|
Size: 21741
Comment:
|
Size: 21832
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 4: | Line 4: |
| The Effects of Hypoxia and Reperfusion on the Central Nervous System | = The Effects of Hypoxia and Reperfusion on the Central Nervous System = |
| Line 21: | Line 22: |
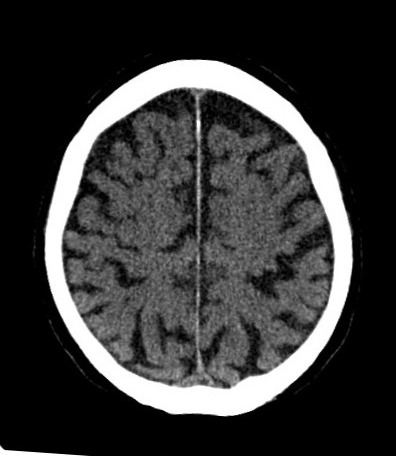
| Chronic hypoxia prompts structural changes to restore the baseline PtO2. Structural changes include an increase in capillary density resulting in a decrease in inter-capillary distance and therefore a decrease in the diffusion distance (LaManna et al., 2004). Lauro and LaManna (1997) found that in the rat brain, the capillary distance almost doubled in response to chronic hypoxia and the average distance between capillaries decreased from 50 to 40 m. It is not known whether brain oxygen consumption is decreased in hypoxic adapted rats (LaManna et al., 2004), however it is known that cytochrome oxidase is decreased by 15% in the rat brain (Caceda et al., 2001; Chávez et al., 1995; LaManna et al., 1996). Not only this but, prolonged mild hypoxia also causes transient CBF increase, increased glucose transport across the blood-brain barrier, incregased glycolysis to maintain tissue acid-base balance, decreased mitochondrial energy consumption and restoration of tissue oxygen tension profiles (LaManna et al., 2004). | Chronic hypoxia prompts structural changes to restore the baseline PtO2. Structural changes include an increase in capillary density resulting in a decrease in inter-capillary distance and therefore a decrease in the diffusion distance (LaManna et al., 2004). Lauro and LaManna (1997) found that in the rat brain, the capillary distance almost doubled in response to chronic hypoxia and the average distance between capillaries decreased from 50 to 40 m. It is not known whether brain oxygen consumption is decreased in hypoxic adapted rats (LaManna et al., 2004), however it is known that cytochrome oxidase is decreased by 15% in the rat brain (Caceda et al., 2001; Chávez et al., 1995; LaManna et al., 1996). Not only this but, prolonged mild hypoxia also causes transient CBF increase, increased glucose transport across the blood-brain barrier, incregased glycolysis to maintain tissue acid-base balance, decreased mitochondrial energy consumption and restoration of tissue oxygen tension profiles (LaManna et al., 2004). || {{https://upload.wikimedia.org/wikipedia/commons/9/91/CCT_Hypoxie-29.jpg}} <<BR>>'''Fig 1.'''<<BR>>''Profound hypoxia brain CT scan in human.'' || |
| Line 23: | Line 25: |
| ||{{https://upload.wikimedia.org/wikipedia/commons/9/91/CCT_Hypoxie-29.jpg}}<<BR>>'''Fig 1.'''<<BR>>''Profound hypoxia brain CT scan in human.''|| | |
| Line 25: | Line 26: |
| ||{{https://upload.wikimedia.org/wikipedia/commons/e/e9/CCT_Hypoxie-12.jpg}}<<BR>> '''Fig 2.'''<<BR>>''Generalised hypoxia brain CT scan in human.''|| | || {{https://upload.wikimedia.org/wikipedia/commons/e/e9/CCT_Hypoxie-12.jpg}} <<BR>> '''Fig 2.'''<<BR>>''Generalised hypoxia brain CT scan in human.'' || |
| Line 33: | Line 35: |
| Downing et al (1963) found that ischemia, hypoxia or hypercapnia of the CNS resulted in an elevation of systemic arterial blood pressure. Elevation of the Pco2 of the CNS perfusate also resulted in large increases of peripheral vascular resistance. | Downing et al (1963) found that ischemia, hypoxia or hypercapnia of the CNS resulted in an elevation of systemic arterial blood pressure. Elevation of the Pco2 of the CNS perfusate also resulted in large increases of peripheral vascular resistance. |
| Line 36: | Line 38: |
| After testing the response on 8 animals, Downing et al (1968) found that the response to CNS hypoxia is a significant tachycardia, the average mean increase being 20 beats per minute. The responses to CNS ischemia were found to be even greater with the average mean change being 31 beats per minute. The tachycardia in response to hypoxia and ischemia were reversible. | After testing the response on 8 animals, Downing et al (1968) found that the response to CNS hypoxia is a significant tachycardia, the average mean increase being 20 beats per minute. The responses to CNS ischemia were found to be even greater with the average mean change being 31 beats per minute. The tachycardia in response to hypoxia and ischemia were reversible. |
| Line 41: | Line 43: |
| Line 45: | Line 46: |
| It could therefore be concluded that cerebral ischemia produced a marked increase of blood pressure, peripheral vascular resistance, heart rate and atrial and ventricular contractility in the body. The cerebral ischemia response was induced by alterations of pCO2, PO2 or pH content of the blood. It was also found that the gas composition of the blood perfusing the central nervous system may importantly influence the response of the organism to systemic hypoxia. | It could therefore be concluded that cerebral ischemia produced a marked increase of blood pressure, peripheral vascular resistance, heart rate and atrial and ventricular contractility in the body. The cerebral ischemia response was induced by alterations of pCO2, PO2 or pH content of the blood. It was also found that the gas composition of the blood perfusing the central nervous system may importantly influence the response of the organism to systemic hypoxia. |
