|
Size: 19887
Comment:
|
← Revision 6 as of 2017-05-02 13:50:12 ⇥
Size: 19857
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 3: | Line 3: |
| Itt írjon a(z) MindParasites-ról/ről | |
| Line 13: | Line 12: |
| ---- |
|
| Line 20: | Line 21: |
| Parasitism refers to an interspecies relationship where one organism (the parasite) benefits and one organism (the host) suffers.This means it is a non-mutualistic relationship. (D. Dantic) Parasitism is one of the three main branches of Symbiotic relationships observed in the natural kingdom. In contrast Mutualism refers to two species with a positive effect on the other (e.g. an Ox-pecker bird gains food from cleaning a buffalo of parasitic insects and the buffalo is rid of the parasitic infection).(A. Miller, 2015) The third type of Symbiotic relationship is Commensalism, in which case, the commensal benefits from the relationship, and the host is unaffected, with no positive or negative effects (e.g. a Remora fish rides with Sharks, gaining food from the leftovers of the sharks prey, meanwhile, the shark has no impact from being accompanied by the Remora).(G. Larson, D. Q. Fuller, 2014) Typical features of parasites in general include: parasites being far smaller than their host (sometimes allowing for large numbers of parasites sharing a single host, e.g. Tics on a dog) and reproducing at a much faster rate than their host species, this allows many generations of parasites to be propagated and live their life cycles using just one host. (D. Dantic) Parasites can be classified into Macroscopic and Microscopic. They can also be split into their phylogenic groups, which is remarkably diverse (as is the range of hosts): Protozoa, Virus, Bacteria, Plant and Animal. Alternatively, parasitic infections can be described by their dependence level on their host. Obligate parasitesare totally dependent on their hosts to complete their life cycles. The opposite to this beingfacultative parasites whichare not dependant. It appears most likely that Facultative Parasites simply use parasitism as a method of improving their reproductive success by providing a more niche role with fewer competitors, for example‘facultative parasite, Macrocheles subbadius. Lifetime reproductive success was higher among female mites that fed on fruit fly haemolymph as parasites compared to free-living mites. The per capita lifetime fecundity for parasitic females was 2.4 times that of free-living females.’ (Luong LT., Subasinghe D., 2017) A direct parasite has only one host while an indirect parasite has multiple hosts. For indirect parasites, there will always be a definitive host (final host) and an intermediate host. The parasites discussed in this essay are being discussed due to their ‘Mind-controlling’ behaviors and therefore will all belong to the group termed ‘Endo-parasites’.Examples of Endo-parasites include the parasitic worms, rabies virus, and Toxoplasma gondii.The opposite to Endo-parasitesare ‘Ecto-parasites’. Ecto-parasites include lice, fleas and ticks (CE. Hopla, L.A. Durden, 1994), referring to the fact that they live and take effect inside the host body, rather than being an external force. These Endo-parasites are then further sub-divided into Intra- (viruses etc.) and Extracellular (parasitic worms) parasites, referring to their ability to reside within or without of an individual cell. This may also provide some clues as to their reproductive pathway (viruses are intracellular parasites as they are obligate parasites in this way, requiring a host cell in order to provide the necessary enzymes and proteins to reproduce). (D. Dantic) Parasitism follows a typical cycle- ‘Transmission through the food chain, where a parasite is immature in an intermediate host that must be eaten by a predatory definitive host before the parasite can reach maturity and complete its life cycle’ (J.P. Webster, 2007). This is referring to the fact that Parasitism is an uncertain method, completely dependent on the actions of other species. Parasitism is commonly used to enable an easier and lower energy-consuming life and propagation technique for the parasitic species. |
Parasitism refers to an interspecies relationship where one organism (the parasite) benefits and one organism (the host) suffers. This means it is a non-mutualistic relationship (D. Dantic). Parasitism is one of the three main branches of Symbiotic relationships observed in the natural kingdom. In contrast Mutualism refers to two species with a positive effect on the other (e.g. an Ox-pecker bird gains food from cleaning a buffalo of parasitic insects and the buffalo is rid of the parasitic infection)(A. Miller, 2015). The third type of Symbiotic relationship is Commensalism, in which case, the commensal benefits from the relationship, and the host is unaffected, with no positive or negative effects (e.g. a Remora fish rides with Sharks, gaining food from the leftovers of the sharks prey, meanwhile, the shark has no impact from being accompanied by the Remora) (G. Larson, D. Q. Fuller, 2014). Typical features of parasites in general include: parasites being far smaller than their host (sometimes allowing for large numbers of parasites sharing a single host, e.g. Tics on a dog) and reproducing at a much faster rate than their host species, this allows many generations of parasites to be propagated and live their life cycles using just one host (D. Dantic). Parasites can be classified into Macroscopic and Microscopic. They can also be split into their phylogenic groups, which is remarkably diverse (as is the range of hosts): Protozoa, Virus, Bacteria, Plant and Animal. Alternatively, parasitic infections can be described by their dependence level on their host. Obligate parasites are totally dependent on their hosts to complete their life cycles. The opposite to this being facultative parasites which are not dependant. It appears most likely that Facultative Parasites simply use parasitism as a method of improving their reproductive success by providing a more niche role with fewer competitors, for example facultative parasite, Macrocheles subbadius. Lifetime reproductive success was higher among female mites that fed on fruit fly haemolymph as parasites compared to free-living mites. The per capita lifetime fecundity for parasitic females was 2.4 times that of free-living females.’ (Luong LT., Subasinghe D., 2017). A direct parasite has only one host while an indirect parasite has multiple hosts. For indirect parasites, there will always be a definitive host (final host) and an intermediate host. The parasites discussed in this essay are being discussed due to their ‘Mind-controlling’ behaviors and therefore will all belong to the group termed ‘Endo-parasites’. Examples of Endo-parasites include the parasitic worms, rabies virus, and Toxoplasma gondii. The opposite to Endo-parasites are ‘Ecto-parasites’. Ecto-parasites include lice, fleas and ticks (CE. Hopla, L.A. Durden, 1994), referring to the fact that they live and take effect inside the host body, rather than being an external force. These Endo-parasites are then further sub-divided into Intra- (viruses etc.) and Extracellular (parasitic worms) parasites, referring to their ability to reside within or without of an individual cell. This may also provide some clues as to their reproductive pathway (viruses are intracellular parasites as they are obligate parasites in this way, requiring a host cell in order to provide the necessary enzymes and proteins to reproduce) (D. Dantic). Parasitism follows a typical cycle- ‘Transmission through the food chain, where a parasite is immature in an intermediate host that must be eaten by a predatory definitive host before the parasite can reach maturity and complete its life cycle’ (J.P. Webster, 2007). This is referring to the fact that Parasitism is an uncertain method, completely dependent on the actions of other species. Parasitism is commonly used to enable an easier and lower energy-consuming life and propagation technique for the parasitic species. |
| Line 31: | Line 32: |
| Parasitism is considered to be the close relationship between a parasite and its host, where the parasite either lives within or on the host. (Weinersmith & Faulkes, 2014) This relationship resultsin favourof the parasite as there is anincreasedchance of successful transmission, but in turn, at the cost of the host as it becomes infected. The infection from the parasite can result in changes of the host phenotype. This can also be propagated by the host’s natural physiological response, which may have a positive or negative effect on the parasite either by adaptive changes or by the host expelling the parasite itself. (Weinersmith & Faulkes, 2014)As the parasite controls the phenotype (biochemical and physical characteristics) of the host it has to adapt and become specialized in the specific host species. This gives the parasite the potential to change the host’s behaviour by adaptation (where the parasite has the ability to circulate throughout the host) or as a physiological response (where the host triggers a reaction to the parasite due to the infection). Understanding how the parasite alters the host’s phenotype can be a good basis in providing a great deal of information between the immune system, endocrine system and specifically the brain on behaviour. (Poulin, 1998) According to the article“Adaptive changes in the behaviour of parasitized animals”, change in host behaviour after the infection of parasite can only be considered as an adaption if they are complex, if there is an increase in activity of either the host or parasite and if they have arisen independently in several different hosts or parasites. (Poulin, 1995) It also mentions that although the host’s behavioural changes can be complex, most are just an increase or decrease in their original behaviour prior to becoming infected. These parasitic changes are not only confined to the host’s phenotype but also to the population and ecosystem as a whole. (Weinersmith & Faulker, 2014)Involvements between the two enable us to have an understanding of the link between the brain and behaviour. When considering the effects of the parasite on brain function we refer to the CNS.The function of the CNS is based on sensing stimuli from the outer or inner environment - between neurons. This is achieved by the active participation of membranes of different afferent nerve endings. (Department of Physiology, 2017)The specific stimulus evokes a cation reflux, generating an action potential. The amplitude of this potential is proportional with the extent of the stimulus. In the article “The Parasitic Zombifier“, it highlights that some mind altering parasites effect their host by generating or altering these electrical signals directly or by adjusting the chemical signals. (Saleemuddin, 2015) |
Parasitism is considered to be the close relationship between a parasite and its host, where the parasite either lives within or on the host (Weinersmith & Faulkes, 2014). This relationship results in favour of the parasite as there is an increased chance of successful transmission, but in turn, at the cost of the host as it becomes infected. The infection from the parasite can result in changes of the host phenotype. This can also be propagated by the host’s natural physiological response, which may have a positive or negative effect on the parasite either by adaptive changes or by the host expelling the parasite itself (Weinersmith & Faulkes, 2014). As the parasite controls the phenotype (biochemical and physical characteristics) of the host it has to adapt and become specialized in the specific host species. This gives the parasite the potential to change the host’s behaviour by adaptation (where the parasite has the ability to circulate throughout the host) or as a physiological response (where the host triggers a reaction to the parasite due to the infection). Understanding how the parasite alters the host’s phenotype can be a good basis in providing a great deal of information between the immune system, endocrine system and specifically the brain on behaviour (Poulin, 1998). According to the article “Adaptive changes in the behaviour of parasitized animals”, change in host behaviour after the infection of parasite can only be considered as an adaption if they are complex, if there is an increase in activity of either the host or parasite and if they have arisen independently in several different hosts or parasites (Poulin, 1995). It also mentions that although the host’s behavioural changes can be complex, most are just an increase or decrease in their original behaviour prior to becoming infected. These parasitic changes are not only confined to the host’s phenotype but also to the population and ecosystem as a whole (Weinersmith & Faulker, 2014). Involvements between the two enable us to have an understanding of the link between the brain and behaviour. When considering the effects of the parasite on brain function we refer to the CNS. The function of the CNS is based on sensing stimuli from the outer or inner environment - between neurons. This is achieved by the active participation of membranes of different afferent nerve endings (Department of Physiology, 2017). The specific stimulus evokes a cation reflux, generating an action potential. The amplitude of this potential is proportional with the extent of the stimulus. In the article “The Parasitic Zombifier“, it highlights that some mind altering parasites effect their host by generating or altering these electrical signals directly or by adjusting the chemical signals (Saleemuddin, 2015). |
| Line 36: | Line 37: |
