in direct relationship with higher oxygen uptake in the alveolus and therefore higher supply to body tissues (Hopf et al., 2001).
Hyperbaric Oxygen Therapy for acute inflammation and wound healing
Under normal circumstances, oxygen is mainly transported by red blood cells. However after applying a higher atmospheric pressure, oxygen is dissolved and transported in the plasma (Jain, 2004). This factor is enhanced by applying 100% O2 concentration. Ultimately, this maximizes tissue O2 supply. Oxygen can be seen as the limiting factor needed for acute wound and post- rupture inflammation healing. Approximately 80% of oxygen in the blood is used for mitochondrial ATP production through O2 dependent oxidative phosphorylation. This is then stored and further processed for energy related cellular function (Wilson et al., 2006). Healing tissue has higher cellular activity than healthy tissue because reparative processes, such as cell proliferation and collagen synthesis are required. The first aim of the body is to seal the wound as fast as possible to decrease the risk of infection and necrosis. Collagen synthesis takes place in fibroblasts, the most dominant cell type of the connective tissue. Fibroblasts are responsible for the extracellular matrix and collagen production, creating the structural framework or stoma of animal tissue. The hydroxylation is a crucial step in procollagen maturation and requires high levels of O2 tension. This was studied under in-vivo experimental conditions. The enzyme prolyl- hydroxylase (which hydroxylizes procollagen to form stable triple-helixes) was tested to require 150 mmHg oxygen tension in order to work at 90% of its maximal enzymatic speed. Without HBOT at 20mmHg only 50% of maximal enzymatic speed was reached (Hopf et al., 2001). These experimental values prove a higher enzymatic activity. Possibly through earlier mentioned higher ATP synthesis, catalysing holoenzyme activation at an increased rate, and therefore elevate the collagen synthesis ́s rate of reaction.
Once the superficial wound has been sealed from the external environment, the deeper structures are regrown, including blood vessels and capillaries. There is a decline in O2 tension, not only because ruptured blood vessels have been closed through blood clotting, but also by the cellular consumption of oxygen by cells that have become metabolically more active as they are in proliferation state. The efficiency of oxygen supply to the wound site primarily depends on arterial oxygen tension and perfusion. HBOT induces vasoconstriction to a rise in atm (Wilson et al., 2006). 100% oxygen concentration alongside the increased pressure, counteracts the effect of lower blood supply due to vasoconstriction as well as possibly having the positive side effect of decreasing oedema in the patients extremities (Levine and Reichling, 1999). Post-wound inflammation is caused through cytokines, chemical substances such as histamine that are secreted by white blood cells of the immune system. The redness and heat in the affected area are side effects of those molecules which try to protect the body from foreign pathogens. The localised swelling associated with inflammation is simply caused by the accumulation of cells in that area and sometimes a leakage of lymphatic fluid into the tissue, which is known as oedema. For this immune response, Nicotinamide-adenine-dinucleotide phosphate- (NADPH-) linked oxidase is required. NADPH oxidase is a membrane bound enzyme complex producing high amounts of oxidants when leukocytes, especially neutrophils, have been activated by the presence of pathogenic microorganisms. This process is termed respiratory burst. The enzyme has successfully been supported through HBOT. In order to achieve 90% of maximal enzymatic speed, O2 tensions above 400mmHg may be required (Allen et al., 1997). This can only be attained by applying hyperbaric oxygen chamber conditions. Not only the oxidant production but also oxidative killing of pathogens can be beneficially influenced through HBOT as it is directly proportional to local oxygen tension and perfusion (Tandara et al., 2004). These mechanisms are essential for successful wound healing due to their obvious indispensable role in preventing wound infection.