| Line 50: | Line 51: |
| The decrease in the pO2 causes the haemoglobin to have a greater affinity to the O2 leading to less O2 being passed into the tissue of the CNS. This can have detrimental affects on the CNS as the tissues need sufficient amounts of O2 to function (Witt et al., 2003). The decrease in sodium can lead a tissue influx which is related to ischemic oedema (Rothman and Olney, 1986). The decrease may also cause secondary energy failure. (Witt et al., 2003). The blood brain barrier may also be destructed by the increase in sucrose. (Witt et al., 2003). These results demonstrate the negative impact hypoxia has on the CNS, some causing irreversible damage (Witt et al., 2003) | The decrease in the pO2 causes the haemoglobin to have a greater affinity to the O2 leading to less O2 being passed into the tissue of the CNS. This can have detrimental affects on the CNS as the tissues need sufficient amounts of O2 to function (Witt et al., 2003). The decrease in sodium can lead a tissue influx which is related to ischemic oedema (Rothman and Olney, 1986). The decrease may also cause secondary energy failure. (Witt et al., 2003). The blood brain barrier may also be destructed by the increase in sucrose. (Witt et al., 2003). These results demonstrate the negative impact hypoxia has on the CNS, some causing irreversible damage (Witt et al., 2003) |
| Line 56: | Line 57: |
| Both pre and post-conditioning treatments were modelled in a study carried out by Simon (2014) by assessing the artery that is affected by the ischemic stroke. For pre-conditioning, the middle cerebral artery was used and the brain was exposed to thirty minutes of ischemic conditions followed by a further one hundred minutes of ischemia seventy-two minutes later. In response to this sub-lethal stress, “pre-conditioning” is induced in the organ allowing a tolerance to develop to protect the brain from subsequent bouts of ischemia (Simon, 2014). In a study carried out by Wein et al (1999) it was found that patients that were exposed to transient ischemic attacks (TIAA) have strokes leading to milder effects. |
Both pre and post-conditioning treatments were modelled in a study carried out by Simon (2014) by assessing the artery that is affected by the ischemic stroke. For pre-conditioning, the middle cerebral artery was used and the brain was exposed to thirty minutes of ischemic conditions followed by a further one hundred minutes of ischemia seventy-two minutes later. In response to this sub-lethal stress, “pre-conditioning” is induced in the organ allowing a tolerance to develop to protect the brain from subsequent bouts of ischemia (Simon, 2014). In a study carried out by Wein et al (1999) it was found that patients that were exposed to transient ischemic attacks (TIAA) have strokes leading to milder effects. |
| Line 60: | Line 61: |
| Thus, these experiments show that while ischemia can negatively affect the brain, it may also have some positive effects. By exposing the brain to small amounts of ischemic conditions, it can help prepare the brain and minimise the harmful effects of hypoxia (Simon, 2014). Not only this, if the blood flow during reperfusion is interrupted temporarily, the reperfused induced injury can be minimised (Roger Simon, 2014). |
Thus, these experiments show that while ischemia can negatively affect the brain, it may also have some positive effects. By exposing the brain to small amounts of ischemic conditions, it can help prepare the brain and minimise the harmful effects of hypoxia (Simon, 2014). Not only this, if the blood flow during reperfusion is interrupted temporarily, the reperfused induced injury can be minimised (Roger Simon, 2014). || {{http://ischemicstroke.org/wp-content/uploads/2013/05/ischemic-attacks.jpg}} <<BR>>'''Fig 3.'''<<BR>>''Stroke patient'' || |
| Line 64: | Line 69: |
| Although reperfusion techniques are commonly used in treatment of stroke patients, some negative effects may be observed. For effective tissue survival during reperfusion, oxygen must be reintroduced to the ischemic brain, however, this event may also cause oxidative stress (Niatsetskaya et al.,2011). In a study conducted by Niatsetskaya et al (2011) it was observed that when reperfusion was initiated with reoxygenation followed by exposure to oxygen, brain injury and oxidative stress was significantly increased. Niatsetskaya et al (2011) found that mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (HI). By limiting this reperfusion-driven stress to the mitochondria, mild hypoxia will increase the severity of the HI brain injury. During HI, the mitochondria's ability to produce ATP by oxidative phosphorylation is hindered. The reperfusion will restore this capacity to generate ATP (Lorek et al, 1994). The mechanism proposed to explain this is the dissipation of the mitochondrial proton-motive force due to an accumulation of the Ca++ and the opening of the mitochondrial permeanlity transition pore (mPTP). This ultimately results in mitochondrial swelling and the loss of the ability to produce energy. The major trigger for this reperfusion-driven oxidative stress is the reintroduction of oxygen to ischemic tissue at the onset of reperfusion. Overall, it was found that changes in the systemic oxygenation during reperfusion had a significant effect on the degree of the HI injury in neonatal mice. This implied that the mechanisms involved in HI brain injury or cerebral recovery is significantly altered by ranging levels of systemic oxygenation during the initiative stage of reperfusion. The severity this damage can be lowered to preserve the immature brain by maintaining mild hypoxemia during early reperfusion (Niatsetskaya et al., 2011). |
