Effect of manganese on neuroinflammation
Written by: Skinner L., Sigurðarson B., Slapgaard M.
Supervisor: Dr. Kiss D.
Physiology Department, University of Veterinary Medicine, Budapest
Contents
[[|]]
Microglia
Microglia are a form of glial cells within the central nervous system (CNS), responsible for cerebral blood flow, neural development, regulation of synaptic function and brain metabolism (Tjalkens et al, 2017). During an inflammatory response microglia are activated to form an M1 phenotype which moves to the site of damage (Tang et al, 2018). M1 inflammatory activation is regulated by the following proteins (Tjalkens et al, 2017):
Nuclear factor kappa B (NF-kB)
M1 microglial phenotypes respond to damage-associated molecular patterns (DAMPs) such as basic fibroblast growth factor (FGF2) (Woodbury and Ikezu, 2014;Tang et al, 2018), released due to necrotic neurons, and are recognised by pattern recognition receptors (PRRs) (Husemann et al, 2002). Activated M1 induce the release of pro-inflammatory factors such as tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), interleukin 1-alpha/beta (IL-1α/β), complement component 1q (C1Q), and reactive oxygen species (ROS) (Tjalkens et al, 2017).
Mn causes the activation of microglia in the substantia nigra inducing M1 phenotype proliferation (Verina et al., 2011). Mn affects M1 signalling pathways via P2Y and P2X receptors which induce NF-kB transcription (Park and Chun, 2016). NF-kB transcription results in nerve growth factor IB expression which initiates pro-inflammatory factor release (Tjalkens et al, 2017) leading to dopaminergic neuronal damage and astrocyte activation via the feed-forward effect (Zhang et al., 2010).
In addition, output of extracellular excitatory neurotransmitter glutamate is directly and indirectly increased by Mn induced M1 phenotype. Directly this is because of cystine/glutamate exchanger (xCT) dysfunction (Kirdajova et al, 2020) and hemichannel gap junctions (Umebayashi et al, 2014). Indirectly this is due to their feed-forward effect on astrocyte glutamate uptake (Takaki et al 2012). Glutamate accumulation results in excitotoxicity (Milewski et al, 2019).
Astrocytes
Astrocytes form the most abundant type of cells within the CNS, providing close contact with microvasculature and neighbouring neurons and forming physical barriers between neuronal synapses known as the tripartite synapse. Their close relationship enables active modulation of synaptic plasticity via gliotransmitter release, as well as homeostatic regulation and provisions of glucose and oxygen for neurons (Tjalkens et al, 2017).
Glucose is stored as glycogen and glutamate, the latter is transaminated into glutamine, used in the neuronal glutamate-glutamine cycle enabling gamma aminobutyric acid (GABA) production. Excess neurotransmitter glutamate released in neuronal synapses are taken up by astrocytes reducing excitotoxicity in neurons (Tjalkens et al, 2017).
In addition, lactate produced by astrocytes forms adenosine triphosphate (ATP) and pyruvate in neurons via the citric acid cycle (Tjalkens et al, 2017).
Astrocytes are pivotal to the blood-brain barrier, forming a semipermeable border between brain parenchyma and vasculature due to their astrocytic end feet, preventing solutes from non-selectively crossing into the CNS (Tjalkens et al, 2017). Mn can move across the blood-brain barrier (Yang et al, 2018).
Due to their ability to accumulate high levels of Mn they play an important role in Mn associated neuroinflammation (Tjalkens et al, 2017;Yang et al, 2018). Spranger et al (1998) found that astrocytes cannot cause neuronal apoptosis in response to Mn without pro-inflammatory factors produced by microglia (Araque et al, 2001;Park and Chun, 2016;Tjalkens et al, 2017).
DAMPs released by injured neurons and pro-inflammatory factors released by M1 microglia cause the activation of astrocytes due to PRRs promoting inflammation (Glass et al, 2010;Tjalkens et al, 2017).These factors result in glial scar formation known as astrogliosis within the basal ganglia (Tjalkens et al, 2017). Astrogliosis leads to the proliferation of the reactive astrocyte (A1) phenotype (Liddelow et al, 2017). Resting A2 astrocyte phenotypes promote neural tissue repair and survival by upregulation of neurotrophic factors whereas A1 proliferation leads to a lack of normal functions which is neurotoxic to both neurons and oligodendrocytes (Liddelow et al, 2017).
Astrocyte cystine-glutamate exchangers (xCT) cause efflux of glutamate and the influx of cystine, a precursor for the antioxidant glutathione (GSH), produced internally (Kitagawa et al, 2019). GSH reduces oxidative stress by scavenging of ROS (Yang et al, 2018). Mn activated A1s exhibit an increase in astrocyte glutaminase expression, as well as glutamate transporter excitatory amino acid carrier-1 (EAAC1) and xCT dysfunction. These dysfunctions result in glutamate accumulation and GSH synthesis inhibition increasing oxidative stress (Xu et al, 2010;Yang et al, 2018), causing astrocytes to become mediators in the excitotoxic events of inflammation (Milewski et al, 2019).