Hyperbaric oxygen therapy creates hyperoxic conditions, a situation where the breathing gas mixture contains more than the atmospheric 21% oxygen. Under normal physiological conditions oxygen is mostly delivered to the areas of vital importance such as the brain. However, when excess O2 is supplied, less essential and thereby slower physiological processes can be boosted; processes such as neovascularization of mending tissue. The natural development of new blood vessels at the site of wound healing is advantageous, as constant oxygen and nutrient supply through circulation is ensured not only during the patient’s treatment phase but also in the future. Experimental results indicate a high responsiveness to oxygen under HBO treatment signifying that blood flow was increased. Evidence supporting this hypothesis has been tested on experimentally induced ischemic rabbit ears. During a patients HBO therapy session the blood oxygen tension continued to rise due to elevated O2 uptake. O2 washout (measured O2 levels left in the tissue at specific time intervals) decreased from 1 hour before therapy to 4 hours after seven daily treatments of 90 minutes each in the hyperbaric oxygen chamber. The control group were exposed to 100% O2 in a 1 atm environment, showing no therapeutic effect (Siddiqui et al, 1997). This evidently indicates the direct relationship between atm and O2 tension, but also expresses a link between HBOT and elevated angiogenesis. Although the evidence is sufficient to prove that HBOT has positive results in wound healing and inflammation. further studies in this areas should be obtained.
Hyperbaric Oxygen Therapy in the case of Toxic Agents
Hyperbaric oxygen therapy has been tested for efficacy in animals suffering from exposure to a variety of toxic agents. HBOT is often used as adjunctive therapy with other treatments in the case of different toxic agents. This section will present successful cases of HBOT use and the physiological mechanisms of healing from poisoning cases in different animals by the following six substances: carbon monoxide, the antibiotic amphotericin B, the insecticide paraoxan, as well as the venoms of the brown recluse spider, rattlesnake and sea nettle.
Carbon monoxide (CO) poisoning commonly occurs in animals (and people) that have been exposed to fires and other sources. The main effects of CO are the inhibition of cytochrome C oxidase (an essential complex in oxidative phosphorylation and therefore ATP production), a decrease in the oxygen carrying capacity of the blood and finally, lipid peroxidation (the destruction of cell components made from lipids by oxidative activity). A study on rats with carbon dioxide poisoning focused on the mitochondrial respiration levels before and after treatment with HBOT (Jang et al., 2018). After treatment, the measurements showed a decreased half-life of CO, decreased lipid peroxidation and an increase in the respiration rate of mitochondria. It can be supposed that the effects of greater oxygen supply to tissues due to the higher atmospheric pressure used in HBOT therapy contributed to these positive findings.
The antibiotic amphotericin-B has nephrotoxic effects that can be mitigated with the use of HBOT, as explored in a study on rats by Berkovitch et al., 2015. Nephrotoxic effects caused by amphotericin-B can be oxidative and histiopathologic damage, a decrease in glomerular filtration rate and an increase in blood plasma creatine levels (which are commonly associated with kidney problems and cause a variety of medical issues). The main mechanism of the successful HBOT in the case of nephrotoxicosis was discovered to be renal vasodilation and subsequently, increased blood flow (Berkovitch et al., 2017).
Another successful case of HBOT used on a toxic agent is that of paraoxon, in the case of rabbits. Paraoxon is an organophosphorous compound that is used in a very potent insecticide and also as a drug against the group of eye dieases known as glaucoma (PubChem Compound Database, 2004). Toxicosis with paraoxon results in hypoxia by a decease in the oxygen tension in muscles and venous blood as well as acidosis. Subsequent convulsions and death usually follow. With the application of HBOT at 3 ATA for 2-4 hours, the blood oxygen tension was increased by 50% compared to the levels at poisoning. The treatment resulted in prolonging the rabbits’ lives from only 3 hours to 5 days of survival after being poisoned, but showed no effect on the acidosis (PubChem Compound Database, 2004).
