|
Size: 21442
Comment:
|
← Revision 22 as of 2014-12-04 15:17:01 ⇥
Size: 21442
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 63: | Line 63: |
| Brenner, B. M.; Garcia, D. L.; Anderson, S. (1988). Glomeruli and blood pressure. Less of one, more the other? ''American Journal of Hypertension''. 1: 335-347 | Brenner, B. M.; Garcia, D. L.; Anderson, S. (1988): Glomeruli and blood pressure. Less of one, more the other? ''American Journal of Hypertension''. 1: 335-347 |
| Line 111: | Line 111: |
| Vio, C. P.; Jeanneret, V. A. (2003). Local Induction of Angiotensin-Converting enzyme in the kidney as a mechanism of progressive renal diseases. ''Kidney International Supplements''. S57-S63 | Vio, C. P.; Jeanneret, V. A. (2003): Local Induction of Angiotensin-Converting enzyme in the kidney as a mechanism of progressive renal diseases. ''Kidney International Supplements''. S57-S63 |
Introduction
Several studies have shown that malnutrition as an embryo at mid gestation may cause an increased risk of several chronic diseases in the fully matured animal (Brennan et al. 2005). The effect of maternal diet on the glomerulus number, mean arterial pressure, medullary angiotensin II receptor and expression of ACE in the renal cortex will be discussed.
The Kidney
The kidney is a vital organ that has many functions, including stabilization of osmolarity and extracellular fluid volume, regulation of ion concentration in the extracellular fluid, removal of waste substances, contribution to the acid-base balance (via removal of bicarbonate and hydrogen ions) and production of renin (Sjaastad et al, 2003).
The Nephron
The functional unit of the kidney is the nephron, which is composed of a glomerulus, proximal tubule, loop of Henle, thick ascending limb, and a distal convoluted tubule (Cunningham and Klein, 2007). The glomerulus is composed of a glomerular tuft which is a network of capillaries composed of 3 layers; the capillary epithelium (which is fenestrated), the basement membrane (which is composed of glycoproteins) and visceral epithelium (which is composed of podocytes) (Cunningham and Klein, 2007). This glomerular tuft is encased in the Bowman’s Capsule (Cunningham and Klein, 2007). Most of the reabsorption of solutes and water occur in the proximal tubule, with electrolytes being reabsorbed in the thick ascending limb and the distal convoluted tubule (Cunningham and Klein, 2007).
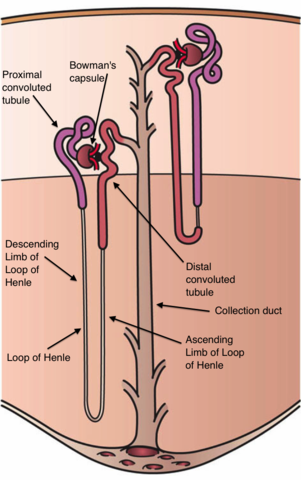
Figure 1. The Nephron
Glomerular Filtration Rate (GFR)
The Glomerular filtration rate (GFR) is “the volume of fluid filtered from the glomerular capillaries into the Bowman’s space per minute” (Sjaastad et al. 2003) and is determined by the permeability of the filtration surface, the area available for filtration (i.e. number of nephrons) and Mean Net filtration Pressure (Reece, 2004). The GFR may be maintained by both systemic factors (such as renal modulation of systemic blood pressure and intravascular volume) and intrinsic factors (such as control of renal blood flow and glomerular capillary pressure) (Cunningham and Klein, 2007).
One of the most important regulators of GFR and renal blood flow is the Renin-Angiotensin-aldosterone system (Cunningham and Klein, 2007). Renin is released by mesangial cells in the kidney when stimulated by a decrease in renal perfusion pressure and so catalyses the transformation of angiotensinogen into angiotensin I (Reece, 2004). Angiotensin I is then converted to Angiotensin II by angiotensin-converting enzyme (ACE) in either the endothelium of the kidney or the endothelium of the lung (Cunningham and Klein, 2007). Angiotensin II in a vasoconstrictor meaning that systemic blood pressure will increase and so renal perfusion pressure will also increase (Cunningham and Klein, 2007).
