|
Size: 12374
Comment:
|
Size: 12382
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 54: | Line 54: |
| Perception can be defined as central processing of nociceptive impulses in order to interpret pain.In the perception neurons are located in the thalamus and project to somatosensory areas II and I in the post-central gyrus and superior wall of the sylvian fissure. Perception and discrete localization of pain take place in these cortical areas. Some fibres project to the anterior cingulated gyrus and are likely to mediate the suffering and emotional components of pain. Perception of pain is the end result of the neuronal activity of pain transmission and where pain becomes a conscious multidimensional experience. The multidimensional experience of pain has affective-motivational, sensory-discriminative, emotional and behavioural components.So as a summary for the above four steps; for an animal to experience pain, nociceptive information must be sent to higher centers in the CNS to be integrated, modulated, and interpreted into the conscious perception of pain. Noxious stimuli (heat, cold, mechanical, chemical) activate free sensory nerve endings known as nociceptors. A-δ and C-fibers transmit sensory information from nociceptors to the dorsal horn of the spinal cord, which directs and modulates input from the periphery and higher centers. Nociceptive information arriving in the dorsal horn of the spinal cord may activate motor neurons responsible for the reflex responses to noxious stimuli. | Perception can be defined as central processing of nociceptive impulses in order to interpret pain.In the perception neurons are located in the thalamus and project to somatosensory areas II and I in the post-central gyrus and superior wall of the sylvian fissure. Perception and discrete localization of pain take place in these cortical areas. Some fibres project to the anterior cingulated gyrus and are likely to mediate the suffering and emotional components of pain. Perception of pain is the end result of the neuronal activity of pain transmission and where pain becomes a conscious multidimensional experience. The multidimensional experience of pain has affective-motivational, sensory-discriminative, emotional and behavioural components. So as a summary for the above four steps; for an animal to experience pain, nociceptive information must be sent to higher centers in the CNS to be integrated, modulated, and interpreted into the conscious perception of pain. Noxious stimuli (heat, cold, mechanical, chemical) activate free sensory nerve endings known as nociceptors. A-δ and C-fibers transmit sensory information from nociceptors to the dorsal horn of the spinal cord, which directs and modulates input from the periphery and higher centers. Nociceptive information arriving in the dorsal horn of the spinal cord may activate motor neurons responsible for the reflex responses to noxious stimuli. {{{ |
| Line 57: | Line 58: |
| }}} |
Itt írjon a(z) Pain_physiology-ról/ről
Physiology Of Pain
Project by:
Supervisor Dr. Zoltan Balazs Barany
Physiology Department, University of Veterinary Medicine, Budapest
Contents
What is pain?
Pain is defined by the International Association for the Study of Pain (IASP) as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage".Pain is a subjective experience made up of two complementary aspects, the first one is localized sensation in a particular body part, while the other is an unpleasant quality of varying severity commonly associated with behaviors directed at relieving or terminating the experience, the pain has much in common with other sensory modalities according to National Academy of Sciences (1985). There are specific pain receptors as these are nerve endings, present in most body tissues, that only respond to damaging or potentially damaging stimuli. Which the messages initiated by these noxious stimuli are transmitted by specific, identified nerves to the spinal cord. The perception of pain results from the brain’s processing of new sensory input with existing memories and emotions, is the same way that other perceptions are produced. Pain become painful is when it reaches out the threshold. However, most studies have found that the pain tolerance threshold, the point at which pain becomes unbearable, varies significantly among different people and species.
Two types of pain, the fast pain which is acute, well localized, short duration, and in thin myelinated fiber, while the slow pain is chronic throbbing, localized long, normal duration, and in unmyelinated fiber.
Pain process;
The pain process is made up of four steps (1) transduction, (2) transmission, (3) modulation, and (4) perception
1-Transduction:
Transduction is a process of converting noxious stimulus to action potential. As it refers to the processes by which tissue-damaging stimuli activate nerve endings. The nociceptors equal to pain receptors, they are capable of transducing and encoding noxious stimuli, free nerve ending, and they can respond to one stimulus, many stimulus (called poly modal), or not responding for any stimulus (called silent). So Transduction begins when peripheral terminals of nociceptive C fibers and A-delta(Aδ) fibers are depolarized. Normally, nociceptor terminals have a high activation threshold. They requiring intense stimulation to generate an action potential. Four types of stimuli can activate pain receptors in peripheral tissues: mechanical, heat, cold, and chemical. Mechanical and heat stimuli are usually short, whereas chemical stimuli are usually long lasting. Nothing yet is known about how these stimuli activate nociceptors.