Deaths and illness in animals from other animals’ bites and/or stings is a common concern in veterinary medicine. HBOT has been tested in the case of rattlesnake, the brown recluse spider and sea nettle venom with favorable effects. In an experiment by Kelly et al.,1991 mice were envenomated with rattlesnake venom, creating hypoxic conditions. The hypoxic conditions caused by the mycotoxic effects of the venom were capillary hemorrhage, tissue damage and edema. When treating snakebites, antivenom is necessary to neutralize the lethality of the venom. HBOT is a useful adjunctive therapy because anitvenom cannot stop the prominent tissue damage from rattlesnake venom (Kelly et al, 1991). After HBOT, the regeneration of myocytes was seen as well as neovascularization through new collagen formation, epitheliazation and the increased activity of polymorphonuclear phagocytes, cell that contribute to wound healing (by further increasing collagen production) and infection prevention.
In the case of the venom of the brown recluse spider on rabbits, hyperbaric oxygen therapy was also successfully used. The venom caused necrotic lesions from the coagulation of blood vessels as polymorphonuclear leukocytes collected on the capillary walls (Strain, 1991). After HBOT treatment, improvements of the necrotic lesions could be seen at a histological level with effects such as re-epithilialization and escalated angiogenesis. Angiogensis requires collagenous support, which is low around wound centers because blood oxygen levels are reduced by the wound trauma. Macrophages aid in the regeneration of collagen by producing factors that encourage fibroblast conglomeration, but this process requires a partial oxygen pressure of 40-55 mmHg to occur (Strain et al., 1991). The HBOT therapy increases partial oxygen pressure and enhanced macrophage numbers and the collagen rebuilding process to aid the healing of necrotic lesions.
A study conducted on rats that were envenomated with sea nettle (a species of jelly fish) venom caused death by central respiratory and cardiac arrest (Muhvitch et al., 1991). The mechanism of action of the sea nettle venom is that is allows more Na+ and Ca2+ into smooth muscle and myocytes. This causes a myriad of issues such as necrosis of the renal and hepatic epithelial cells, as well as ischemia in the brain. The drug ‘Verapamil’ was used to inhibit the calcium channels in conjunction with HBOT at 2 atm. Rats that received the HBOT treatment compared to the rats only receiving the venom were reported to have increased blood flow to the brain, relieving ischemia (Muhvitch et al., 1991). The blood that flowed to the brain after HBOT had higher levels of oxygen than the original condition, relieving the ischemia.
Hyperbaric Oxygen Therapy and its Neurological impacts
Hyperbaric oxygen therapy is being applied to neurological disorders and injuries in humans and more recently, in animals as well. The brain functions almost completely by aerobic mechanisms and since the main mechanism of action of HBOT is to increase the intake of oxygen by the organism and deliver that oxygen around the body, it makes sense that it could have very positive effects in the brain (Kraitsy et al., 2014). Also, compared with other forms of therapy, no side effects have been discovered in treating neurological disorders and injuries with HBOT. In the past, neurological disorders have been mainly cured with different medical and physical therapies, which both have considerable limitations. In the case of medicine, there are many possible side effects such as cytotoxic effects, depressive disorders and conducting signal errors. The limitations of physical therapy is that it can take a long time to effect the neural signal and can be limited to only delaying the progression of neurological diseases. As the studies on HBOT in treating neurological disorders show, this classical treatment for decompression sickness may start a new era for the treatment of neurological disorders.
Research has shown that long-term HBOT treatment can cure demyelination, specifically through remyelination. It is useful to understand the basic components of myelinated neurons and how damage to the myelin will effect the brain functioning. A neuron consists of a cell body, a dendrite and an axon. These small tissues have vital roles in the brain and entire body. The neuron is the conducting signal center and feeds back all the information that is vital for survival. An important structure of signal conduction is the myelin sheath and each axon is coated by it. Not all neurons have a myelin sheath. Myelinated neurons are characterized by very fast conduction. Our brain requires faster conduction relative to the other parts of the body, so most of the myelin medical issues occur in the brain area or CNS. If there is damage to the myelin sheath, it may be degraded to the point that the conducting signal becomes very slow or sends back incorrect information to the brain. The damage of the myelin sheath is called demyelination, which has many neural effects (Cammer et al., 1978). Some symptoms of demyelination are as follows; numbness, loss of reflex responses, loss of blood pressure regulation, blurry vision, dizziness, heart palpitations, memory disorders, pain, constipation and fatigue. A major factor in causing demyelination is inflammation, which can be caused by different viruses (i.e rabies, canine distemper), metabolic problems, a decrease in oxygen and physical compression, such as in brain trauma (Canto and Rabinowitz, 1982). In order to highlight the positive effects of HBOT on neurological issues, three case studies will be reviewed as follows; HBOT’s effect on patients who inhaled carbon monoxide, on rhino cerebral fungal infections and in mice with brain trauma.