Maternal Dietary Restriction
Over the past twenty years there has been significant evidence to support the theory that offspring exposed to poor nutrition in utero are at an increased risk of developing adult onset chronic disease (Cleal et al, 2007; Gilbert et al, 2005). This phenomenon of asserting that conditions experienced during gestation will have consequential effects in adulthood can be referred to as perinatal programming (Luft, 2003). In the ovine species, studies have found that maternal nutrient restriction during pregnancy may result in abnormal physiological changes. Specifically, there is a noted decrease in both nephron and glomerular endowment, which arguably increases the risk of developing diseases later in life (Lloyd et al. 2012).
Most studies exploring these effects used broad-spectrum dietary restriction; typically feeding test subjects 50% of their recommended energy requirements from early to mid gestation. Brennan et al (2005) ascertain that this time frame represents maximal placental growth and is crucial for fetal kidney development. As adverse environmental factors have the greatest impact during the period of active nephrogenesis, early to mid gestation is ideal for studying maternal nutrient intake in the ovine species (Zandi-Nejad et al, 2006). Studies conducted on sheep outside this time frame yielded less dramatic results.
The progression of ovine nephrogenesis is similar to that which is found in humans, where kidney formation is completed and the adult nephron complement is achieved by the time of mid gestation (Brennan et al, 2005). No new nephrons are formed after birth (Mitchell et al, 2004). Fetal ovine kidney development usually begins around days 16 to 18, in which the mesonephros first become functional. This is followed by development of the full nephron compliment between days 27-30 (Brennan et al, 2005), with peak nephrogenesis typically occurring around day 65 (Lloyd et al, 2012).
Impacts on the Nephron and Glomeruli
In most cases, a reduced number of both nephrons and glomeruli were detected in the tested offspring. These findings were notably prominent in the studies that specifically explored the effects of gestational protein restriction. Detriment to nephron and glomerular numbers in diets specifically restricting protein were comparable to studies using overall nutrient restrictions, suggesting that maternal protein intake plays a crucial role in fetal kidney health (Gopalakrishnan et al, 2005; Woods et al, 2004). There has been suggestion that this reduction of nephrons causes hyperperfusion in the nephrons that are present as their individual demand is increased. This then causes compensatory glomerular sclerosis, and a cyclic pattern of hypertension and nephron death (Luft, 2003). In rats, low nephron numbers have been associated with a decreased Glomerular Filtration Rate [GFR], reduced excretion of sodium, and increased glomerular hypertrophy (Zandi-Nejad et al, 2006). The reduction in sodium excretion caused by the reduction in the number of nephrons will cause increased water retention thereby increasing the blood pressure. Painter et al (2005) further argues that a reduced number of glomeruli in humans can act as a precursor for developing further glomerular harm. Glomeruli are forced to work harder due to increased filtration, and will eventually succumb to hypertrophy and may even later contribute to impaired renal function. It is plausible from these findings to ascertain that nephron and glomeruli deficient kidneys and their decrease in functional ability may amplify propensity for renal injury and further functional decline (Painter et al, 2005; Zandi-Nejad et al, 2006).
Impacts on the Renin-Angiotensin-Aldosterone System
The Renin-Angiotensin-Aldosterone system is very important in regulating the GFR (Cunningham and Klein, 2007). Angiotensin II has several roles in this system, it acts as a strong vasoconstrictor and it also increases the intravascular volume, both directly (via stimulating the uptake of sodium ions from the proximal tube and the collecting duct) and indirectly (by stimulating release of aldosterone which in turn stimulates sodium and water retention)(Cunningham and Klein, 2007). These effects are accepted to be mediated by the angiotensin 1 receptor (Gilbert et al, 2005). The increase in vascular resistance caused by the vasoconstriction and the increased intravascular volume cause the increase in systemic blood pressure and consequently the increase in renal perfusion pressure (Cunningham and Klien, 2007). Angiotensin II also stimulates the release of prostaglandins which act as vasodialtors and so moderate the Renin-Angiotensin-Aldosterone system (Reece, 2004). 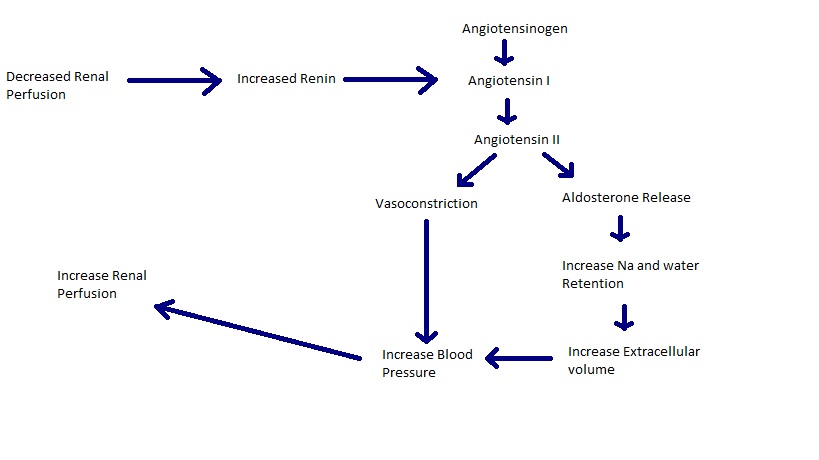
Figure 2. The Renin-Angiotensin-Aldosterone System.