As each of these stimuli have different receptors:
- Heat – VR1, VRL, TRPV1receptors
- Cold –TRPM8, TRPA1 receptors, KCNK family
- Mechanical – ATP release, other possible receptor candidates
- Chemical – TRPV1, TRPM8, TRPA1 receptors
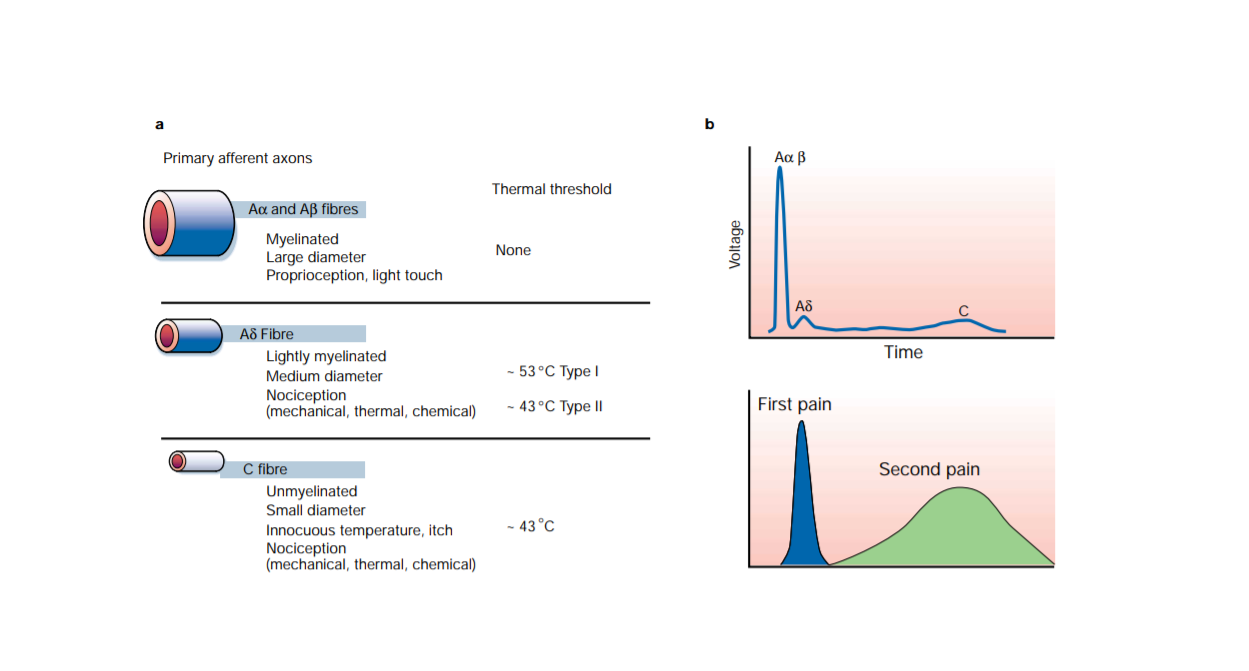
Figure 1 Shows different nociceptors detect different types of pain. (a) Peripheral nerves include small-diameter (Aδ) and medium- to large-diameter (Aδ,B) myelinated afferent fibers, as well as small-diameter unmyelinated afferent fibers (C). (b) shows the fact that conduction velocity is directly related to fiber diameter is highlighted in the compound action potential recording from a peripheral nerve. (This Diagram taken from book: Pain. By Howard L. Fields, MD, PhD New York, McGraw‐Hill, 1987, and it was used also in Molecular mechanisms of nociception Review Article)
2-Transmissiom:
Pain impulses are transmitted by two fiber systems. The presence of two pain pathways explains the existence of two components of pain: fast by Aδ fibers; and a duller slower (second pain) which is conducted by C fibers. Aδ fibers are myelinated, 2 – 5 µm in diameter and conduct at rates of 12 – 30 m/s, whereas C fibers are unmyelinated, 0.4 – 1.2 µm in diameter and conduct at rates of 0.5 to 2 m/s. As the nociceptive message is transmitted from the periphery to the central nervous system by the axon of the primary afferent nociceptor. This neuron has its cell body in the dorsal root ganglion and a long process, the axon, that divides and sends one branch out to the periphery and one into the spinal cord. There are four physiological mechanisms have been proposed to explain referred pain:
- Activity in sympathetic nerves,
- Peripheral branching of primary afferent nociceptors,
- Convergence projection,
- Convergence facilitation,
Transmission of the pain signal effect of voltage gated ion channels and the pharmacological relevance:
- Sodium gated ion channels (TTX (tetrodotoxin)) sensitive,
- TTX insensitive) Calcium gated ion channels (P/Q, T and N type)
- Potassium gated ion channels
3-Modulation:
Modulation is one of the most important discoveries in pain research was that the brain contains substances that have the same pharmacological properties as plant-derived opiates and synthetic opioid drugs. These substances, called endogenous opioid peptides, are present within nerve cells of the peripheral and central nervous systems (Palkovits, 1984).The opioid peptides modulate nociceptive input in two ways: (1) block neurotransmitter release by inhibiting Ca2+ influx into the presynaptic terminal, or (2) open potassium channels, which hyperpolarizes neurons and inhibits spike activity. They act on various receptors in the brain and spinal cord three classes of opioid receptors have been identified: μ-mu, δ-delta and κ-kappa. So it can be divided to Peripheral or Central modulation. Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, or in supra spinal structures. This modulation can either inhibit or facilitate pain.
