|
Size: 22580
Comment:
|
← Revision 82 as of 2017-05-05 09:47:18 ⇥
Size: 22580
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 74: | Line 74: |
| LPA-6 is involved with human hair growth. Its mRNA expression is localised in Hurleys layer of the hair follicle inner root sheath and epidermis is humans (Yanagida and Ishii, 2011). | LPA-6 is involved with human hair growth. Its mRNA expression is localised in Hurleys layer of the hair follicle inner root sheath and epidermis in humans (Yanagida and Ishii, 2011). |
The Role of Lysophosphatidic Acid in Vasoconstriction
K. Vagg, N. McSharry, J. Foley.
Lysophosphatidic acid (LPA) is a member of the lysophospholipid class of phospholipids (Zhou et al., 2009).
LPA can be found in every tissue including plasma, serum, saliva, cerebrospinal fluid, follicular fluid and aqueous humour. LPA synthesis has three main pathways, these are regulated by the availability of precursors and catalytic enzyme expression. LPA can be released from activated platelets, injured cells and growth factor stimulated cells and concentrates in the neointima of human atherosclerotic plaque. Crucially it stimulates proliferation and migration of vascular smooth muscle cells among many other functions (Sheng et al., 2015).
By definition vasoconstriction is the contraction of the vascular smooth muscle cells, resulting in a narrowing of the internal diameter of the lumen.
Contents
|
Figure 1: Structure of LPA (Self Made Figure) |
Synthesis and Transport
LPA can be synthetized from a wide variety of precursors and also acts as a precursor for other molecules. The most significant molecule LPA acts as a precursor for is glycerolipid synthesis. The enzyme GPAT (glycerophosphate acyltransferase) is located in the endoplasmic reticulum and mitochondria, it functions as a catalyst for the formation of LPA by the acylation of glycerol 3-phosphate (Pagès et al., 2001).
The synthetized LPA is then quickly acylated into phosphatidic acid by monoacylglycerolphosphate acetyltransferase (MGAT) in the mitochondria which is a precursor of all glycerolipids. Additional to the the above precursor of LPA glycerol 3-phosphate, the two other main precursors are phosphatidic acid (PA) and lysophosphatidylcholine.The enzyme responsible for the synthesis of LPA from PA is phospholipase A which catalyses the deacylation of phosphatidic acid. The exposure of fibroblasts to Streptomyces chromofocus phospholipase D elicits a quick generation of LPA from the reduction of lysphosphatidylcholine (Pagès et al., 2001).
LPA can be produced both intra and extracellularly. Lipid binding proteins are necessary to avoid toxicity from LPA and transport from one organelle to another. The main extracellular protein is albumin. Intracellularly LPA binds to fatty acid binding proteins. Gelsolin is another binding protein, which is circulated in the blood (Pagès et al., 2001).
LPA Receptors
LPA Receptors are located on the plasma membranes of various cells. Their receptors are known as G protein coupled receptors (Noguchi et al., 2009).
The LPA Receptors can be divided into two categories: EDG family G type receptors and non EDG family G type receptors. EDG stands for endothelial differentiation gene and the two groups are distinguished from each other by their chemical structure (Yanagida and Ishii, 2011).
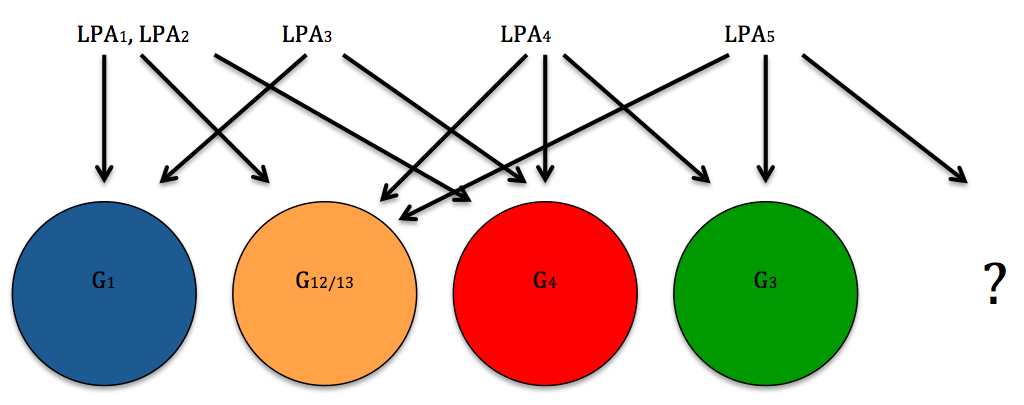
Six LPA structures are known to exist. The EDG family includes LPA-1, LPA-2 and LPA-3 whereas the non EDG family includes LPA-4, LPA-5 and LPA-6 (Yanagida and Ishii, 2011).