Angiotenson-Converting Enzyme (ACE)
Studies have found that the angiotensin-converting enzyme (ACE) expression in the renal cortex is increased in animals that experienced restricted nutrition as a fetus in comparison to animals who did not receive restricted nutrition in utero (Gilbert et al, 2005). The elevated ACE levels within the renal cortex is known to be an indicator in the progression of chronic renal disease (Vio and Jeanneret, 2003) however the mechanism by which increased ACE levels contribute to renal disease or hypertension is unclear (Gilbert et al, 2005). However there may be indications that the restriction of blood to the cortico-medullary interface could be involved (Fine et al, 2000) or it may be due to an imbalance between bradykinin and angiotensin II in the kidney (Vio and Jeanneret, 2003).
Numbers Angiotensin 1 receptors (AT1) were found to be the same in both the control group of sheep and in the nutrient restricted group of sheep (Gilbert et al, 2005). However, it was found in rats that the number of AT1 were increased in animals who had experienced nutrient restriction as a fetus (Vehaskari et al, 2004), although this is thought to be due to the different nutrient requirements of each species (Gilbert et al, 2005).
Angiotenson Receptors
Although the function of the Angiotensin 2 receptor (AT2) is not fully understood, it is thought to have a role in nitric oxide release, apoptosis (Tejera et al, 2004), decreasing pressure natriuresis (pressure natriuresis is an increase in water and sodium excretion in the kidney to reduce arterial blood pressure) (Liu et al, 1999) and as an antagonist to AT1 (Horiuchi et al, 1997). Gilbert et al, (2005) found that in sheep that had undergone nutrient restriction before birth, the number of AT2 was significantly higher than in the control group. A similar study on rats also had the same result (Vehaskari et al, 2004). It has been shown that in humans, damaged or diseased kidneys also have an increased AT2 number compared to non-damaged/diseased kidneys (Ruiz-Ortega et al, 2003). Guyton, (1990) found that abnormal pressure-natriuresis was associated with chronic high blood pressure and so the increase in medullary AT2 may be a maladaptive phenotype with the decrease in sodium and water excretion from the kidney contributing to the hypertension (Gilbert et al, 2005). It has also been proposed that the increase in AT2 may be an adaption to counteract the effects of the impaired kidney (Vasquez et al, 2005).
Mean Arterial Pressure
As previously discussed studies have indicated that offspring who experienced poor nutrition in utero have an increased risk of developing heart disease and hypertension as adults. Hypertension [HTN] is the medical term used to describe an elevation in blood pressure. Sustaining this elevation has the potential to cause occlusion and ischemic necrosis of blood vessel walls, which can lead to serous exudation, hemorrhages, and edema (Wingfield and Raffe, 2002).
Hypertension is also closely associated with the renal system, as the kidney is a primary organ in relation to the development of HTN (Zandi-Nejad et al, 2006; Wintour et al, 2003)
It is now widely accepted that maternal nutritional status in pregnancy is a major programming influence on the offspring. One of the most notable consequences is the relationship of gestational nutrition and it's effect on the fetal programming. Hypertension deficiency in pregnancy has an impact upon the development of cardiovascular functions and also appears to impact the nephron number (Langly et al, 2003 )
The results of Gilbert et al (2005) have shown that blood pressure and heart rate mean arterial pressure (MAP) were approximately 17 mmHg higher in the offspring exposed to nutrient restriction during development than in animals whose mothers had no dietary restrictions. There was no difference in heart rate (HR) between the dietary groups. Strong negative correlation between MAP and nephron number is evident only in the nutrient-restricted group of offspring. These results are consistent with the hypothesis advanced by (Brenner et al, 1988) who proposed that nephron number and blood pressure are inversely related. Wintour et al. (2003) used glucocorticoid treatment early in kidney development to create hypertension. Glucocorticoids are steroid hormones that have an important role in the metabolism of glucose (Cunningham et al, 2013). Hypertension is associated with impaired glucose metabolism and insulin resistance (Mahfoud et al, 2011; Gatford et al, 2000 ). Therefore, manipulating of glucocorticoid level can induce hypertension. The results points that the hypertension developed, at least in part, from impaired nephrogenesis. This support the hypothesis mention above.