The ascending pain pathway refers to the path of the pain signal from the site of stimulus towards the spinal cord via primary axon fibres, and then consequently the brain via secondary axon fibres in the spinothalmic tract. The neurotransmitter in this synapse is substance P. The spinothalmic tract terminates at the thalamus. The thalamus sends on the signal through tertiary axons to the area of the somatosensory cortex corresponding to the affected body part, where the pain is perceived. The descending pain pathway is responsible for modulation of the ascending pathway. Modulation refers to the processing of the pain signal, and will attenuate or strengthen the effects that are consciously experienced due to the stimulus. In central modulation there are several areas of the brain that are implicated in pain modulation, some responsive to opioids, and some apparently responsive to the “placebo or nocebo effect”. These include among them the amygdala, the thalamus, the periaqueductal gray and the nucleus raphe magus. Periaqueductal gray lines the mesencephalic aquaduct between the third and fourth ventricles, and is responsible for modulation of the descending pain signal, as well as fear and some autonomous responses. The amygdala is part of the limbic system controlling emotions, and the thalamus is a relay station for the sensory pathways of the nervous system, which enables focusing on relevant or imminent information. The pain experienced can be altered by emotional state, expectation, and level of cognition/excitement (Ossipov et al. 2010). The nucleus raphe magnus sends axons to the dorsal horn of the spinal cord to provide inhibition to the synapse of the ascending pathway. It also stimulates opioid releasing interneurons, which inhibit the release of substance P neurotransmitter.
4-Perception:
Perception can be defined as central processing of nociceptive impulses in order to interpret pain.In the perception neurons are located in the thalamus and project to somatosensory areas II and I in the post-central gyrus and superior wall of the sylvian fissure. Perception and discrete localization of pain take place in these cortical areas. Some fibres project to the anterior cingulated gyrus and are likely to mediate the suffering and emotional components of pain. Perception of pain is the end result of the neuronal activity of pain transmission and where pain becomes a conscious multidimensional experience. The multidimensional experience of pain has affective-motivational, sensory-discriminative, emotional and behavioural components. So as a summary for the above four steps; for an animal to experience pain, nociceptive information must be sent to higher centers in the CNS to be integrated, modulated, and interpreted into the conscious perception of pain. Noxious stimuli (heat, cold, mechanical, chemical) activate free sensory nerve endings known as nociceptors. A-δ and C-fibers transmit sensory information from nociceptors to the dorsal horn of the spinal cord, which directs and modulates input from the periphery and higher centers. Nociceptive information arriving in the dorsal horn of the spinal cord may activate motor neurons responsible for the reflex responses to noxious stimuli.
{{attachment:image5.png|alt|width="1000"}}
The General Pain Pathway
Within the pain pathway there are 3 orders of neurons which carry action potentials:
First order → The pseudo unipolar neurons which have cells bodies within the dorsal root ganglion, they have one axon which splits into two branches, a peripheral and a central branch.
Second order → The cell bodies of these neurons are found in the Rexed laminae of the spinal cord so decussate in the anterior white commissure and ascend cranially in the spinothalamic tract.
Third order → The cell bodies of third order neurons lie within the VPL of the thalamus. They project via the posterior limb of the internal capsule to terminate in the ipsilateral postcentral gyrus.
Types of Pain
Physicians and neuroscientists generally classify pain in the following ways:
►Acute pain
Acute pain is caused by an injury to the body. It warns of potential damage that requires action by the brain, and it can develop slowly or quickly it can last for a few minutes to six months and goes away when the injury heals.Common causes of acute pain include:broken bones / surgery / burns ...
►Chronic pain
This type persists long after the trauma has healed and in many cases, it occurs in the absence of any trauma. Chronic pain does not warn the body to respond, and it usually lasts longer than six months. Some common examples of chronic pain include: nerve damage pain / vertebrates pain / arthritis pain ...
►Cancer pain
Or it can be named as malignant this pain is associated with malignant tumors. Tumors invade healthy tissues and exert pressure on nerves or blood vessels, producing pain. Cancer pain can also be associated with invasive procedures or treatments and note that some neuroscientists classify cancer pain with chronic pain.
►Neuropathic pain
This pain results from damage to a nerve or some other part of the central nervous system. This type of pain is not frequently diagnosed in veterinary medicine, mainly because animals cannot communicate a problem such as a tingling sensation.
Michael H. Ossipov, Gregory O. Dussor, Frank Porreca. (2010). Central modulation of pain. The Journal of Clinical Investigation. 120 (11), 3779-3787.
https://www.physio-pedia.com/Pain_Mechanisms
https://teachmephysiology.com/nervous-system/sensory-system/pain-pathways/