EDG Family G Type Receptors
This group is shown to mediate intracellular Ca2+ mobilization, adenylyl cyclase inhibition and mitogen activated kinase activation through PTX-sensitive and also insensitive G-proteins. In studies carried out with mice it has been demonstrated that this family has vital roles in areas such as the nervous system, cancer progression, cardiovascular function and other processes (Yanagida and Ishii, 2011).
LPA-1
Was first discovered from overexpression studies which used cortical neuroblast cell lines. LPA-1 was isolated in this way as it was discovered that overexpressing the LPA produced an increased number of LPA binding sites, more receptor protein and also increased efficacy of LPA for the stimulation of cell rounding and inhibition of cAMP production. This was the basis of proving LPA-1 as a receptor (Fukushima and Chun, 2001).
LPA-2
A second EDG family G type receptor was later discovered. It was shown to have an approximately 60% amino acid similarity to LPA-1. It was suggested that LPA-1 and LPA-2 were formed from a common ancestral gene as the structure of LPA-2 is made of three axons with an intron inserted within a putative transmembrane. This is structurally similar to LPA-1 (Fukushima and Chun, 2001).
LPA-3
A final EDG family receptor was assumed to exist by many as some LPA responsive cells did not express LPA-1 or LPA-2.As a consequence LPA-3 was discovered with a 50% amino acid similarity to human LPA-1 and LPA-2 (Fukushima and Chun, 2001).
Non EDG Family G Type Receptors
After the discovery of the EDG family G type receptors it was implied that the existence of additional LPA receptors was probable. This was for a number of reasons. Firstly, the EDG family receptor mediated responses did not account for distinct ligand specificity that was demonstrated in human platelet aggregation which was LPA induced. Secondly as discussed later in this essay there is a more potent platelet aggregation response to alkyl-ether linked LPA (alkyl LPA) than to ester linked LPA. The stereoselectivity of this was similarly seen in rat hepatoma RH7777 cells. These cells do not express any EDG family LPA receptors. Despite this they produced a mitogenic response to LPA and its analogs (Yanagida and Ishii, 2011).
LPA-4
LPA-4 was the first non EDG family G type receptor to be identified. It shares a reduced 20-24% amino acid similarity with EDG family LPA receptors (Yanagida and Ishii, 2011).
LPA-4 was shown to have a crucial role in normal blood and lymphatic vessel development. In studies completed in LPA-4 knockout embryos with bleeding blood vessels were found to be dilated. In addition there was a detected problem in recruiting smooth muscle cells and pericytes (Yanagida and Ishii, 2011).
An interesting discovery was ATX (autotaxin) which is a LPA producing enzyme with a role in vascular development. In embryos which were each ATX knocked out, none survived and were later seen to have massive vascular defects. G13 coupled LPA receptors are assumed to be responsible for this phenomenon as the phenotypes of G13 knockout mice were analogous to ATX knockout mice. However, five out of the six LPA receptors are known to couple G12/13 proteins so this may be a combined receptor process (Yanagida and Ishii, 2011).
LPA-5
LPA-5 shares an approximate 35% amino acid similarity to LPA-4. The gene expression profiling of human platelets for GPCRs has shown that LPA-5 receptors are the major type of LPA receptors expressed of all LPA receptors. It has been dubbed the "platelet type" LPA receptor (Yanagida and Ishii, 2011).
