|
Size: 25703
Comment: 500 internal server error
|
Size: 25712
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 94: | Line 94: |
| Sodium gated Ion Channels | __Voltage Gated Sodium Channels __ |
Itt írjon a(z) Pain_physiology-ról/ről
Physiology Of Pain
Project by:
Supervisor Dr. Zoltan Balazs Barany
Physiology Department, University of Veterinary Medicine, Budapest
Contents
What is pain?
Pain is defined by the International Association for the Study of Pain (IASP) as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage". Therefore it holds functions in creating awareness of, and behaviours and reactions to avoid or terminate, stimuli which can cause harm to the organism. The sensory and emotional aspects go to make up the subjective experience of pain, creating a strong and lasting learnt avoidance reaction against similar stimuli that may be encountered in the future. This involves processing of the novel sensory input with memories and established emotional patterns.
The nerve endings sensing painful stimuli are distinct from those which detect other sensations, such as light touch. (Julius, Basbaum 2001). These nerve endings lead to specific primary sensory afferent nerve fibres termed nociceptors, which transmit the stimulus to the dorsal horn of the spinal cord. Here it will synapse to a secondary afferent neuron in the ascending pathway to the brain. The detection of pain itself is termed Nociception, and can be classed as a sense similar to vision or olfaction due to its selectivity for stimuli, in this case those that are harmful not benign (Julius, Basbaum 2001).
There are two types of pain occurring in response to injury. The first is acute, fast pain which is well localised, the second is slow pain, which tends to be dull with a longer duration, and to be less well localised. In response to acute injury there will first be acute first pain indicating the location of injury, and a need to remove or avoid the damaging stimulus. This will be followed by the second pain, which has a function in changing behaviour, such as inducing cleaning behaviours such as licking, and avoiding pressure or impact which could affect the healing and recovery of the injured area.
Pain process;
The pain process is made up of four steps (1) transduction, (2) transmission, (3) modulation, and (4) perception
|
1-Transduction:
Transduction is a process of converting noxious stimuli to a neuronal signal. Receptors for noxious stimuli can be generally categorized as cation channels which de-polarise the peripheral terminals of nociceptors, creating an action potential (Du, Gamper, 2013). Nociceptor terminals may require intense stimulation to generate the action potential, meaning that the threshold potential that must be reached is high. There are several types of noxious stimuli that elicit a pain reaction in the peripheral tissues. These include heat, cold, mechanical, and chemical. Mechanical, heat and cold stimuli are generally short lasting, but chemical stimuli last longer and can also represent the reactions within the tissues to the injury, such as inflammation, contributing to the slow-type second pain.
►Heat
Studies on the sensing of heat induced pain have suggested several specific receptors that may be involved in initiating the pain pathways.
TRPV1,2,4 - transient receptor potential TRP – Vanilloid receptors. These are receptive to vanilloid compounds such as Capsaicin. Capsaicin is the compound found in chilli peppers that activates the same receptors as high temperatures, leading to “heat”. These receptors act as a plasma membrane channel for cations that are only activated above certain temperatures. The temperature varies based on the specific receptor type (Julius, Basbaum 2009), (Basbaum et al. 2009).
VRL1 - Vanilloid receptor like channels. These are similar to vanilloid receptor channels but don’t respond to vanilloid compounds, only to heat. The temperature of activation is 52°C (Julius, Basbaum 2009).
►Cold
The receptors and afferents involved in nociception of cold stimuli are different and less numerous than those implicated in the detection of heat (Julius, Basbaum 2009). Menthol and eucalyptol are chemicals used in the detection of cold based pain, as they contain similar properties.
TRPM8 – Transient receptor potential (Menthol) - a channel that is sensitive to both cold and menthol.
TRPA1 – Transient receptor potential that may detect cold below 15°C. This channel is sensitive to icilin and menthol at high concentrations.
KCNK – a family of voltage gated K+ channels, including KCNK2 (TREK-1) and KCNK4 (TRAAK) which have some function in nociception, including possible response to cold (Basbaum et al. 2009).