| Parasites which infect invertebrates or host’s body cavity will alter the host’s behaviour by exposing themselves more to their potential predators. Whereas in vertebrates, the hosts tissues and CNS become infected resulting in an impaired host response to their predators. (Poulin, 1995)These parasite that have effects on the CNS are said to be more restricted to the number of hosts they can infect compared to other parasites that can infect other types of tissues and more hosts. An example of this is the Helminth parasite. (Saleemuddin, 2015)The difference is that the parasites affecting the CNS have a larger and more specific effect on the host compared to the others in which the manipulation of the infected tissues are less specific. The type of host and the location of infection have a big impact on how the parasite alters the host’s behaviour. (Saleemuddin, 2015) | Parasites which infect invertebrates or host’s body cavity will alter the host’s behaviour by exposing themselves more to their potential predators. Whereas in vertebrates, the hosts tissues and CNS become infected resulting in an impaired host response to their predators (Poulin, 1995). These parasite that have effects on the CNS are said to be more restricted to the number of hosts they can infect compared to other parasites that can infect other types of tissues and more hosts. An example of this is the Helminth parasite (Saleemuddin, 2015). The difference is that the parasites affecting the CNS have a larger and more specific effect on the host compared to the others in which the manipulation of the infected tissues are less specific. The type of host and the location of infection have a big impact on how the parasite alters the host’s behaviour (Saleemuddin, 2015). |
| Line 38: | Line 39: |
| ---- | |
| Line 45: | Line 45: |
| Line 52: | Line 51: |
| Rabies is an acute viral encephalitis that is spread through the saliva of infected hosts (Rupprecht et al, (2002). Clinical manifestations vary, but the neurological phase often includes increased aggression and the tendency to bite and thereby transmit infection; rapid progression to death is inevitable (Hemachudha et al (2002). These distinctive signs make transmission of rabies easier to track than that of most other diseases.This provides an unusual opportunity to explore epidemiological patterns at the scale of the individual. A quantitative understanding of rabies transmission dynamics in domestic dog populations is crucial to determining whether global elimination can be achieved (Hampson, et al. (2009). Once it is inside the host’s bloodstream, it quickly starts taking over cells, transforming them into rabies factories that churn out thousands of copies of the virus. As the attackers grow in number, they make their way to the host’s central nervous system and head for the brain. Rabies viruses don’t just settle down anywhere in the brain, they specifically seek out the hippocampus, amygdala and hypothalamus brain structures that play central roles in memory, fear and emotion (Thomas, 2015). They don’t just devour brain cells indiscriminately either, instead they alter the way these cells release neurotransmitters like serotonin, GABA, and endogenous opioids. In other words, they turn their hosts’ own brain chemistry against them.(Thomas, 2015) In the altered states brought on by a rabies infection, animals often lash out at any nearby living thing. This may be more out of fear than anger. Human rabies patients become terrified of water and puffs of air, both of which make them flinch and twitch uncontrollably. (Thomas, 2015) If the infection goes untreated, rabies patients can fall deeper into confusion and hallucination. This can lead tostriking out at imagined threats and bystanders. They lose their ability to sleep, sweat profusely, and finally fall into a paralyzed state as their brain function slips into chaos (Thomas, 2015). After a few days as the paralysis reaches their hearts and lungs, they fall into a coma and die. Once rabies has infected a human, survival is all-but impossible. | Rabies is an acute viral encephalitis that is spread through the saliva of infected hosts (Rupprecht et al, 2002). Clinical manifestations vary, but the neurological phase often includes increased aggression and the tendency to bite and thereby transmit infection; rapid progression to death is inevitable (Hemachudha et al, 2002). These distinctive signs make transmission of rabies easier to track than that of most other diseases. This provides an unusual opportunity to explore epidemiological patterns at the scale of the individual. A quantitative understanding of rabies transmission dynamics in domestic dog populations is crucial to determining whether global elimination can be achieved (Hampson, et al. , 2009). Once it is inside the host’s bloodstream, it quickly starts taking over cells, transforming them into rabies factories that churn out thousands of copies of the virus. As the attackers grow in number, they make their way to the host’s central nervous system and head for the brain. Rabies viruses don’t just settle down anywhere in the brain, they specifically seek out the hippocampus, amygdala and hypothalamus brain structures that play central roles in memory, fear and emotion (Thomas, 2015). They don’t just devour brain cells indiscriminately either, instead they alter the way these cells release neurotransmitters like serotonin, GABA, and endogenous opioids. In other words, they turn their hosts’ own brain chemistry against them (Thomas, 2015). In the altered states brought on by a rabies infection, animals often lash out at any nearby living thing. This may be more out of fear than anger. Human rabies patients become terrified of water and puffs of air, both of which make them flinch and twitch uncontrollably (Thomas, 2015). If the infection goes untreated, rabies patients can fall deeper into confusion and hallucination. This can lead to striking out at imagined threats and bystanders. They lose their ability to sleep, sweat profusely, and finally fall into a paralyzed state as their brain function slips into chaos (Thomas, 2015). After a few days as the paralysis reaches their hearts and lungs, they fall into a coma and die. Once rabies has infected a human, survival is all-but impossible. |
| Line 70: | Line 69: |