In this way, LPA-5 may be used in the future as an anti-thrombotic therapy for those with myocardial infarction and stroke as it is the main platelet activating lipid of mildly oxidised low density lipoprotein and human atherosclerotic lesions (Yanagida and Ishii, 2011).
LPA-6
LPA-6 is involved with human hair growth. Its mRNA expression is localised in Hurleys layer of the hair follicle inner root sheath and epidermis in humans (Yanagida and Ishii, 2011).
|
G- Protein Mechanism
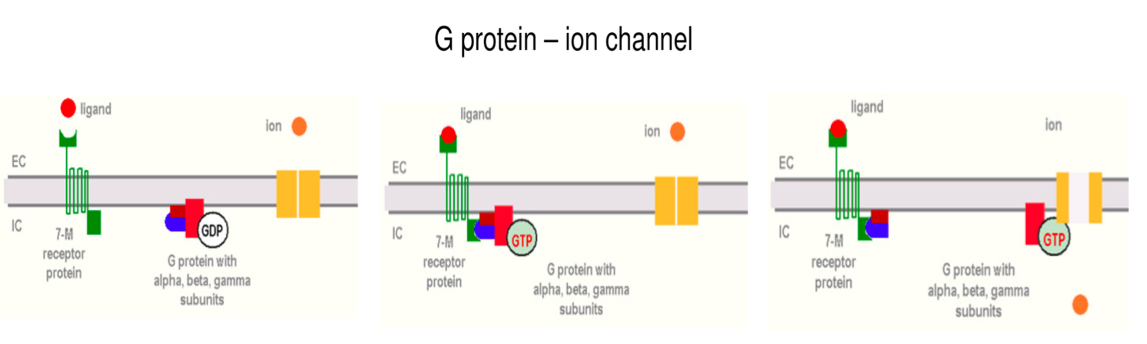
A G-protein is an intracellular molecule. It consists of three subunits. An alpha subunit binds a GDP and forms a complex with beta and gamma subunits. G proteins connect to 7-M transmembrane proteins. These are so called as they cross the membrane seven times.
A conformational change is brought about in the 7-M protein when the ligand binds to the receptor on the EC side. This change allows beta and gamma subunits to bind to the IC side of the receptor. The alpha subunit can then bind GTP as conformational changes to its structure takes place. The alpha-GTP complex in its now activated state is liberated and is able to stimulate (Gs protein) or inhibit (Gi) an ion channel or enzyme on the IC side of the membrane.
The mechanism is ended when GTP is converted to GDP, the alpha subunit binds to gamma and beta again and G protein returns to its resting state.
|
Role of LPA in Atherosclerosis & Thrombosis
Atherosclerosis is a vascular disease involving hardening of the arteries and is a major cause of heart attack and stroke. Atherosclerosis is characterised by the aggregation of lipids, macrophages, smooth muscle cells (SMCs), T Lymphocytes and fibrous tissue in the inner lining of large and medium sized arteries. The formation of an atherosclerosis arises from endothelial cell activation which produces adhesion molecules in the EC plasma membrane (Cui, 2011).
Proinflammatory chemokines produced in the intima attract leukocytes that have adhered on the endothelium. These leukocytes secrete cytokines and growth factors which promote smooth muscle cell migration into the intima which form a fibrous matrix. Ultimately, inflammatory mediators can inhibit collagen synthesis, evoke the expression of collagenases, and induce tissue factor (TF) expression in the plaques. When these plaques rupture TF activates factor VII initiating the blood coagulation pathway (Cui, 2011).
LPA's Effect on Vascular Endothelial Cells
LPA causes the adhesion molecule present in endothelial cells (ECs) to become activated. Adhesion molecules such as E-selectin, VCAM-1 and ICAM-1 are released on the cell surface. LPA receptor 1 (LPA1) is responsible for LPA-induced ICAM-1 expression. LPA induction of the expression of ICAM-1 and VCAM-1 in ECs is mediated through the Rho kinase-2-triggered NF-κB pathway. These adhesion molecules help bind the lymphocytes onto the endothelium (Cui, 2011).