Mn activation of P2Y receptors and alteration of TRPC3 cation channels disrupts ATP dependent calcium signalling in astrocyte membranes leading to local hypoxia due to release of vasoactive compounds and insufficient metabolic support (Tjalkens et al, 2017). These effects lead to depolarisation of cell membranes, calcium ion overload, and increased extracellular glutamate production (Kirdajova et al, 2020).
According to Yang et al (2018) additional astrocytic changes in response to Mn include:
Increased peripheral-type benzodiazepine receptor(PTBR) binding sites in mitochondria
Cytotoxic oedema due to Aquaporin 4 (AQP4) channels
Nucleolus collapse
Intense chromatin condensation
Increased activity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) initiating apoptosis
Inhibition of glutamine synthetase enzyme (Verkhratsky et al, 2016)
Increased reactive nitrogen species(RNS) production, due to L-Arginine uptake, inducible nitric oxide synthase (iNOS) and stimulation of NF-kB as a result of inflammatory cytokine modulation (Moreno et al, 2008;Zhang et al, 2010)
ROS and RNS increase vulnerability of dopaminergic cell groups to Mn induced toxicity (Zhang et al, 2010), contributing towards neuronal-induced cell death by nitrosylation and nitration of protein residues (Tjalkens et al, 2017).
In addition to the effect of microglia on astrocytes, astrocytes perpetuate microglial activation by the crosstalk feed-forward effect (Glass et al, 2010;Kokaia et al, 2012).
Bhatia et al (2019) found that, if introduced in an increasing manner, healthy astrocytes and neurons in the absence of pro-inflammatory microglia are capable of moderate toleration of oxidative stress, allowing astrocytes to fulfil their neuroprotective role. However these astrocytes are A2 phenotypes, suggesting that induced A1 phenotypic astrocytes may not be as tolerant.
Neuroinflammatory diseases
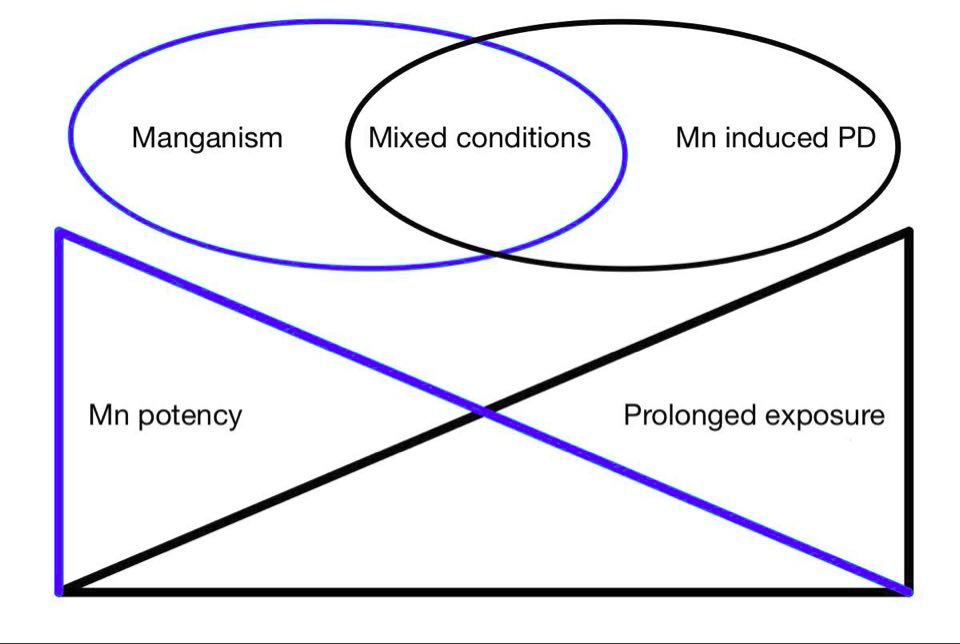
Manganism, Parkinson’s disease and manganese induced parkisonism
|
Manganism is a result of excessive or chro nic inhalation of Mn (Kwakye et al,2015). DMT1 in the olfactory epithelium provides access to the olfactory nerve, leading to basal ganglia and other DTM1 rich structures of the CNS (Harascharda et al 2019).
While Manganism and Parkinson’s disease are different, they share many similarities. Both affect the substantia nigra pars compacta (SNpc) and cause neuroinflammation followed by dopaminergic cell death (Andruska and Racette, 2015;Wang et al, 2015). Microglial activation is frequently observed during post mortem research of Parkison's disease (Wang et al, 2015), correlating to the effect of Mn on microglia through NF-kB and MAPk pathways (Tjalkens et al 2017).