Lo et al.,2007 found that in patients with neuropsychiatric syndrome caused by carbon monoxide inhalation, HBOT had a significant positive effect. The carbon monoxide caused neuropsychiatric syndrome by demyelination. In this study, fraction anistrophy (FA) values were different between groups; higher in the control group, which didn’t inhale carbon monoxide and lower in the group that did. The FA value is the conduction route of white matter. If the value is higher, it means white matter is showing higher conduction activity.After treatment with HBOT for 3 months, this group showed a higher FA value than before. Also, MRI showed that the white matter was recovering its function. Mucormycosis is a fungal infection that begins at the sinus and can spread to the eyes, brain and all other organs caused by fungi of the Mucorales order. When the fungus has spread into the brain it is very aggressive and can be fatal. Diabetic and immuno-compromised patients are most susceptible. In a study by Kajs-Wyllie et al.,1995 rhino cerebral fungal infections were treated with the typical antibiotics amphotericin B and Parconazole as well as HBOT. The combined antibiotics and HBOT treatment helped reduce the toxicosis of fungal effect on the brain and increase the survival duration. The HBOT was successful because the increased atmospheric pressure applied allows more oxygen to be taken up by the lungs and increases the plasma oxygen concentration and deliverance to tissues. At higher enough levels, oxygen has antifungal and effects against acidosis, thereby reducing the toxicosis of the fungus.
Lastly, a study conducted on artificially caused trauma in the mouse brain treated with long term HBOT showed many positive results, continuing even post treatment. The results of the treatments were measured for 3 weeks post treatment (Kraitsy et al., 2014). It was found that the HBOT administered mice showed decreased conduction time compared to the control group not treated with HBOT. Conducting time is the length of time that stimuli-brain communication takes. When the conducting time increases, the processing takes a long time and visa versa. Along with the improved conduction speed, there were no measured side effects seen on the MRI. The mice that received the treatment also showed more active sites (site where brain activity is occurring) for a wide range of the brain whereas the control group showed a very narrow range of active sites. Remyelination was also observed, apparent by the increase in myelin isoform proteins, an increase in the presence of myelin and an increase in the expression of proteolipid proteins (PLP), which are one of the main protein types that central nervous system myelin is composed of (Kraitsy et al., 2014).
Conclusion
HBOT can successfully be administered in many different fields of application, including both acute and chronic cases even though it is only used in conjunction to other treatments. In Figure 1., a summary of the major uses and physiological mechanisms of healing in HBOT case studies that were highlighted in this paper is presented for simplified understanding. Although this paper presented many positive case studies and the unarguable physiological benefits, there are some negative aspects to take into consideration. A downside of HBOT is the often long duration time of therapy that may result in death of the patient before treatment time is complete. The high expenses of the equipment required is also a negative factor of HBOT, especially in the field of veterinary medicine where the funding for expensive medical technologies can be significantly less. When considering the negative and positive implications in HBOT, the successful applications of this technology are very promising. Given that it is a relatively modern method and is still very much in the development phase, growth within this scientific field is to be expected, resulting in increased HBOT being used as a reliable and valuable tool in both human and veterinary medicine.