LPA induces inflammatory cytokine secretion from ECs. LPA stimulates ECs to generate proinflammatory cytokines and chemoattractant cytokines. Furthermore, ECs stimulated by LPA have been demonstrated to enhance monocytic cell recruitment to the surface of ECs via LPA-induced IL-8 and MCP-1 secretion from ECs (Cui, 2011).
LPA stimulates angiogenesis. Angiogenesis is the formation of new blood vessels. This process involves the migration, growth, and differentiation of endothelial cells, which line the inside wall of blood vessels. This is essential for the growth and development of an animal (Cui, 2011).
LPA's Effect on Vascular SMCs
LPA induces SMC migration and proliferation. As the process of atherogenesis takes place SMCs begin to line the inner layer of the intima. In animal models of vascular injury, intimal and medial thickening is thought to be attributable to vascular SMC migration as well as proliferation from media to intima. In atherosclerotic lesions SMCs are the most common cell type (Cui, 2011).
LPA promotes the production of inflammatory cytokines and chemokines from vascular SMCs. LPA induces the secretion of IL-6 and MCP-1 from human aortic SMCs. MCP-1 plays a vital role during the induction of atherosclerosis. IL-6 is a multifunctional cytokine whose presence in the circulation is linked with diverse types of cardiovascular disease and is an independent risk factor for atherosclerosis (Cui, 2011).
LPA's Effect on Fibroblasts
Lysophosphatidic acid promotes fibroblast cell proliferation and fibroblast cell migration. Although as is discussed above it is well recognized that vascular SMC migration and proliferation are essential components of atherogenesis, recent data also indicates that excessive proliferation and migration of adventitial fibroblasts plays an important role in the pathobiology of atherosclerosis and restenosis (Cui, 2011).
LPA Effects on Platelet Function
LPA in humans has shown to be a weak activator of platelets. It is able to stimulate platelet shape change, fibronectin matrix assembly and also platelet-monocyte coaggregation formation. Thrombus forming and stability is enhanced in this way (Smyth et al., 2008).
Epinephrine and adenosine diphosphate (ADP) have a synergistic relationship with LPA to increase platelet aggregation and adhesion (Smyth et al., 2008).
These effects combined with LPA receptor antagonists stopping or inhibiting the platelet aggregation brought about by atherosclerotic plaque lipid rich cores serve a major role in bringing about platelet responses during acute thrombosis during which LPA is produced at sites of platelet activation after plaque rupture or erosion (Smyth et al., 2008).
mRNAs for five of the G protein coupled receptors can be found in human platelets with LPA-4 and LPA-5 being the most common. However the LPA receptor most likely to be majorly responsible for the effects of LPA on human platelets is LPA-5 which is the most prolific or an as of yet unidentified LPA receptor due to a difference in the common structure of those molecules which are proven to have a more potent effect (Smyth et al., 2008).
Furthermore, LPA has a much stronger effect on isolated platelets than on whole blood in causing platelet aggregation. This could be due to albumin or gelsolin in the plasma (LPA binding proteins) adjusting platelet LPA responses (Smyth et al., 2008).
The Role of LPA in Vasoconstriction Through The Action of Integrin
LPA can induce integrin activation in vascular smooth muscle cells and alter myogenic vasoconstriction (Staiculescu et al., 2014).
Integrins are transmembrane proteins and function as links between the cytoskeleton and extra cellular matrix. Linkage of the extra cellular matrix to cell requires these transmembrane adhesion proteins that act as matrix receptors. Most EC proteins (collagen, fibronectin and laminins) can be classified as integrins - fibronectin being the most important integrin in the role of LPA in vasoconstriction of vascular smooth muscle cells (Staiculescu et al., 2014).
Integrin stimulation and signalling are imperative for cell survival, proliferation and motility under physiological and pathological conditions. Integrins generally have no enzymatic activity themselves but many of the scaffolding proteins that attach them to the IC areas do have enzymes. This is what allows integrins to transmit signals intracellularly (from the EC to the cytoskeleton), making them a good example of proteins to take part in mechanotransduction of signals across plasma membranes of many cell types (Staiculescu et al., 2014).