Although reperfusion techniques are commonly used in treatment of stroke patients, some negative effects may be observed. For effective tissue survival during reperfusion, oxygen must be reintroduced to the ischemic brain, however, this event may also cause oxidative stress (Niatsetskaya et al.,2011). In a study conducted by Niatsetskaya et al (2011) it was observed that when reperfusion was initiated with reoxygenation followed by exposure to oxygen, brain injury and oxidative stress was significantly increased. Niatsetskaya et al (2011) found that mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (HI). By limiting this reperfusion-driven stress to the mitochondria, mild hypoxia will increase the severity of the HI brain injury. During HI, the mitochondria's ability to produce ATP by oxidative phosphorylation is hindered. The reperfusion will restore this capacity to generate ATP (Lorek et al, 1994). The mechanism proposed to explain this is the dissipation of the mitochondrial proton-motive force due to an accumulation of the Ca++ and the opening of the mitochondrial permeanlity transition pore (mPTP). This ultimately results in mitochondrial swelling and the loss of the ability to produce energy. The major trigger for this reperfusion-driven oxidative stress is the reintroduction of oxygen to ischemic tissue at the onset of reperfusion. Overall, it was found that changes in the systemic oxygenation during reperfusion had a significant effect on the degree of the HI injury in neonatal mice. This implied that the mechanisms involved in HI brain injury or cerebral recovery is significantly altered by ranging levels of systemic oxygenation during the initiative stage of reperfusion. The severity this damage can be lowered to preserve the immature brain by maintaining mild hypoxemia during early reperfusion (Niatsetskaya et al., 2011). |
| Line 69: | Line 72: |
| Hypoxia and reperfusion have a myriad of effects on central nervous system as illustrated in the above points. Such effects are so extensive that they elicit responses from the cardiovascular system, energy metabolism of the body and blood-brain barrier permeability. Furthermore, the body can adapt to hypoxic conditions according to the severity of the condition. However, acclimatizing responses to hypoxia may be impaired with increasing age and metabolic or vascular disease (LaManna, 2007). Due to the complex relationship between hypoxia and reperfusion in the central nervous system, modern medicine has begun utilising hypoxic conditions and reperfusion techniques in the study of stroke patients. It was found that reperfusion may help in the recovery of those affected. However, this is not to say that reperfusion techniques have had solely positive effects on the central nervous system, in fact, reperfusion was recorded to cause oxidative stress on the central nervous system (Niatsetskaya et al.,2011). Surprisingly, although ischemia was found to found to generally have negative effects on the central nervous system, when coupled with reperfusion it in fact had a positive effect on the recovery of stroke patients. Exposure of the brain to minimal amounts of ischemic conditions helped minimise the effects of hypoxia later (Simon, 2014). Not only this but mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (Niatsetskaya et al.,2011). | Hypoxia and reperfusion have a myriad of effects on central nervous system as illustrated in the above points. Such effects are so extensive that they elicit responses from the cardiovascular system, energy metabolism of the body and blood-brain barrier permeability. Furthermore, the body can adapt to hypoxic conditions according to the severity of the condition. However, acclimatizing responses to hypoxia may be impaired with increasing age and metabolic or vascular disease (LaManna, 2007). Due to the complex relationship between hypoxia and reperfusion in the central nervous system, modern medicine has begun utilising hypoxic conditions and reperfusion techniques in the study of stroke patients. It was found that reperfusion may help in the recovery of those affected. However, this is not to say that reperfusion techniques have had solely positive effects on the central nervous system, in fact, reperfusion was recorded to cause oxidative stress on the central nervous system (Niatsetskaya et al.,2011). Surprisingly, although ischemia was found to found to generally have negative effects on the central nervous system, when coupled with reperfusion it in fact had a positive effect on the recovery of stroke patients. Exposure of the brain to minimal amounts of ischemic conditions helped minimise the effects of hypoxia later (Simon, 2014). Not only this but mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (Niatsetskaya et al.,2011). |