►Mechanical
Mechanical pain can be due to such stimuli as intense pressure, deformation, destruction of tissue, or swelling. There are several candidate mechanisms and receptors which may be responsible for mechanical nociception. These include mechanosensitive (channels opened by cell membrane deformation) and mechanochemical (releasing chemical messenger in response to stretching or deformation)(Julius, Basbaum 2001).
Another possible way that mechanical nociception can occur is through the release of ATP through a damaged or distorted cell membrane, which when existing extracellularly can function in ATP gated ion channels, or G-protein coupled ATP receptors, leading to the activation of surrounding nociceptor terminals in the tissues (Julius, Basbaum 2001).
Other unproven but possible receptor mechanotransducers include:
ASIC1,2,3 – Acid sensing ion channels. These are expressed in mechanosensitive neurons but their role in nociception hasn’t been proven.(Basbaum et al 2009)
TRPV2,4 – These are heat/vanilloid receptors which can also respond to stretching stimuli.(Basbaum et al 2009)
KNCK2,4 – these are also implicated in cold nociception but may have a mechanoreceptor role too. KNCK18 responds to compounds in Szechuan peppercorns which cause tingling sensations, but beyond this mechanoreceptive response to noxious mechanical stimuli hasn’t been proven. (Basbaum et al 2009).
Piezo componants
►Chemical
Chemical nociception takes place as a result of environmental irritants or physiological stress (Basbaum et al 2009). Some receptors for plant derived chemical irritants have already been mentioned in the context of temperature based nociception, for example TRPV1 (capsaicin), and TRPM8 (menthol).
Physiological stress can involve the chemical factors involved in inflammation, of which they or their precursors are released by cells in response to tissue damage, and are known collectively as “inflammatory soup”. Components of the inflammatory soup include H+ ions K+ ions, histamines, ATP and serotonin which affect neuronal excitability, and Bradykinin, prostaglandins and nerve growth factors/neurotrophins which bind to receptors which are metabotropic (leading to second messenger cascades) (Julius, Basbaum 2001). One of the effects of these chemicals is to create a long lasting secondary pain sensation, leading to behavioural protection of the healing tissues.
2-Transmission:
|
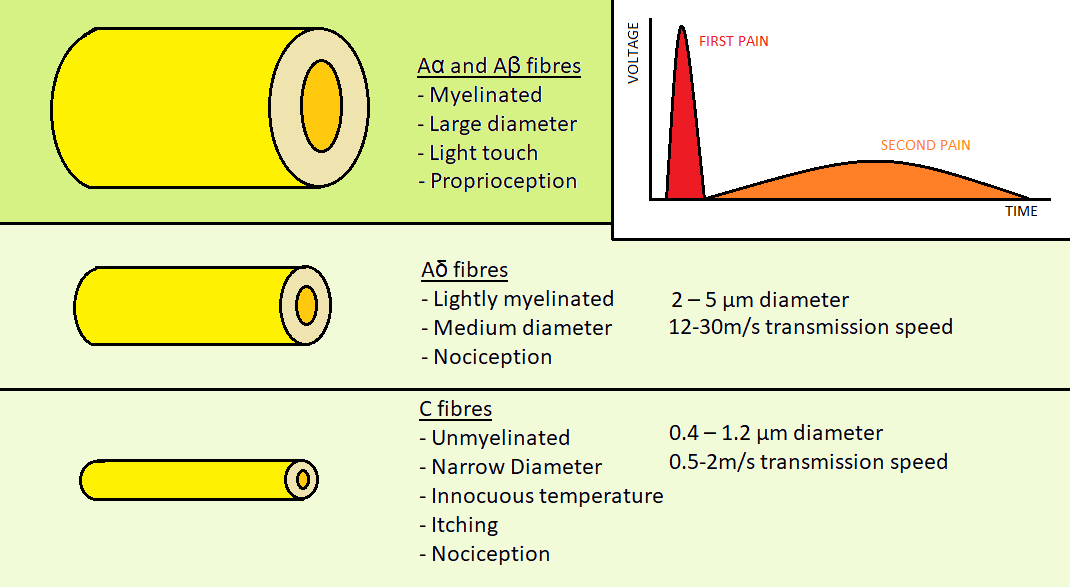
After stimulation of the peripheral nerve ending a depolarisation will occur in the nociceptor axon. As previously mentioned there are nociceptor fibres for acute first pain, and others for slower, dull second pain. There are four different types of primary afferent fibres for skin sensation, two of which are involved in nociception. Aα and Aβ fibres are myelinated fibres with a large diameter that are involved in transmission of stimuli from light, non-painful touch.