Vascular smooth muscle cells integrin activity has been associated with pathological and physiological processes including modulation of myogenic phenomena and vascular remodelling. In vascular smooth muscle cells increasing adhesion to the extra cellular matrix proteins as well as production of reactive O2 species are strongly stimulated by LPA, in particular increasing adhesion to fibronectin (Staiculescu et al., 2014).
Additionally exposure to LPA doubles the integrin fibronectin adhesion compared to normal pathological conditions in vascular smooth muscle cells. This increased adhesion augments the myogenic constriction response to increase the intraluminal pressure in vascular smooth muscle cells (40-100mmHg) by 71%. LPA induced integrin vasoconstriction action involves the G-protein coupled receptors LPAR1-LPAR6 as previously mentioned (Staiculescu et al., 2014).
In vivo, administration of LPA results in acute increases in blood pressure in different animal species, suggesting a role for LPA in both normal blood pressure regulation and hypertension. (Staiculescu et al., 2014).
Experiment: The Effect of LPA on Fibrogen Activated Vasoconstriction
Vascular smooth muscle cells were enzymatically isolated from cremasteric arterioles using papain, collagenase, and elastase. The isolated vascular smooth cells were maintained in culture conditions with DMEM/F-12 and fetal bovine serum. The solution was kept in a humidified incubator at 37 degrees Celsius and 5% CO2 before being plated on 60mm tissue culture dishes (Staiculescu et al., 2014).
The luminal diameter was measured with a wide caliper system and changes in the inner diameter were measured. Two groups of isolated VSMCs were used - a control and a test group. The test group was exposed to LPA for 2 hours with no LPA exposure for the control group, each group were subjected to 20mmHg increasing increments in pressure which were maintained in the range of 40-100mmHg in intravascular pressure (Staiculescu et al., 2014).
|
Results
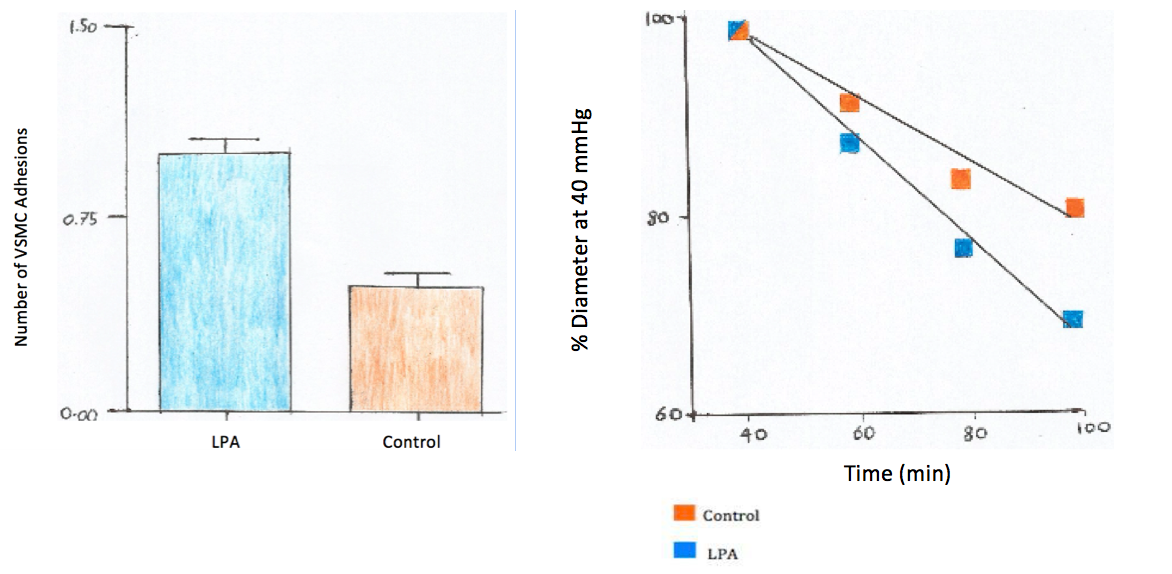
To quantify the cell based adhesion, fibronectin coated beads were applied to the surface of VSMCs. The results presented that the occurance of adhesion events was 52% lower in the control group of isolated vsmc then that of the group treated with LPA, as shown in the graph on the left: (Staiculescu et al., 2014).