| Line 73: | Line 75: |
Abbruscato, T. and Davis, T. (1999). Combination of Hypoxia/Aglycemia Compromises In Vitro Blood-Brain Barrier Integrity. Journal of Pharmacology and Experimental Therapeutics, 289(2), pp.668-675. |
Abbruscato, T. and Davis, T. (1999). Combination of Hypoxia/Aglycemia Compromises In Vitro Blood-Brain Barrier Integrity. Journal of Pharmacology and Experimental Therapeutics, 289(2), pp.668-675. |
| Line 83: | Line 83: |
| Bottom of Form | Downing, S., Mitchell, J. and Wallace, A. (1963). Cardiovascular responses to ischemia, hypoxia, and hypercapnia of the central nervous system. American Journal of Physiology-Legacy Content, 204(5), pp.881-887. |
| Line 85: | Line 85: |
| Downing, S., Mitchell, J. and Wallace, A. (1963). Cardiovascular responses to ischemia, hypoxia, and hypercapnia of the central nervous system. American Journal of Physiology-Legacy Content, 204(5), pp.881-887. Fisher, M. (1995). Diffusion and perfusion imaging for acute stroke. Surgical Neurology, 43(6), pp.606-609. Halestrap, A. (2010). A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochemical Society Transactions, 38(4), pp.841-860. |
Fisher, M. (1995). Diffusion and perfusion imaging for acute stroke. Surgical Neurology, 43(6), pp.606-609. Halestrap, A. (2010). A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochemical Society Transactions, 38(4), pp.841-860. |
| Line 94: | Line 91: |
| Khurshid, S., Trupe, L., Newhart, M., Davis, C., Molitoris, J., Medina, J., Leigh, R. and Hillis, A. (2012). Reperfusion of specific cortical areas is associated with improvement in distinct forms of hemispatial neglect. Cortex, 48(5), pp.530-539. | Khurshid, S., Trupe, L., Newhart, M., Davis, C., Molitoris, J., Medina, J., Leigh, R. and Hillis, A. (2012). Reperfusion of specific cortical areas is associated with improvement in distinct forms of hemispatial neglect. Cortex, 48(5), pp.530-539. |
| Line 100: | Line 97: |
| LaManna, J. (2004). Structural and functional adaptation to hypoxia in the rat brain. Journal of Experimental Biology, 207(18), pp.3163-3169. | LaManna, J. (2004). Structural and functional adaptation to hypoxia in the rat brain. Journal of Experimental Biology, 207(18), pp.3163-3169. |
| Line 102: | Line 99: |
| LaManna, J. (2007). Hypoxia in the central nervous system. Essays In Biochemistry, 43, pp.138-15 Lauro, K. L. and LaManna, J. C. (1997). Adequacy of cerebral vascular remodeling following three weeks of hypobaric hypoxia. Examined by an integrated composite analytical model. Adv. Exp. Med. Biol. 411, 369- 376. |
LaManna, J. (2007). Hypoxia in the central nervous system. Essays In Biochemistry, 43, pp.138-15 Lauro, K. L. and LaManna, J. C. (1997). Adequacy of cerebral vascular remodeling following three weeks of hypobaric hypoxia. Examined by an integrated composite analytical model. Adv. Exp. Med. Biol. 411, 369- 376. |
| Line 109: | Line 105: |
| Lum, H., Barr, D., Shaffer, J., Gordon, R., Ezrin, A. and Malik, A. (1992). Reoxygenation of endothelial cells increases permeability by oxidant-dependent mechanisms. Circulation Research, 70(5), pp.991-998. | Lum, H., Barr, D., Shaffer, J., Gordon, R., Ezrin, A. and Malik, A. (1992). Reoxygenation of endothelial cells increases permeability by oxidant-dependent mechanisms. Circulation Research, 70(5), pp.991-998. |
| Line 111: | Line 107: |
| Mark, K. and Davis, T. (2002). Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. American Journal of Physiology-Heart and Circulatory Physiology, 282(4), pp.H1485-H1494. | Mark, K. and Davis, T. (2002). Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. American Journal of Physiology-Heart and Circulatory Physiology, 282(4), pp.H1485-H1494. |
| Line 115: | Line 111: |
| Nyakas, C., Buwald, B. and Luiten, P. (1996). Hypoxia and brain development. Progress in Neurobiology, 49(1), pp.1-51. | Nyakas, C., Buwald, B. and Luiten, P. (1996). Hypoxia and brain development. Progress in Neurobiology, 49(1), pp.1-51. |
| Line 119: | Line 115: |
| Rothman, S. and Olney, J. (1986). Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Annals of Neurology, 19(2), pp.105-111. | Rothman, S. and Olney, J. (1986). Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Annals of Neurology, 19(2), pp.105-111. |
| Line 123: | Line 119: |
| Simon, R. (2014). Post-Conditioning and Reperfusion Injury in the Treatment of Stroke. Dose-Response, 12(4), p.dose-response.1. | Simon, R. (2014). Post-Conditioning and Reperfusion Injury in the Treatment of Stroke. Dose-Response, 12(4), p.dose-response.1. |
| Line 125: | Line 121: |
| Witt, K., Mark, K., Hom, S. and Davis, T. (2003). Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. American Journal of Physiology-Heart and Circulatory Physiology, 285(6), pp.H2820-H2831. | Witt, K., Mark, K., Hom, S. and Davis, T. (2003). Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. American Journal of Physiology-Heart and Circulatory Physiology, 285(6), pp.H2820-H2831. |