Extrapyramidal symptoms result in both diseases, although tremors are not as severe or frequent in Manganism. Individuals with Manganism have the tendency to fall backwards, hypersalivate, and Babinski-like responses can be observed more frequently than in Parkison's disease (Harischandra et al, 2019).
α-synuclein is believed to play a role in Parkison's disease, when misfolded or an excessive amount gets released from the degenerating dopaminergic cells, microglia release cytokines enhancing the inflammatory response. (Wang et al, 2015). According to Harascharda et al (2019) a long term exposure to Mn can also result in increased misfolding of α-synuclein leading to aggregation and amyloid formation.
Previously Manganism was commonly caused by high exposure of Mn, typically throughmining, however chronic low dose exposure is more typical today. It is theorized that chronically acquired toxicity may increase the risk of developing Parkison's disease. Chronic low dose toxicity may be acquired through external exposure, genetic mutation of Mn regulation (e.g. transport proteins ATP13A2 and SLC30A10) or liver failure (Lucchini et al 2009). Children ingesting high levels of manganese have shown to be at higher risk to develop cognitive dysfunction due to higher gastrointestinal absorption and lower liver excretion (Tjalkens et al, 2019).
Hepatic encephalopathy
An important development of impaired liver function is hepatic encephalopathy. While ammonia and its effect on astrocytes is suspected to be the primary cause for hepatic encephalopathy, it can not account for all symptoms in patients with hepatic encephalopathy. Activation of microglia causing proinflammatory cytokines release plays a significant role in the inflammation and early development of hepatic encephalopathy. Although ammonia-cytokine synergism has been observed to increase neuroinflammatory effect ammonia has little effect on the microglial activation (Prakash and Mullen, 2010) unlike manganese (Butterworth, 2013).
Manganese excretion occurs due to liver metabolism whereby it enters the liver from the blood through the portal system and is recirculated into the gastrointestinal tract. Within the gastrointestinal tract 1-5% is reabsorbed, and the majority is excreted in faeces. (Davis et al, 1993;Aschner and Aschner, 2005). Lack of liver metabolism due to disease, such as cirrhosis or portosystemic shunt [[|]]may lead to the accumulation of manganese in blood. A study by Prakash and Mullen, 2010 found that levels of manganese in the brain were increased by chronic liver failure but not the acute form (Butterworth, 2013), this was confirmed by Parkison's disease like symptoms in human patients with hepatic encephalopathy, caused by either cirrhosis and portosystematic shunt (Prakash and Mullen, 2010).
Various dog and cat breeds suffer from congenital PSS, putting them at risk of developing hepatic encephalopathy. Dog breeds include (Van den Bossche et al, 2012):
MRI images of basal ganglia of patients with hepatic encephalopathy, as well as observed dopaminergic apoptosis suggested manganese involvement. Due to the effect of manganese on the activation of microglial cells causing neuroinflammation chronic liver failure can be linked with neuroinflammation due to manganese accumulation (Butterworth, 2013).
Alzheimers Disease
High Mn levels in the CNS of patients with Alzheimer's disease suggest that Mn might be a risk factor. Overexposure of Mn and other metals can cause changes in amyloid-β aggregation in the frontal cortex, hinting towards cognitive and memory defects(Ijomone et al, 2019).
Mn induces oxidative stress mostly in astrocytes by promoting [[|]]ROS due to mitochondrial respiratory chain dysfunction. Consequently, inhibition of antioxidant production and calcium signal changes occur. Resulting decreased mitochondrial activity and increased calcium levels within the mitochondria leads to neuron apoptosis, thought to cause neurodegeneration (Ijomone et al, 2019). Mn induced apoptosis involves PKC-δ, an enzyme involved in Alzheimer's, Parkinson’s and prion diseases (Harischarda et al, 2019). According to a study by Du et al (2018) PKC-δ levels were high in patients with Alzheimer’s, this link was confirmed by reduction of amyloid-β levels due to inhibition of PKC-δ.
While Alzheimers disease is commonly known to be a human disease, alzheimer like neurodegeneration has been observed in dolphins, sea lions and to lesser extents, catscats (Gunn-More et al, 2018). More research is required to understand the link between high Mn levels and Alzheimer's.
Mn accumulation in different species
Increased inflammatory genes have been measured in rodent and non-human primate studies of Mn exposure ((Moreno et al, 2008);(Verina et al, 2011)). An experiment by Bock et al (2008) on marmosets and rats demonstrated different levels of Mn accumulation patterns in brain areas between these two species. Marmosets showed accumulation of Mn in more brain structures than rats which showed accumulation within the striatum and the basal ganglia (Xu et al. 2010), this is hypothesised as being because of ventricle differences between species. Most current information has been obtained using rodent models (Tjalkens et al, 2017).