The test group also showed significant myogenic constriction when exposed to LPA, through all pressure incriments when compared to the control. The graph on the right shows the varations in diameter expressed as a percent of the vascular diameter attained at 40mmHg( the initial pressure). The luminal diameter of the LPA test group showed a 71% increase in myogenic vasoconstriction (Staiculescu et al., 2014).
Clinical Uses
LPA’s role as an endogenous mediator in the cells of the vascular system can be seen if administered by exogenous means to animals. Rats which were intravenously injected with LPA showed increased hypertension. Locally infusing the common carotid artery had a positive effect on neointimal forming (Panchatcharam et al., 2008).
Similarly in an experiment conducted with swine cerebral vasoconstriction was a result of local application (Panchatcharam et al., 2008).
Conclusion
It can be concluded that lysophosphatidic acid has an integral role in vasoconstriction. It achieves this through multiple actions as discussed extensively above leading to acute pathological conditions. It is clear that this is a bioactive molecule with huge potential in therapy of cases such as myocardial infarction and stroke in the future. However to comprehend its full uses more research must be done in this field.
References
Cui, M. (2011). Lysophosphatidic acid effects on atherosclerosis and thrombosis. Clinical Lipidology, 6(4), pp.413-426.
Fukushima, N. and Chun, J. (2001). The LPA receptors. Prostaglandins & Other Lipid Mediators, 64(1-4), pp.21-32.
Lin, M., Herr, D. and Chun, J. (2010). Lysophosphatidic acid (LPA) receptors: Signaling properties and disease relevance. Prostaglandins & Other Lipid Mediators, 91(3-4), pp.130-138.
Noguchi, K., Herr, D., Mutoh, T. and Chun, J. (2009). Lysophosphatidic acid (LPA) and its receptors. Current Opinion in Pharmacology, 9(1), pp.15-23.
Pagès, C., Simon, M., Valet, P. and Saulnier-Blache, J. (2001). Lysophosphatidic acid synthesis and release. Prostaglandins & Other Lipid Mediators, 64(1-4), pp.1-10.
Panchatcharam, M., Miriyala, S., Yang, F., Rojas, M., End, C., Vallant, C., Dong, A., Lynch, K., Chun, J., Morris, A. and Smyth, S. (2008). Lysophosphatidic Acid Receptors 1 and 2 Play Roles in Regulation of Vascular Injury Responses but Not Blood Pressure. Circulation Research, 103(6), pp.662-670.
Sheng, X., Yung, Y., Chen, A. and Chun, J. (2015). Lysophosphatidic acid signalling in development. Development, 142(8), pp.1390-1395.
Smyth, S., Cheng, H., Miriyala, S., Panchatcharam, M. and Morris, A. (2008). Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1781(9), pp.563-570.
Staiculescu, M., Ramirez-Perez, F., Castorena-Gonzalez, J., Hong, Z., Sun, Z., Meininger, G. and Martinez-Lemus, L. (2014). Lysophosphatidic acid induces integrin activation in vascular smooth muscle and alters arteriolar myogenic vasoconstriction. Frontiers in Physiology, 5: 413 doi: 10.3389/fphys.2014.00413.
Yanagida, K. and Ishii, S. (2011). Non-Edg family LPA receptors: the cutting edge of LPA research. Journal of Biochemistry, 150(3), pp.223-232.
Zhou, Z., Niu, J. and Zhang, Z. (2009). Receptor-Mediated Vascular Smooth Muscle Migration Induced by LPA Involves p38 Mitogen-Activated Protein Kinase Pathway Activation. International Journal of Molecular Sciences, 10(7), pp.3194-3208.
Figure List
Figure 1: Structure of LPA (Self Made Figure)
Figure 2: G- Protein's Coupled to LPA Receptors (Self Made Figure Based on Lin et al., 2010)
Figure 3: G- Protein Mechanism. Bartha, T. (2009). Endocrinology Lecture Notes. University of Veterinary Medicine Budapest, pp. 48-50.
Figure 4: The Effect of LPA on Fibrogen Activated Vasoconstriction Experiment Results (Self Made Figure Based on Staiculescu et al., 2014)