Itt írjon a(z) HypoxiaReperfusionBrain-ról/ről
The Effects of Hypoxia and Reperfusion on the Central Nervous System
Sorcha Delargy, Beth Dunne and Ciara Redmond
Contents
- The Effects of Hypoxia and Reperfusion on the Central Nervous System
Introduction
The central nervous system, especially the brain, requires a sufficient supply of oxygen for its normal functioning. Hypoxia in the CNS occurs when metabolic activity is not balanced with adequate oxygen supply (LaManna et al., 2004) and it is a threat to brain functioning during the entire life span from the foetal stage and beyond (Nyakas et al, 1996). Although the mammalian central nervous system can tolerate low oxygen environments for periods of time, prolonged exposure to low oxygen environments provokes systemic, cardiovascular and respiratory adaptations in order to preserve oxygen delivery to the brain (LaManna et al., 2004). Low oxygen environments coupled with an increase in neuronal activity will also cause physiological changes (LaManna et al., 2004). In addition, Hypoxia is also known to cause neuronal cell injury, neurodegeneration and cell death (Nyakas et al, 1996). It is also understood that hypoxia may alter blood-brain barrier permeability (Witt et al., 2003). Therefore, the study and understanding of this condition is of paramount importance in Medical studies. In this essay we will discuss the various effects of hypoxia and reperfusion on the central nervous system, with particular attention to the treatment of stroke patients. We will also mention ischemia in our discussions as it often accompanies hypoxia (Nyakas et al, 1996).
Acute and Chronic hypoxia
It is argued that when hypoxemia in the body is ‘mild’, successful long term adaptation is possible (LaManna et al, 2004). The brain can respond to mild hypoxia naturally by utilizing acute and chronic adaptive mechanisms (LaManna, 2007). LaManna et al (2004) describes theses adaptations using the rat brain as an example, which we will discuss in the following paragraphs.
Acute brain effects of hypoxia
In the CNS, there are notable alterations in energy metabolism and cerebrovasculature that act to preserve tissue oxygen and the energy supply required to maintain sufficient neurological function (LaManna et al., 2004). Such adaptations include an increase in cerebral blood flow (CBF) along with an increase in the consumption of glucose (Beck and Krieglstein, 1987) although Pto2 still decreases (Sick et al., 1982). Other such adaptations include a faster capillary mean transit time and haemoglobin disoxygenation (LaManna et al., 2004).
Chronic brain effects of hypoxia
Chronic hypoxia is often seen during foetal development, often as a result of placental dysfunctions (Nyakas et al, 1996). After early hypoxia, behavioural and psychological dysfunctioning may be observed (Nyakas et al, 1996). More significant neuronal damage such as cell loss has been known to cause motor disabilities, cerebral palsy or epilepsy (Nyakas et al, 1996).
Chronic hypoxia prompts structural changes to restore the baseline PtO2. Structural changes include an increase in capillary density resulting in a decrease in inter-capillary distance and therefore a decrease in the diffusion distance (LaManna et al., 2004). Lauro and LaManna (1997) found that in the rat brain, the capillary distance almost doubled in response to chronic hypoxia and the average distance between capillaries decreased from 50 to 40 m. It is not known whether brain oxygen consumption is decreased in hypoxic adapted rats (LaManna et al., 2004), however it is known that cytochrome oxidase is decreased by 15% in the rat brain (Caceda et al., 2001; Chávez et al., 1995; LaManna et al., 1996). Not only this but, prolonged mild hypoxia also causes transient CBF increase, increased glucose transport across the blood-brain barrier, incregased glycolysis to maintain tissue acid-base balance, decreased mitochondrial energy consumption and restoration of tissue oxygen tension profiles (LaManna et al., 2004).
|
|
The cardiovascular response to hypoxia on the CNS
Downing et al (1963) carried out a series of experiments on animals exploring the cardiovascular response to ischemia, hypoxia and hypercapnia of the central nervous system. In their studies, Downing et al (1963) found that there were 4 main physiological responses to these experimentally induced conditions;
Systemic blood pressure and peripheral resistance responses
Downing et al (1963) found that ischemia, hypoxia or hypercapnia of the CNS resulted in an elevation of systemic arterial blood pressure. Elevation of the Pco2 of the CNS perfusate also resulted in large increases of peripheral vascular resistance.
Heart rate responses
After testing the response on 8 animals, Downing et al (1968) found that the response to CNS hypoxia is a significant tachycardia, the average mean increase being 20 beats per minute. The responses to CNS ischemia were found to be even greater with the average mean change being 31 beats per minute. The tachycardia in response to hypoxia and ischemia were reversible.