Appendix 1
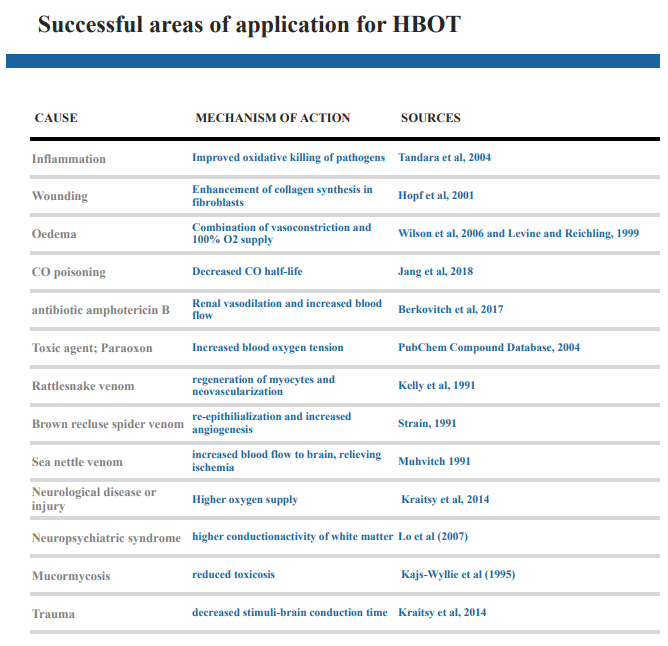
Figure 1:A summary of health issues/toxic agents and the physiological mechanisms of healing with HBOT
References
Allen, D. B.; Maguire, J. J.; Mahdavian, M. (1997): Wound Hypoxia and Acidosis Limit Neutrophil Bacterial Killing Mechanisms. Archive of Surgery132: (9) 991–996
Berkovitch, M.; Shain, Y.; Kozer, E.; Goldman, M.; Abu-Kishk, I. (2017): Hyperbaric oxygen treatment and nephrotoxicity induced by gentamicinin rats. BMC Nephrology 18: 374
Cammer,G.W.; Bloom, B. R.; Norton, W. T.; Gordon, S. (1978): Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages-A possible mechanism of inflammatory demyelination. Proceeding of the National Academy of Sciences of the United States of America 75: (3) 1554-1558
Canto, Mauro C. Dal and Rabinowitz, Stanley G. (1982): Experimental models of virus-induced demyelination of the central nervous system. Annals of Neurology 11: (2)
DeStefano, F.; Verstraeten, T.; Jackson, L. A.; Chen, R. T. (2003):Vaccinations and Risk of Central Nervous System Demyelinating Diseases in Adults. Archives of Neurology 60: (4) 504-9
Gill, A. L and Bell, C. N. A (2004): Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM: An International Journal of Medicine 97: (7) 385-395
Hopf, H. W.; Humphrey, L. M.; Puzziferri, N.; West, J. M.; Attinger, C. E.; Hunt, T. K. (2001): Adjuncts to preparing wounds for closure- Hyperbaric oxygen, growth factors, skin substitutes, negative pressure wound therapy (vacuum-assisted closure). Foot Ankle Clinic North America 6: (4) 661–682
Hopf, H. W.; Viele, M.; Watson, J. J. (2000): Subcutaneous Perfusion and Oxygen During Acute Severe Isovolemic Hemodilution in Healthy Volunteers. Archive of Surgery 135: (12) 1443–1449
Jang, D. H.; Khatri, U. G.; Shortal, B. P.; Kelly, M.; Hardy, K.; Lambert, D. S.; Eckmann, D. M. (2018): Alterations in mitochondrial respiration and reactive oxygen species in patients poisoned with carbon monoxide treated with hyperbaric oxygen. Intensive Care Medicine Experimental 6: (4) 2-14
Kajs-Wyllie, M. (1995): Hyperbaric oxygen therapy for rhinocerebral fungal infection. Journal of Neuroscience Nursing 27: (3) 174-81
Kelly, J. J.; Sadeghani, K.; Gottlieb, S. F.; Ownby, C. L.; Van Meter, K. W.; Torbati, D. (1991): Reduction of Rattlesnake-Induced-Myonecrosis in Mice by Hyperbaric Oxygen Therapy. The Journal of Emergency Medicine 9: 1-7