| Toxoplasma gondii (T. gondii) is one of the most famous and most controversial neurological parasites. The parasite Toxoplasma gondii manipulates the behaviour of its intermediate rat host in order to increase its chance of being predated by cats, its feline definitive host, thereby ensuring the completion of its life cycle (Berdoy et al 2000). According to the manipulation hypothesis, a parasite may alter the behaviour of its host for its own benefit, usually by enhancing its transmission rate. The hypothesis implies that such host behaviour modification represents a sophisticated product of parasite evolution aimed at host manipulation, rather than an accidental side-effect of infection (Barnard & Behnke 1990; Poulin 1994). T. gondii is an intracellular protozoan (Beverley 1976) capable of infecting all mammals. Its associate disease, toxoplasmosis, is of significant economic, veterinary and medical importance (Luft & Remington 1986) T. gondii has an indirect life cycle, where members of the cat family are the definitive hosts of the parasites and the only mammals known to shed T. gondii oocysts with their faeces (Hutchinson et al. 1969). If the oocysts are ingested by another mammal such as a wild rodent (the intermediate host), small thin-walled cysts form in various tissues, most commonly the brain Moreover. Since sexual reproduction of T. gondii can be accomplished only in the feline, there might be strong selective pressure on the parasite to evolve such a mechanism. Accordingly, recent studies on both wild and laboratory hybrid rats have demonstrated that T. gondii causes an increase in activity (Webster 1994b) and a decrease in neophobic (fear of novelty) behaviour (Webster et al. 1994). Both of which can be argued to facilitate transmission to the felid definitive host. Of its host places T. gondii is in a privileged position to manipulate behaviour (Werner et al. 1981). Rats have evolved an innate and pronounced defensive reaction to predator odours, including cat (Vernet-Maury et al. 1984). To test the potential effect of T. gondii on the rat’s perception of predation risk Berdoy et al. (2000) observed the nocturnal exploratory behaviour of rats in outdoor pens (2m x 2m). Humans represent a dead-end host for the parasite. Their results suggest that the reports of altered personality and IQ levels in T. gondii-infected patients represent the outcome of a parasite evolved to manipulate the behaviour of another mammal. | Toxoplasma gondii (T. gondii) is one of the most famous and most controversial neurological parasites. The parasite Toxoplasma gondii manipulates the behaviour of its intermediate rat host in order to increase its chance of being predated by cats, its feline definitive host, thereby ensuring the completion of its life cycle (Berdoy et al 2000). According to the manipulation hypothesis, a parasite may alter the behaviour of its host for its own benefit, usually by enhancing its transmission rate. The hypothesis implies that such host behaviour modification represents a sophisticated product of parasite evolution aimed at host manipulation, rather than an accidental side-effect of infection (Barnard & Behnke 1990; Poulin 1994). T. gondii is an intracellular protozoan (Beverley 1976) capable of infecting all mammals. Its associate disease, toxoplasmosis, is of significant economic, veterinary and medical importance (Luft & Remington 1986). T. gondii has an indirect life cycle, where members of the cat family are the definitive hosts of the parasites and the only mammals known to shed T. gondii oocysts with their faeces (Hutchinson et al. 1969). If the oocysts are ingested by another mammal such as a wild rodent (the intermediate host), small thin-walled cysts form in various tissues, most commonly the brain Moreover. Since sexual reproduction of T. gondii can be accomplished only in the feline, there might be strong selective pressure on the parasite to evolve such a mechanism. Accordingly, recent studies on both wild and laboratory hybrid rats have demonstrated that T. gondii causes an increase in activity (Webster 1994) and a decrease in neophobic (fear of novelty) behaviour (Webster et al. 1994). Both of which can be argued to facilitate transmission to the felid definitive host. Of its host places T. gondii is in a privileged position to manipulate behaviour (Werner et al. 1981). Rats have evolved an innate and pronounced defensive reaction to predator odours, including cat (Vernet-Maury et al. 1984). To test the potential effect of T. gondii on the rat’s perception of predation risk Berdoy et al. (2000) observed the nocturnal exploratory behaviour of rats in outdoor pens (2m x 2m). Humans represent a dead-end host for the parasite. Their results suggest that the reports of altered personality and IQ levels in T. gondii-infected patients represent the outcome of a parasite evolved to manipulate the behaviour of another mammal. |
| Line 81: | Line 80: |
| Line 88: | Line 86: |
| The Nematomorpha is a relatively unknown taxon which contains about 300 species distributed around the world and commonly called hairworms (Schmidt-Rhaesa, 1997). Adult males and females are free-living in aquatic environments and gather to mate in tight masses. Unlike adults, juveniles are parasitic in arthropods. It has often been hypothesized that mature nematomorphs manipulate the behaviour of their terrestrial insect host making them seek water and jumping into it (Dawkins, 1990; Schmidt-Rhaesa, 1997). The aim of Thomas et al (2000) study was to determine whether hairworms altered the behaviour of their host in order to reach an aquatic environment needed for their emergence and reproduction. Crickets harbouring a worm often jumped into the water whereas uninfected crickets were more reluctant to enter it.This behavioural difference is a key step in the manipulative process as it allows the hairworm to emerge immediately after its host enters water. Adaptations can also be recognized at the macro-evolutionary scale. When different parasite lineages evolving under similar selective pressures have independently evolved the ability to cause identical alterations in host behaviour (Poulin, 1998). | The Nematomorpha is a relatively unknown taxon which contains about 300 species distributed around the world and commonly called hairworms (Schmidt-Rhaesa, 1997). Adult males and females are free-living in aquatic environments and gather to mate in tight masses. Unlike adults, juveniles are parasitic in arthropods. It has often been hypothesized that mature nematomorphs manipulate the behaviour of their terrestrial insect host making them seek water and jumping into it (Dawkins, 1990; Schmidt-Rhaesa, 1997). The aim of Thomas et al (2000) study was to determine whether hairworms altered the behaviour of their host in order to reach an aquatic environment needed for their emergence and reproduction. Crickets harbouring a worm often jumped into the water whereas uninfected crickets were more reluctant to enter it. This behavioural difference is a key step in the manipulative process as it allows the hairworm to emerge immediately after its host enters water. Adaptations can also be recognized at the macro-evolutionary scale. When different parasite lineages evolving under similar selective pressures have independently evolved the ability to cause identical alterations in host behaviour (Poulin, 1998). |
| Line 95: | Line 93: |
| Line 104: | Line 101: |
| Line 106: | Line 102: |
| Line 111: | Line 106: |
The Principle of Operation of “Mind-Controlling” parasites on the behaviour of the Host