Ventricular contractility responses
In response to ischemia of the CNS aortic pressure, heart rate and cardiac output were maintained at constant levels (Downing et al., 1963). However, when perfusion of the CNS was temporarily interrupted the end diastolic pressure of the left ventricle fell 3cm H20, the duration of ejection was reduced and the maximal rate of ventricular pressure development increased (Downing et al., 1963). Thus the ventricle was doing the same amount of work from a lower end-diastolic pressure at a higher rate; the contractility was therefore increased. Perfusion of the CNS with hypoxic blood also produced an increase of ventricular contractility (Downing et al., 1963).
Atrial contractility
It was also found that changes in the left arterial pressure occurred as a consequence to ischemia in the CNS (Downing et al., 1963).
It could therefore be concluded that cerebral ischemia produced a marked increase of blood pressure, peripheral vascular resistance, heart rate and atrial and ventricular contractility in the body. The cerebral ischemia response was induced by alterations of pCO2, PO2 or pH content of the blood. It was also found that the gas composition of the blood perfusing the central nervous system may importantly influence the response of the organism to systemic hypoxia.
Effects of hypoxia and reperfusion on the blood-brain barrier
The blood brain barrier is critical in maintaining homeostasis within the brain. Hypoxia and reoxygenation affects the blood brain barrier by increasing its permeability with affiliated changes to the tight junctional protein expression (Witt et al., 2003). Witt et al (2003) used anaesthetised rats that underwent hypoxia insult in O2 controlled hypoxic chamber. This hypoxic insult was then followed by 10 minutes of reoxygenation. The levels of sucrose, blood gas and electrolyte and ATP were used to examine the permeability of the blood brain barrier. There was an increase in sucrose uptake by 32% as well as a decrease in both the oxygen and carbon dioxide partial pressures. A decrease in sodium levels was also observed. The rapid increase in the pH was noted only at the start of the hypoxic conditions while an increase in ATP concentration was only seen during the reoxygenation stage (Witt et al., 2003). This shows that the permeability of the blood brain barrier may increase as a result of acute hypoxia and reoxygenation. This leads to an increase in transport across the intercellular space between cells across the blood brain barrier and a change in order of the tight junctions (Witt et al., 2003). In vitro blood brain barrier models show an increase in cerebrovascular paracellular permeability after hypoxia and post hypoxia reoxygenation (Abbruscato and Davis, 1999), (Lum et al., 1992), (Mark and Davis, 2002).
The decrease in the pO2 causes the haemoglobin to have a greater affinity to the O2 leading to less O2 being passed into the tissue of the CNS. This can have detrimental affects on the CNS as the tissues need sufficient amounts of O2 to function (Witt et al., 2003). The decrease in sodium can lead a tissue influx which is related to ischemic oedema (Rothman and Olney, 1986). The decrease may also cause secondary energy failure. (Witt et al., 2003). The blood brain barrier may also be destructed by the increase in sucrose. (Witt et al., 2003). These results demonstrate the negative impact hypoxia has on the CNS, some causing irreversible damage (Witt et al., 2003)
Hypoxia and reperfusion as treatment in stroke patients
The protection of the brain from stroke has conventionally been by exogenous drug treatment, however, endogenous treatments are now being investigated (Dirnagl et al., 2003). In a study conducted by Khurshid et al (2012), twenty five patients diagnosed with acute right hemisphere stroke were observed in order to determine whether reperfusion to specific right cortical regions results in improvement in cerebral function.
Each administration of the test was coupled with Diffusion Weight Imaging (DWI) and Perfusion Weight Imaging (PWI) in order to access the areas of the brain proving to be dysfunctional and to localise the areas that may have been perfused. DWI establishes the areas of permanent structural damage (Fisher, 1995). While PWI reveals the total area of the brain affected by an insufficient blood flow. These areas, although previously debilitated due to lack of perfusion, are potentially redeemable if the blood flow can be restored (Beaulieu et al. 1999). The “diffusion-perfusion mismatch” is then applicable to certain regions. This miss match region presents the areas where atypical perfusion lesions are generally larger than the DWI lesions, concluding that a severe DWI/PWI mismatch deficits are at considerable risk of lesion enlargement. This ‘diffusion-perfusion mismatch’ allowed identification of areas that were potentially recoverable and showed the areas that became reperfused when compared over a 2-3-day period. Simultaneously behavioural task performance was measured and the changes observed in days 1-3 presented which intellectual abilities improved over time. It was then possible to create a positive correlation between the improvement in behavioural abilities and the tissue recovered via reperfusion.
Both pre and post-conditioning treatments were modelled in a study carried out by Simon (2014) by assessing the artery that is affected by the ischemic stroke. For pre-conditioning, the middle cerebral artery was used and the brain was exposed to thirty minutes of ischemic conditions followed by a further one hundred minutes of ischemia seventy-two minutes later. In response to this sub-lethal stress, “pre-conditioning” is induced in the organ allowing a tolerance to develop to protect the brain from subsequent bouts of ischemia (Simon, 2014). In a study carried out by Wein et al (1999) it was found that patients that were exposed to transient ischemic attacks (TIAA) have strokes leading to milder effects.