The two fibres involved in nociception are the Aδ and C type fibres.
Aδ fibres are the fibres which transmit the acute first pain. They are myelinated for the sending of rapid impulses and thinner than non-nociceptor fibres, at 2 – 5 µm in diameter. The rate of conduction s 12-30m/s.(David Julius & Allan I. Basbaum - 2000)
C type fibres are unmyelinated. They are the thinnest primary afferents at 0.4 – 1.2 µm in diameter. The rate of conduction is 0.5-2m/s, significantly slower than the Aδ fibres.(David Julius & Allan I. Basbaum - 2000)
Laminae of dorsal horn
The primary afferent nociceptors are defined as pseudo-unipolar, as they feature central and peripheral terminals, and can receive analgesics at both ends (Basbaum et al 2009). The soma, or cell body of the sensory neuron is located in the dorsal root ganglion of the spinal cord for innervation of the body, and the axon projects a branch to the periphery and a branch into the spinal cord. For facial innervation the soma of the neuron cell is located at trigeminal gamglia (Julius, Basbaum 2001).
The main neurotransmitter chemicals that are responsible for stimulating nerve terminals and bridging synapses include Glutamate and Substance P. Glutamate receptors are widely present in the body while Substance P is very specific to nociceptive signalling (Zieglgänsberger, 2019).
Transmission of the pain signal along the fibres occurs through the movement of ions through voltage gated ion channels, generating action potentials:
Voltage Gated Sodium Channels
There are two classes of Sodium ion channels: tetrodotoxin (TTX) sensitive, or tetrodotoxin resistant. Tetrodotoxin is a toxin present in some species of marine fish which specifically blocks the sodium ion channels, and has been used to differentiate channel types. The TTX resistant channels NAV1.8 an NAV1.9 are involved in nociception. NAV1.3 is TTX sensitive and has been suggested to sustain high frequency action potentials in chronic pain, due to faster recovery from stimulation.
Voltage Gated Potassium Channels
Potassium channels when open maintain the normal excitability of neurons and are believed to spontaneously induce pain when blocked (Du, Gamper, 2013).
Voltage Gated Calcium Channels
Like sodium channels, calcium channels are classified based on their sensitivity to pharmacological agents, being subdivided into L, N, P/Q, R, T type channels. All are involved in nociception, with the N-type channels playing a prominent role. These channels are active in the dorsal root ganglion and in synapses, where they are stimulated by action potentials to assist the release of vesicles into the synaptic cleft. (Park, Luo, 2010)
As there are four physiological mechanisms have been proposed to explain referred pain which are: (M Osterweis - 1987):
- Activity in sympathetic nerves,
- Peripheral branching of primary afferent nociceptors,
- Convergence projection,
- Convergence facilitation,
3-Modulation:
|
Modulation is one of the most important discoveries in pain research was that the brain contains substances that have the same pharmacological properties as plant-derived opiates and synthetic opioid drugs. These substances, called endogenous opioid peptides, are present within nerve cells of the peripheral and central nervous systems (Palkovits, 1984).The opioid peptides modulate nociceptive input in two ways: (1) block neurotransmitter release by inhibiting Ca2+ influx into the presynaptic terminal, or (2) open potassium channels, which hyperpolarizes neurons and inhibits spike activity. They act on various receptors in the brain and spinal cord three classes of opioid receptors have been identified: μ-mu, δ-delta and κ-kappa. So it can be divided to Peripheral or Central modulation. Modulation of pain occurs peripherally at the nociceptor, in the spinal cord, or in supra spinal structures. This modulation can either inhibit or facilitate pain (M Osterweis - 1987).