Matthew Dixon
Chameen Zurfluh
Ciara Quill
Contents
Background information of general Parasitic Infections:
Parasitism refers to an interspecies relationship where one organism (the parasite) benefits and one organism (the host) suffers. This means it is a non-mutualistic relationship (D. Dantic). Parasitism is one of the three main branches of Symbiotic relationships observed in the natural kingdom. In contrast Mutualism refers to two species with a positive effect on the other (e.g. an Ox-pecker bird gains food from cleaning a buffalo of parasitic insects and the buffalo is rid of the parasitic infection)(A. Miller, 2015). The third type of Symbiotic relationship is Commensalism, in which case, the commensal benefits from the relationship, and the host is unaffected, with no positive or negative effects (e.g. a Remora fish rides with Sharks, gaining food from the leftovers of the sharks prey, meanwhile, the shark has no impact from being accompanied by the Remora) (G. Larson, D. Q. Fuller, 2014). Typical features of parasites in general include: parasites being far smaller than their host (sometimes allowing for large numbers of parasites sharing a single host, e.g. Tics on a dog) and reproducing at a much faster rate than their host species, this allows many generations of parasites to be propagated and live their life cycles using just one host (D. Dantic). Parasites can be classified into Macroscopic and Microscopic. They can also be split into their phylogenic groups, which is remarkably diverse (as is the range of hosts): Protozoa, Virus, Bacteria, Plant and Animal. Alternatively, parasitic infections can be described by their dependence level on their host. Obligate parasites are totally dependent on their hosts to complete their life cycles. The opposite to this being facultative parasites which are not dependant. It appears most likely that Facultative Parasites simply use parasitism as a method of improving their reproductive success by providing a more niche role with fewer competitors, for example facultative parasite, Macrocheles subbadius. Lifetime reproductive success was higher among female mites that fed on fruit fly haemolymph as parasites compared to free-living mites. The per capita lifetime fecundity for parasitic females was 2.4 times that of free-living females.’ (Luong LT., Subasinghe D., 2017). A direct parasite has only one host while an indirect parasite has multiple hosts. For indirect parasites, there will always be a definitive host (final host) and an intermediate host. The parasites discussed in this essay are being discussed due to their ‘Mind-controlling’ behaviors and therefore will all belong to the group termed ‘Endo-parasites’. Examples of Endo-parasites include the parasitic worms, rabies virus, and Toxoplasma gondii. The opposite to Endo-parasites are ‘Ecto-parasites’. Ecto-parasites include lice, fleas and ticks (CE. Hopla, L.A. Durden, 1994), referring to the fact that they live and take effect inside the host body, rather than being an external force. These Endo-parasites are then further sub-divided into Intra- (viruses etc.) and Extracellular (parasitic worms) parasites, referring to their ability to reside within or without of an individual cell. This may also provide some clues as to their reproductive pathway (viruses are intracellular parasites as they are obligate parasites in this way, requiring a host cell in order to provide the necessary enzymes and proteins to reproduce) (D. Dantic). Parasitism follows a typical cycle- ‘Transmission through the food chain, where a parasite is immature in an intermediate host that must be eaten by a predatory definitive host before the parasite can reach maturity and complete its life cycle’ (J.P. Webster, 2007). This is referring to the fact that Parasitism is an uncertain method, completely dependent on the actions of other species. Parasitism is commonly used to enable an easier and lower energy-consuming life and propagation technique for the parasitic species.
Background information specifically regarding ‘Mind-Controlling’ Parasites:
Parasitism is considered to be the close relationship between a parasite and its host, where the parasite either lives within or on the host (Weinersmith & Faulkes, 2014). This relationship results in favour of the parasite as there is an increased chance of successful transmission, but in turn, at the cost of the host as it becomes infected. The infection from the parasite can result in changes of the host phenotype. This can also be propagated by the host’s natural physiological response, which may have a positive or negative effect on the parasite either by adaptive changes or by the host expelling the parasite itself (Weinersmith & Faulkes, 2014). As the parasite controls the phenotype (biochemical and physical characteristics) of the host it has to adapt and become specialized in the specific host species. This gives the parasite the potential to change the host’s behaviour by adaptation (where the parasite has the ability to circulate throughout the host) or as a physiological response (where the host triggers a reaction to the parasite due to the infection). Understanding how the parasite alters the host’s phenotype can be a good basis in providing a great deal of information between the immune system, endocrine system and specifically the brain on behaviour (Poulin, 1998). According to the article “Adaptive changes in the behaviour of parasitized animals”, change in host behaviour after the infection of parasite can only be considered as an adaption if they are complex, if there is an increase in activity of either the host or parasite and if they have arisen independently in several different hosts or parasites (Poulin, 1995). It also mentions that although the host’s behavioural changes can be complex, most are just an increase or decrease in their original behaviour prior to becoming infected. These parasitic changes are not only confined to the host’s phenotype but also to the population and ecosystem as a whole (Weinersmith & Faulker, 2014). Involvements between the two enable us to have an understanding of the link between the brain and behaviour. When considering the effects of the parasite on brain function we refer to the CNS. The function of the CNS is based on sensing stimuli from the outer or inner environment - between neurons. This is achieved by the active participation of membranes of different afferent nerve endings (Department of Physiology, 2017). The specific stimulus evokes a cation reflux, generating an action potential. The amplitude of this potential is proportional with the extent of the stimulus. In the article “The Parasitic Zombifier“, it highlights that some mind altering parasites effect their host by generating or altering these electrical signals directly or by adjusting the chemical signals (Saleemuddin, 2015).