Timing is an important factor to consider for the success of fetal nephron endowment. Significantly compromised nephrogenesis occurs when the intrauterine growth restriction (IUGR) takes place early in development, but not during late gestation. Therefore the period of early to mid-gestation represents an important time frame for monitoring maternal diet.
Benefits of Future Research
Knowing the effects of a restricted maternal diet is of paramount importance because it can help us reduce the chance of congenital problems, such as renal and heart conditions. Further research, however, may help us predict what other chronic issues may develop. During a time when obesity (which can exacerbate congential problems) is a rising problem in both animal and human populations, further reasearch is critical (Lloyed et al. 2012); allowing professionals to provide the best possible advice to their clients.
References
Brennan, K. A.; Gopalakrishnan, G. S.; Kurlak, L.; Rhind, S. M.; Kyle, C. E.; Brooks, A. N.; Rae, M. T.; Olson, D. M.; Stephenson, T.; Symonds, M. E. (2005): Impact of maternal undernutrition and fetal number on glucocorticoid, growth hormone and insulin-like growth factor receptor mRNA abundance in the ovine fetal kidney. Reproduction, 129: 151-159
Brenner, B. M.; Garcia, D. L.; Anderson, S. (1988): Glomeruli and blood pressure. Less of one, more the other? American Journal of Hypertension. 1: 335-347
Cleal, J. K.; Poore, K. R.; Boullin, J. P.; Khan, O.; Chau, R.; Hambidge, O.;Torrens, C.; Newman, J. P.; Poston, L.; Noakes, D. E.; Hanson, M. A.; Green, L. R. (2007): Mismatch pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. PNAS, 104: (22) 9529-9533.
Cunningham, J. G.; Klein, B. G. (2007): Textbook of Veterinary Physiology. 4th edition, Saunders Elsevier, Missouri (pp528)
Cunningham, J. G.; Klein, B. G. (2013): Textbook of Veterinary Physiology. 5th edition, Saunders Elsevier, Missouri (pp383-385)
Fine, L. G.; Bandyopadhay, D.; Norman, J. T. (2000): Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney international supplements, 75: S22-S26
Gilbert, J. S.; Lang, A. L.; Grant, A. R.; Nijland, M. J. (2005): Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. Journal of Physiology, 565:137-147
Gopalakrishnan, G. S.; Gardner, D. S.; Dandrea, J.; Langley-Evans, S.C.;Pearce, S.; Kurlak, L. O.; Walker, R. M.; Seetho, I. W.; Keisler, D. H.; Ramsay, M. M.; Stephenson, T.; Symonds, M. E. (2005): Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. British Journal of Nutrition, 94: 938-947
Guyton, A. C. (1990): Long term arterial pressure control: an analysis from animal experiments and computer and graphic models. American Journal of Physiology: Regulatory, Intergrative and Comparitive Physiology. 259: R865-R877
Heasman, L.; Clarke, L.; Stephenson, T. J.; Symonds, M. E. (1999): Theinfluence of maternal nutrient restriction in early to mid-pregnancy on placental and fetal development in sheep. Proceedings of the Nutrition Society. 58: 283-288
Horiuchi, M.; Hayashida, W.; Kambe, T.; Yamada, T.; Dzau, V. J. (1997): Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. Journal of Biological Chemistry. 272: 19022-19026
Gatford, K.L.; Wintour, E. M.; De Blasio, M. J.; Owens, J. A.; Dodi, M. (2000): Differential timing for programming of glucose homoeostasis, sensitivity to insulin and blood pressure by in utero exposure to dexamethasone in sheep. Clinical Science, 98, 553–560.
Langley-Evans, S.C.; Langley-Evans, A.J.; and Marchand, M.C. (2003): Nutritional Programming of Blood Pressure and Renal Morphology. Archives of Physiology and Biochemistry. 111: (1) 8-16
Lloyd, L. J.; Foster, T.; Rhodes, P.; Rhind, S. M.; Gardner, D. S. (2012): Protein energy malnutrition during early gestation in sheep blunts fetal renal vascular and nephron development and compromises adult renal function. The Journal of Physiology. 590: 377-393.