Post-conditioning refers to the decrease in harmful processes which occur during reperfusion of ischemic brain (Simon, 2014). In post-conditioning treatments, the brain was exposed to equal lengths of reperfusion and occlusion (Simon, 2014). Reperfusion of the region of the brain affected by ischemia is the prevailing treatment for acute stroke. Post conditioning is most efficient during the 1st 30mins of reperfusion (Pignalaro et al., 2008).
Thus, these experiments show that while ischemia can negatively affect the brain, it may also have some positive effects. By exposing the brain to small amounts of ischemic conditions, it can help prepare the brain and minimise the harmful effects of hypoxia (Simon, 2014). Not only this, if the blood flow during reperfusion is interrupted temporarily, the reperfused induced injury can be minimised (Roger Simon, 2014).
|
Negative effects of reperfusion
Although reperfusion techniques are commonly used in treatment of stroke patients, some negative effects may be observed. For effective tissue survival during reperfusion, oxygen must be reintroduced to the ischemic brain, however, this event may also cause oxidative stress (Niatsetskaya et al.,2011). In a study conducted by Niatsetskaya et al (2011) it was observed that when reperfusion was initiated with reoxygenation followed by exposure to oxygen, brain injury and oxidative stress was significantly increased. Niatsetskaya et al (2011) found that mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (HI). By limiting this reperfusion-driven stress to the mitochondria, mild hypoxia will increase the severity of the HI brain injury. During HI, the mitochondria's ability to produce ATP by oxidative phosphorylation is hindered. The reperfusion will restore this capacity to generate ATP (Lorek et al, 1994). The mechanism proposed to explain this is the dissipation of the mitochondrial proton-motive force due to an accumulation of the Ca++ and the opening of the mitochondrial permeanlity transition pore (mPTP). This ultimately results in mitochondrial swelling and the loss of the ability to produce energy. The major trigger for this reperfusion-driven oxidative stress is the reintroduction of oxygen to ischemic tissue at the onset of reperfusion. Overall, it was found that changes in the systemic oxygenation during reperfusion had a significant effect on the degree of the HI injury in neonatal mice. This implied that the mechanisms involved in HI brain injury or cerebral recovery is significantly altered by ranging levels of systemic oxygenation during the initiative stage of reperfusion. The severity this damage can be lowered to preserve the immature brain by maintaining mild hypoxemia during early reperfusion (Niatsetskaya et al., 2011).
Conclusions
Hypoxia and reperfusion have a myriad of effects on central nervous system as illustrated in the above points. Such effects are so extensive that they elicit responses from the cardiovascular system, energy metabolism of the body and blood-brain barrier permeability. Furthermore, the body can adapt to hypoxic conditions according to the severity of the condition. However, acclimatizing responses to hypoxia may be impaired with increasing age and metabolic or vascular disease (LaManna, 2007). Due to the complex relationship between hypoxia and reperfusion in the central nervous system, modern medicine has begun utilising hypoxic conditions and reperfusion techniques in the study of stroke patients. It was found that reperfusion may help in the recovery of those affected. However, this is not to say that reperfusion techniques have had solely positive effects on the central nervous system, in fact, reperfusion was recorded to cause oxidative stress on the central nervous system (Niatsetskaya et al.,2011). Surprisingly, although ischemia was found to found to generally have negative effects on the central nervous system, when coupled with reperfusion it in fact had a positive effect on the recovery of stroke patients. Exposure of the brain to minimal amounts of ischemic conditions helped minimise the effects of hypoxia later (Simon, 2014). Not only this but mild hypoxia in reperfusion lowers oxidative brain injury prior to hypoxia-ischemia (Niatsetskaya et al.,2011).
References
Abbruscato, T. and Davis, T. (1999). Combination of Hypoxia/Aglycemia Compromises In Vitro Blood-Brain Barrier Integrity. Journal of Pharmacology and Experimental Therapeutics, 289(2), pp.668-675.
Beaulieu, C., De Crespigny, A., Tong, D., Moseley, M., Albers, G. and Marks, M. (1999). Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: Evolution of lesion volume and correlation with clinical outcome. Annals of Neurology, 46(4), pp.568-578Beck, T. and Krieglstein, J. (1987). Cerebral circulation, metabolism, and blood–brain barrier in rats in hypocapnic hypoxia. Am. J. Physiol. 252, H504-H512
Beck, T. and Krieglstein, J. (1987). Cerebral circulation, metabolism, and blood–brain barrier in rats in hypocapnic hypoxia. Am. J. Physiol. 252, H504-H512
Caceda, R., Gamboa, J. L., Boero, J. A., Monge, C. and Arregui, A. (2001). Energetic metabolism in mouse cerebral cortex during chronic hypoxia. Neurosci. Lett. 301, 171-174.