Parasites which infect invertebrates or host’s body cavity will alter the host’s behaviour by exposing themselves more to their potential predators. Whereas in vertebrates, the hosts tissues and CNS become infected resulting in an impaired host response to their predators (Poulin, 1995). These parasite that have effects on the CNS are said to be more restricted to the number of hosts they can infect compared to other parasites that can infect other types of tissues and more hosts. An example of this is the Helminth parasite (Saleemuddin, 2015). The difference is that the parasites affecting the CNS have a larger and more specific effect on the host compared to the others in which the manipulation of the infected tissues are less specific. The type of host and the location of infection have a big impact on how the parasite alters the host’s behaviour (Saleemuddin, 2015).
Specific examples of Mind-controlling Parasites and commonly infected hosts (with exhibited effects):
Rabies
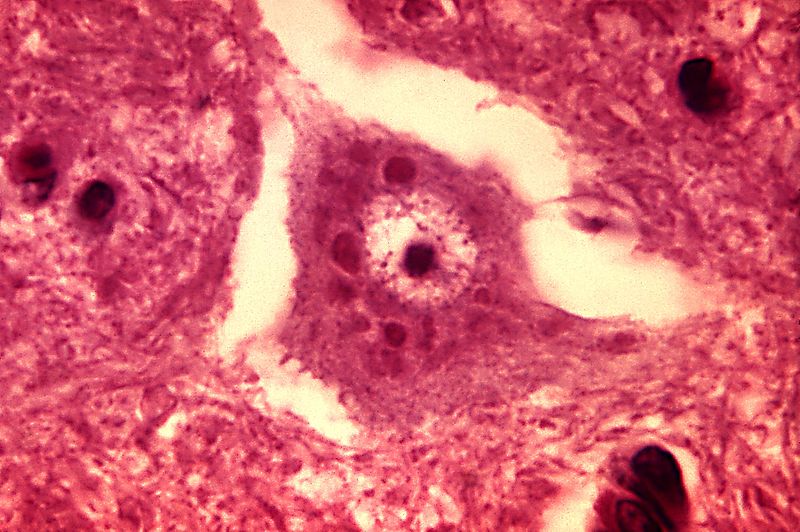
Figure 1 Rabies Encephalitis Negri bodies
(Dr Daniel P. Perl, 1971)
Rabies is an acute viral encephalitis that is spread through the saliva of infected hosts (Rupprecht et al, 2002). Clinical manifestations vary, but the neurological phase often includes increased aggression and the tendency to bite and thereby transmit infection; rapid progression to death is inevitable (Hemachudha et al, 2002). These distinctive signs make transmission of rabies easier to track than that of most other diseases. This provides an unusual opportunity to explore epidemiological patterns at the scale of the individual. A quantitative understanding of rabies transmission dynamics in domestic dog populations is crucial to determining whether global elimination can be achieved (Hampson, et al. , 2009). Once it is inside the host’s bloodstream, it quickly starts taking over cells, transforming them into rabies factories that churn out thousands of copies of the virus. As the attackers grow in number, they make their way to the host’s central nervous system and head for the brain. Rabies viruses don’t just settle down anywhere in the brain, they specifically seek out the hippocampus, amygdala and hypothalamus brain structures that play central roles in memory, fear and emotion (Thomas, 2015). They don’t just devour brain cells indiscriminately either, instead they alter the way these cells release neurotransmitters like serotonin, GABA, and endogenous opioids. In other words, they turn their hosts’ own brain chemistry against them (Thomas, 2015). In the altered states brought on by a rabies infection, animals often lash out at any nearby living thing. This may be more out of fear than anger. Human rabies patients become terrified of water and puffs of air, both of which make them flinch and twitch uncontrollably (Thomas, 2015). If the infection goes untreated, rabies patients can fall deeper into confusion and hallucination. This can lead to striking out at imagined threats and bystanders. They lose their ability to sleep, sweat profusely, and finally fall into a paralyzed state as their brain function slips into chaos (Thomas, 2015). After a few days as the paralysis reaches their hearts and lungs, they fall into a coma and die. Once rabies has infected a human, survival is all-but impossible.