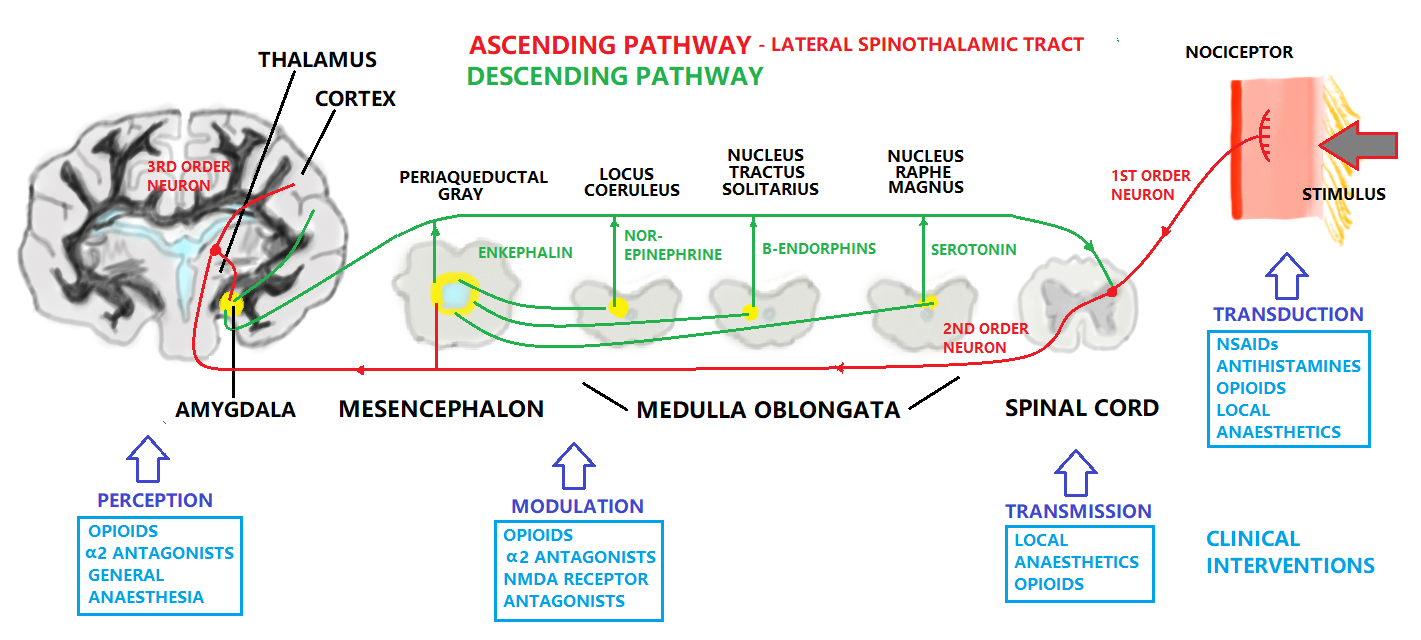
The ascending pain pathway refers to the path of the pain signal from the site of stimulus towards the spinal cord via primary axon fibres, and then consequently the brain via secondary axon fibres in the spinothalmic tract. The spinothalmic tract terminates at the thalamus. The thalamus sends on the signal through tertiary axons to the area of the somatosensory cortex corresponding to the affected body part, where the pain is perceived. The descending pain pathway is responsible for modulation of the ascending pathway. Modulation refers to the processing of the pain signal, and will attenuate or strengthen the effects that are consciously experienced due to the stimulus. In central modulation there are several areas of the brain that are implicated in pain modulation, some responsive to opioids, and some apparently responsive to the “placebo or nocebo effect”. These include among them the amygdala, the thalamus, the periaqueductal gray and the nucleus raphe magus. Periaqueductal gray lines the mesencephalic aquaduct between the third and fourth ventricles, and is responsible for modulation of the descending pain signal, as well as fear and some autonomous responses. The amygdala is part of the limbic system controlling emotions, and the thalamus is a relay station for the sensory pathways of the nervous system, which enables focusing on relevant or imminent information. The pain experienced can be altered by emotional state, expectation, and level of cognition/excitement (Ossipov et 2010). The nucleus raphe magnus sends axons to the dorsal horn of the spinal cord to provide inhibition to the synapse of the ascending pathway. It also stimulates opioid releasing interneurons, which inhibit the release of substance P neurotransmitter.