Downing, S., Mitchell, J. and Wallace, A. (1963). Cardiovascular responses to ischemia, hypoxia, and hypercapnia of the central nervous system. American Journal of Physiology-Legacy Content, 204(5), pp.881-887.
Fisher, M. (1995). Diffusion and perfusion imaging for acute stroke. Surgical Neurology, 43(6), pp.606-609. Halestrap, A. (2010). A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochemical Society Transactions, 38(4), pp.841-860.
Hillis, A., Barker, P., Beauchamp, N., Gordon, B. and Wityk, R. (2000). MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology, 55(6), pp.782-788.
Karnath, H., Zopf, R., Johannsen, L., Berger, M., Nägele, T. and Klose, U. (2005). Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain, 128(10), pp.2462-2469.
Khurshid, S., Trupe, L., Newhart, M., Davis, C., Molitoris, J., Medina, J., Leigh, R. and Hillis, A. (2012). Reperfusion of specific cortical areas is associated with improvement in distinct forms of hemispatial neglect. Cortex, 48(5), pp.530-539.
Kim, J., Jin, Y. and Lemasters, J. (2006). Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. American Journal of Physiology-Heart and Circulatory Physiology, 290(5), pp.H2024-H2034.
Kuroda, S., Katsura, K., Tsuchidate, R. and Siesjo, B. (1996). Secondary bioenergetic failure after transient focal ischaemia is due to mitochondrial injury. Acta Physiologica Scandinavica, 156(2), pp.149-150.
LaManna, J. (2004). Structural and functional adaptation to hypoxia in the rat brain. Journal of Experimental Biology, 207(18), pp.3163-3169.
LaManna, J. (2007). Hypoxia in the central nervous system. Essays In Biochemistry, 43, pp.138-15 Lauro, K. L. and LaManna, J. C. (1997). Adequacy of cerebral vascular remodeling following three weeks of hypobaric hypoxia. Examined by an integrated composite analytical model. Adv. Exp. Med. Biol. 411, 369- 376.
Leibovitch, F., Black, S., Caldwell, C., McIntosh, A., Ehrlich and, L. and Szalai, J. (1999). Brain SPECT imaging and left hemispatial neglect covaried using partial least squares: The sunnybrook stroke study. Human Brain Mapping, 7(4), pp.244-253.
Lorek, A., Takei, Y., Cady, E., Wyatt, J., Penrice, J., Edwards, A., Peebles, D., Wylezinska, M., Owen-Reece, H., Kirkbride, V., Cooper, C., Aldridge, R., Roth, S., Brown, G., Delpy, D. and Reynolds, E. (1994). Delayed (“Secondary”) Cerebral Energy Failure after Acute Hypoxia-Ischemia in the Newborn Piglet: Continuous 48-Hour Studies by Phosphorus Magnetic Resonance Spectroscopy. Pediatric Research, 36(6), pp.699-706.
Lum, H., Barr, D., Shaffer, J., Gordon, R., Ezrin, A. and Malik, A. (1992). Reoxygenation of endothelial cells increases permeability by oxidant-dependent mechanisms. Circulation Research, 70(5), pp.991-998.
Mark, K. and Davis, T. (2002). Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. American Journal of Physiology-Heart and Circulatory Physiology, 282(4), pp.H1485-H1494.
Niatsetskaya, Z., Charlagorla, P., Matsukevich, D., Sosunov, S., Mayurasakorn, K., Ratner, V., Polin, R., Starkov, A. and Ten, V. (2011). Mild Hypoxemia during Initial Reperfusion Alleviates the Severity of Secondary Energy Failure and Protects Brain in Neonatal Mice with Hypoxic-Ischemic Injury. Journal of Cerebral Blood Flow & Metabolism, 32(2), pp.232-241.
Nyakas, C., Buwald, B. and Luiten, P. (1996). Hypoxia and brain development. Progress in Neurobiology, 49(1), pp.1-51.
Puka-Sundvall, M., Wallin, C., Gilland, E., Hallin, U., Wang, X., Sandberg, M., Karlsson, J., Blomgren, K. and Hagberg, H. (2000). Impairment of mitochondrial respiration after cerebral hypoxia–ischemia in immature rats: relationship to activation of caspase-3 and neuronal injury. Developmental Brain Research, 125(1-2), pp.43-50.
Rothman, S. and Olney, J. (1986). Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Annals of Neurology, 19(2), pp.105-111.
Sick, T. J., Lutz, P. L., LaManna, J. C. and Rosenthal, M. (1982). Comparative brain oxygenation and mitochondrial redox activity in turtles and rats. J. Appl. Physiol. 53, 1354-1359.
Simon, R. (2014). Post-Conditioning and Reperfusion Injury in the Treatment of Stroke. Dose-Response, 12(4), p.dose-response.1.
Witt, K., Mark, K., Hom, S. and Davis, T. (2003). Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. American Journal of Physiology-Heart and Circulatory Physiology, 285(6), pp.H2820-H2831.