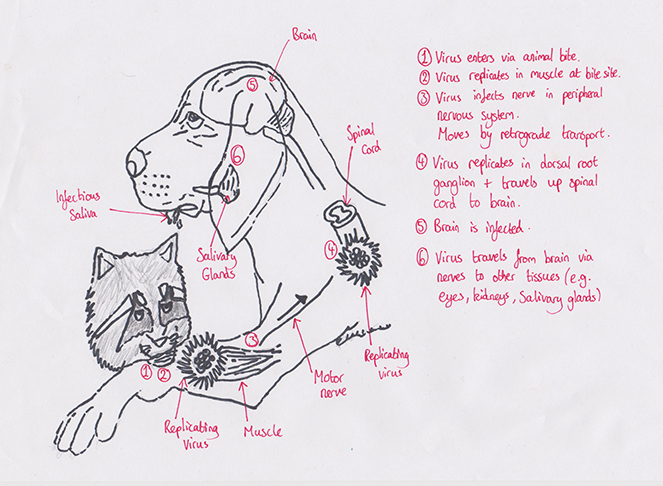
Figure 2 Rabies Life cycle
(Adapted from M. N. O. Uwamose (2013))
Toxoplasma gondii
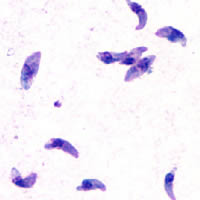
Figure 3 Toxoplasma gondii cells
(Adapted from DPDx Image Library (2004))
Toxoplasma gondii (T. gondii) is one of the most famous and most controversial neurological parasites. The parasite Toxoplasma gondii manipulates the behaviour of its intermediate rat host in order to increase its chance of being predated by cats, its feline definitive host, thereby ensuring the completion of its life cycle (Berdoy et al 2000). According to the manipulation hypothesis, a parasite may alter the behaviour of its host for its own benefit, usually by enhancing its transmission rate. The hypothesis implies that such host behaviour modification represents a sophisticated product of parasite evolution aimed at host manipulation, rather than an accidental side-effect of infection (Barnard & Behnke 1990; Poulin 1994). T. gondii is an intracellular protozoan (Beverley 1976) capable of infecting all mammals. Its associate disease, toxoplasmosis, is of significant economic, veterinary and medical importance (Luft & Remington 1986). T. gondii has an indirect life cycle, where members of the cat family are the definitive hosts of the parasites and the only mammals known to shed T. gondii oocysts with their faeces (Hutchinson et al. 1969). If the oocysts are ingested by another mammal such as a wild rodent (the intermediate host), small thin-walled cysts form in various tissues, most commonly the brain Moreover. Since sexual reproduction of T. gondii can be accomplished only in the feline, there might be strong selective pressure on the parasite to evolve such a mechanism. Accordingly, recent studies on both wild and laboratory hybrid rats have demonstrated that T. gondii causes an increase in activity (Webster 1994) and a decrease in neophobic (fear of novelty) behaviour (Webster et al. 1994). Both of which can be argued to facilitate transmission to the felid definitive host. Of its host places T. gondii is in a privileged position to manipulate behaviour (Werner et al. 1981). Rats have evolved an innate and pronounced defensive reaction to predator odours, including cat (Vernet-Maury et al. 1984). To test the potential effect of T. gondii on the rat’s perception of predation risk Berdoy et al. (2000) observed the nocturnal exploratory behaviour of rats in outdoor pens (2m x 2m). Humans represent a dead-end host for the parasite. Their results suggest that the reports of altered personality and IQ levels in T. gondii-infected patients represent the outcome of a parasite evolved to manipulate the behaviour of another mammal.
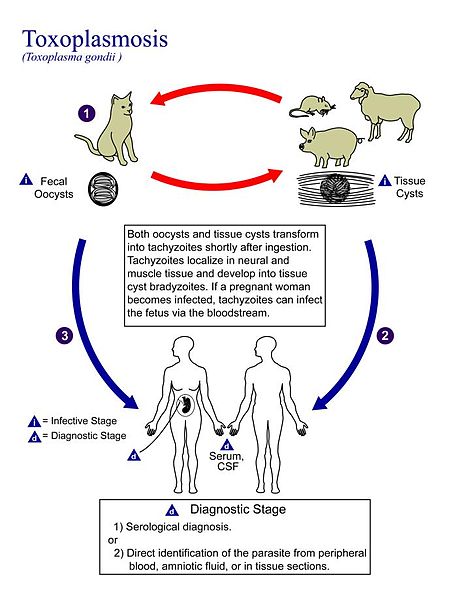
Figure 4 Toxoplasma gondii Life cycle
(A. J. de Silva, M. Moser (2002))
Nematomorpha Worms
Figure 5 Nematomorpha Worms
(B. Von D. et al. (2004))
The Nematomorpha is a relatively unknown taxon which contains about 300 species distributed around the world and commonly called hairworms (Schmidt-Rhaesa, 1997). Adult males and females are free-living in aquatic environments and gather to mate in tight masses. Unlike adults, juveniles are parasitic in arthropods. It has often been hypothesized that mature nematomorphs manipulate the behaviour of their terrestrial insect host making them seek water and jumping into it (Dawkins, 1990; Schmidt-Rhaesa, 1997). The aim of Thomas et al (2000) study was to determine whether hairworms altered the behaviour of their host in order to reach an aquatic environment needed for their emergence and reproduction. Crickets harbouring a worm often jumped into the water whereas uninfected crickets were more reluctant to enter it. This behavioural difference is a key step in the manipulative process as it allows the hairworm to emerge immediately after its host enters water. Adaptations can also be recognized at the macro-evolutionary scale. When different parasite lineages evolving under similar selective pressures have independently evolved the ability to cause identical alterations in host behaviour (Poulin, 1998).