Luft, F. C. (2003): Baa, baa, black sheep, are your kidneys full?. The Journal of Physiology. 549: 665
Liu, K. L.; Lo, M.; Grouzmann, E.; Mutter, M.; Sassard, J. (1999): The subtype 2 of angiotensin II receptors and pressure-natriuresis in adult rat kidneys. British Journal of Pharmacology. 126: 826-832
Mahfoud, F.; Schlaich, M.; Kindermann, I.; Ukena, C.; Cremers, B.; Brandt, M.C.; Hoppe, U.C.; Vonend O.;Rump, L.C; Sobotka, P.A.; Krum,H.; Esler,M.; Bo¨hm, M. (2011): Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation, 123, 1940-1946.
Mitchell, E. K. L.; Louey, S.; Cock, M. L.; Harding, R.; Black, M. J. (2004): Nephron endowment and filtration surface area in the kidney after growth restriction of fetal sheep. Pediatric Research. 55: (5) 769-773.
Moritz, K. M.; Dodic, M.; Wintour, E. M. (2003): Kidney development and the fetal programming of adult disease. Bioessays. 25: 212-220
Painter, R. C.; Roseboom, T. J.; van Montfrans, G. A.; Bossuyt, P. M. M.; Krediet, R. T.; Osmond, C.; Barker, D. J. P.; Bleker, O. P. (2005): Microalbuminuria in adults after renatal exposure to the Dutch famine. Journal of the American Society of Nephrology. 16: 189-194.
Reece, W.O.(ed), (2004): Dukes’ Physiology of Domestic Animals. 12th edition. Comstock Publishing Associates, United States of America, (pp73). Ruiz-Ortega, M.; Esteban, V.; Suzuki, Y.; Ruperez, M.; Mezzano, S.; Ardiles, L.; Justo, P.; Ortiz, A.; Egido, J. (2003): Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney international supplements. S21-S26
Sjaastad, O. V.; Hove, K.; Sand, O.; (2003): Physiology of Domestic Animals. Scandinavian Veterinary Press, Oslo. (pp429)
Tejera, N.; Gomez-Garre, D.; Lazaro, A.; Gallego-Delgado, J.; Alonso, C.; Blanco, J.; Ortiz, A.; Egido, J. (2004): Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells. American journal of Pathology.164: 1817-1826
Vazquez, E.; Coronel, I.; Bautista, R.; Romo, E.; Villalon, C. M.; Vila-Casado, M. C.; Soto, V.; Escalante, B. (2005): Angiotensin II-dependent induction of AT2 receptor expression after renal ablation. American Journal of Physiology: Renal Physiology. 288: F207-F213
Vehaskari, V. M.; Stewart, T.; Lafont, D.; Soyez, C.; Seth, D.; Manning, J. (2004): Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. American Journal of Physiology: Renal Physiology. 287: F262-F267
Vio, C. P.; Jeanneret, V. A. (2003): Local Induction of Angiotensin-Converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney International Supplements. S57-S63
Wingfield, R.R.; Raffe, M.E. (2002): The Veterinary ICU Book. Teton NewMedia.Wyoming. (pp 887)
Wintour, E. M.; Alcorn, D.; Butkus, A.; Congiu, M.; Earnest, L.; Pompolo, S.; Potocnik, S. J. (1996): Ontogeny of hormonal and excretory function of the meso- and metanephros in the ovine fetus. Kidney International. 50: 1624-1633
Wintour, E.M.; Moritz, K.M.; Johnson, K.; Ricardo, S.; Samuel. C.S.; Dodic, M. (2003): Reduced Nephron Number in Adult Sheep, Hypertensive as a Result of Prenatal Glucocorticoid Treatment. Journal of Physiology. 549: 929-935
Woods, L. L.; Weeks, D. A.; Rasch, R. (2004): Programming of adult bloodpressure by maternal protein restriction: Role of nephrogenesis. Kidney International. 65: 1339-1348
Zandi-Nejad, K.; Luyckx, V. A.; Brenner, B. M. (2006): Adult hypertension and kidney disease. Hypertension. 47: 502-508
Figure References
Figure 1. Kidney Nephron. http://en.wikipedia.org/wiki/Nephron#mediaviewer/File:Kidney_Nephron.png Accessed Novermber 22, 2014
Figure 2. Renin-Angiotensin-Aldosternon System. Recreated from Cunningham and Klein, 2007