Kraitsy, K.; Uecal, M.; Grossauer, S.; Bruckmann, L.; Pfleger, F.; Ropele, S.; Fazekas, F.; Gruenbacher, G.; Patz, S.; Absenger, M.; Probsky, C.; Smolle-Juettner, F.; Tezer, I.; Molcanyi, M.; Fasching, U.; Schaefer, U. (2014): Repetitive Long-Term Hyperbaric Oxygen Treatment (HBOT) Administered after Experimental Traumatic Brain Injury in Rats Induces Significant Remyelination and a Recovery of Sensorimotor Function. The Publishing Library of Science 9: (5) https://doi.org/10.1371/journal.pone.0097750
Lo, C. P.; Chen, S. Y.; Chou, M. C.; Wang, C. Y.; Lee, K. W.; Hsueh, C. J.; Chen, C. Y; Huang, K. L.; Huang, G. S. (2007): Diffusion‐tensor MR imaging for evaluation of the efficacy of hyperbaric oxygen therapy in patients with delayed neuropsychiatric syndrome caused by carbon monoxide inhalation. European Journal of Neurology 14: (7) 777-82
Muhvitch, K. H.; Sengottuvelu, S.; Manson, P. N.; Myers, R. A. M.; Burnett, J. W.; Marzella, L. (1991): Pathophysiology of the Sea Nettle (Chrysaora quinquercirrha), Envenomation in a rat model and the effects of Hyperbaric Oxygen and Verapamil Treatment. Toxicon 29: (7) 857-866
Nir, H.; Berkovitch, M.; Youngster, I.; Kozer, E.; Goldman, M.; Abu-Kishk, I. (2015): The effect of hyperbaric oxygen therapy on amphotericin B-induced acute renal failure in rats. The Italian Journal of Urology and Nephrology 67(2): 97–102
Siddiqui, A.; Davidson, J. D.; Mustoe, T. A. (1997): Ischemic Tissue Oxygen Capacitance after Hyperbaric Oxygen Therapy: A New Physiologic Concept. Plastic and Reconstructive Surgery 99: (1) 148–155
Strain, G. M.; Snider, T.G.; Tedford, B. L.; Cohen, G. H. (1991): Hyperbaric Oxygen Effects on Brown Recluse Spider (Loxosceles Reclusa) Envenomation in Rabbits. Toxicon 29: (8) 989-996
Tandara, A. A. and Mustoe, T. A. (2004): Oxygen in Wound Healing- More than a Nutrient. World Journal of Surgery: (28) 294–300
Wilson, Hilary D.; Wilson, Judy R.; Fuchs, Perry N. (2006): Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Research 1098: (1) 126-128
Zhang, T.; Yang, Q. W.; Wang, S. N.; Wang, J. Z.; Wang, Q.; Wang, Y.; Luo, Y. J. (2010): Hyperbaric oxygen therapy improves neurogenesis and brain blood supply in piriform cortex in rats with vascular dementia. Brain Injuries 24: (11) 1350-7
Other Sources
Jain, K.K. 2004: Textbook of Hyperbaric Medicine 4th ed. Cambridge. Hogrefe and Huber. Cambridge. pp 23-25
Jones, M.W.; Wyatt, H. A., 2017: Hyperbaric, Physics. URL: https://knowledge.statpearls.com/chapter/0/23143/. Accessed: April 23, 2018.
Levine, J.D., Reichling, D.B.1999: Textbook of Pain. Peripheral mechanisms of inflammatory pain 4th edition. Edinburgh.Churchill Livingstone. pp. 59–84
PubChem Compound Database, 2004: Paraoxan. URL: https://pubchem.ncbi.nlm.nih.gov/compound/paraoxon#section=Top. Accessed: April 23, 2018.