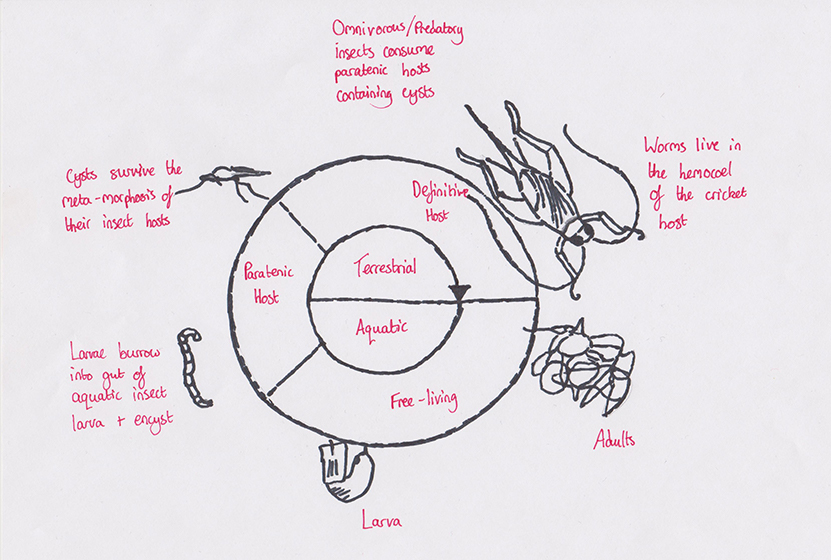
Figure 6 Nematomorpha Worm Life cycle
(B. Hanelt, F. Thomas, A Schmidt-Rhaesa (2005))
Conclusion
In conclusion, a close relation between parasites and host is a necessary feature of parasitism. This brief review serves to illustrate the importance of mind-control as a feature of parasitism, in a wide variety of parasites. This behavioural modification of the host allows for a higher chance of successful transmission and survival of the individual parasite, at the cost of host.
Reference list
Book References
Barnard, C. J. & Behnke, J. M. (ed.) (1990) Parasitism and host behaviour. London, UK: Taylor and Francis 193–229.
Dawkins, R. (1990) Parasites, desiderata lists and the paradox of the organism. Parasitology 100: S63–S73.
Website References
A. Miller, (2015) Intricate Relationship allows the other to flourish, AskNature.org
D. Dantic, Pathogenic Parasitic Infections, peoi.org
Department of Physiology, (2017) Physiology of the Nervous System, Department of Physiology, University of Veterinary Medicine of Budapest
Scientific Paper References
Beverley, J. K. A. (1976) Toxoplasmosis in animals. Vet. Rec. 99, 123-127.
CE. Hopla, L.A. Durden and J.E. Keirans (1994) Ectoparasites and Classification, Rev. sci. tech. Off. int. Epiz. 13 (4), 985-1017
G. Larson, D. Q. Fuller (2014) The Evolution Of Animal Domestication, Annual Reviews, Vol. 45:115-136
Hemachudha T, Laothamatas J, Rupprecht CE (2002) Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neuro 1: 101–109.
Hutchison, W. M., Dunachie, J. F., Siim, J. C. & Work, K. (1969) the life cycle of Toxoplasma gondii. Br. Med. J. 4, 806.
K. Hampson, et al. (2009) Transmission Dynamics and Prospects for the Elimination of Canine Rabies, PLOS Biology
Luft, B. J. & Remington, J. S. (1986) Toxoplasmosis in the central nervous system. Curr. Clin.Top. Infect. Dis. 6, 316-357.
Luong LT., Subasinghe D. (2017) A facultative ectoparasite attains higher reproductive success as a parasite than its free-living conspecifics, PubMed, 71(1):63-70
M. Berdoy, J.P Webster and D.W Macdonald (2000) Fatal attraction in rats infected with Toxoplasma gondii, The Royal Society Publishing, 267:(1452) 1591-4.
M. Saleemuddin (2015) The Parasite Zombifiers, Science Reporter, 52 20-22
Poulin, R., (1994) The evolution of parasite manipulation of host behaviour: a theoretical analysis. Parasitology, 109(S1), pp.S109-S118.
Poulin, R., (1995) “Adaptive” changes in the behaviour of parasitized animals: A critical review. International journal for parasitology, 25(12), pp.1371-1383.
Poulin, R., (1998) Evolution and phylogeny of behavioural manipulation of insect hosts by parasites. Parasitology, 116(S1), pp.S3-S11.
Schmidt-Rhaesa, A. (1997) Nematomorpha. In: Su¨bwasserfauna Mitteleuropas 4/4 (J. Schwoerbel & P. Zwick, eds). Gustav Fischer-Verlag, Stuttgart. 143(1):2-9
Thompson, S.N. and Kavaliers, M., (1994) Physiological bases for parasite-induced alterations of host behaviour. Parasitology, 109(S1), pp.S119-S138
Vernet-Maury, E., Polak, E. H. & Demael, A. (1984) Structure and activity relationship of stress-inducing odorants in the rat. J. Chem. Ecol. 10, 1007-1017.
Webster, J. P. (1994) the effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology 109, 583-589.
Webster, J. P., Brunton, C. F. A. & Macdonald, D. W. (1994) Effect of Toxoplasma gondii on neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology 109, 37-43.
Weinersmith K. et al. (2014) Parasitic manipulation of hosts’ phenotype, or how to make a zombie—an introduction to the symposium, PubMed, 54 (2): 93-100
Werner, H., Masihi, K. N. & Senk, U. (1981) Latent Toxoplasma infection as a possible risk factor for CNS disorders. Zentralblatt fur Bacteriology Microbiology und Hygiene A 250, 368-375.
Figure Bibliography
Figure 4 from: A. J. da Silva, M. Moser (2002) Toxoplasma gondii Life cycle, WikiCommons.
Figure 6 adapted from: B. Hanelt, F. Thomas, A. Schmidt-Rhaesa (2005) Biology of the Phylum Nematomorpha, PubMed.
Figure 5 from: Bildspende von D. Andreas Schmidt-Rhaesa, Veröffentlichung unter (2004) Paragordius tricuspidatus,WikiCommons.
Figure 3 from: DPDx Image Library (2004) Toxoplasma gondii, WikiCommons.
Figure 1 from: Dr. Daniel P. Perl (1971) Rabies encephalitis Negri bodies, WikiCommons.
Figure 2 adapted from: M. N. O. Uwamose (2013) A Presentation on rabies, Department of Environmental Biology, Faculty of Science, Delta State University, Abraka (Slideshare.net).