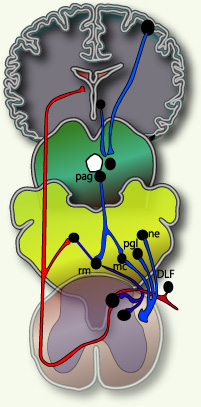
Fields and Basbaum (1984) proposed a diagram of the structural components of the descending opioid-related pain inhibitory system (Figure 3). Generally, findings indicate that several centers are involved in generating analgesia, three of which have received extensive investigation, the periventricular and periaqueductal grey, the rostral ventral medulla, and the spinal cord.Afferent input to the descending pain modulating system is less well known, but certainly hypothalamic and amygdala inputs are involved and possibly the frontal granular and insular cortex. So this figure shows descending pain modulation neurons form a network that extends from the fontal cortex and hypothalamus down through the periaqueductal grey (PGA) to the rostral ventral medulla and then down to the spinal dorsal horn.(rm=nucleus rahe magnus; mc=raticularis magnocellularis; pgl=paragigantocellularis; ne=noradrenergic pontomedullary cell groups; DLF=dorsolateral funiculus).(Fields and Basbaum - 1984)
4-Perception:
In the brain, nociceptive input is perceived as pain. New data suggest that there is no single, precise location where pain perception occurs so instead pain perception involves several brain structures.It is known that the brain is necessary for pain perception hence no brain, no pain. Until it is understood clearly where pain is perceived, prudent nursing practice involves treatment of any noxious stimulus as potentially painful, even in the comatose individual who does not respond to noxious stimuli. Lack of a behavioral response to a noxious stimulus does not indicate that their is lack of pain perception.(M Osterweis - 1987) Moreover perception can be defined as central processing of nociceptive impulses in order to interpret pain.In the perception neurons are located in the thalamus and project to somatosensory areas II and I in the post-central gyrus and superior wall of the sylvian fissure. Perception and discrete localization of pain take place in these cortical areas. Some fibres project to the anterior cingulated gyrus and are likely to mediate the suffering and emotional components of pain. Also it is the end result of the neuronal activity of pain transmission and where pain becomes a conscious multidimensional experience. The multidimensional experience of pain has affective-motivational, sensory-discriminative, emotional and behavioural components.(M Osterweis - 1987)
So as a summary for the above four steps; for an animal to experience pain, nociceptive information must be sent to higher centers in the CNS to be integrated, modulated, and interpreted into the conscious perception of pain. Noxious stimuli (heat, cold, mechanical, chemical) activate free sensory nerve endings known as nociceptors. A-δ and C-fibers transmit sensory information from nociceptors to the dorsal horn of the spinal cord, which directs and modulates input from the periphery and higher centers. Nociceptive information arriving in the dorsal horn of the spinal cord may activate motor neurons responsible for the reflex responses to noxious stimuli.
The General Pain Pathway
Within the pain pathway there are 3 orders of neurons which carry action potentials:
First order → The pseudo unipolar neurons which have cells bodies within the dorsal root ganglion, they have one axon which splits into two branches, a peripheral and a central branch.
Second order → The cell bodies of these neurons are found in the Rexed laminae of the spinal cord so decussate in the anterior white commissure and ascend cranially in the spinothalamic tract.
Third order → The cell bodies of third order neurons lie within the VPL of the thalamus. They project via the posterior limb of the internal capsule to terminate in the ipsilateral postcentral gyrus.
Pain Terminology
The pain Terminology can be classified to:(IASP)
⇒Analgesia: occurs when there is lack of pain sensation(IASP)
⇒Anesthesia: occurs when there is lack to any sensation(IASP)
⇒Allodynia: when the pain due to non-harmful stimulus(IASP)
⇒Hyperalgesia: increased response to harmful stimulus(IASP)
⇒Neuralgia: Painful site innervated by a nerve or group of nerves.(IASP)
Types of Pain
Physicians and neuroscientists generally classify pain in the following ways:
►Acute pain
Acute pain is caused by an injury to the body. It warns of potential damage that requires action by the brain, and it can develop slowly or quickly and goes away when the injury heals.Common causes of acute pain include:broken bones / surgery / burns...(Brigitta Brandne 2010)
►Chronic pain
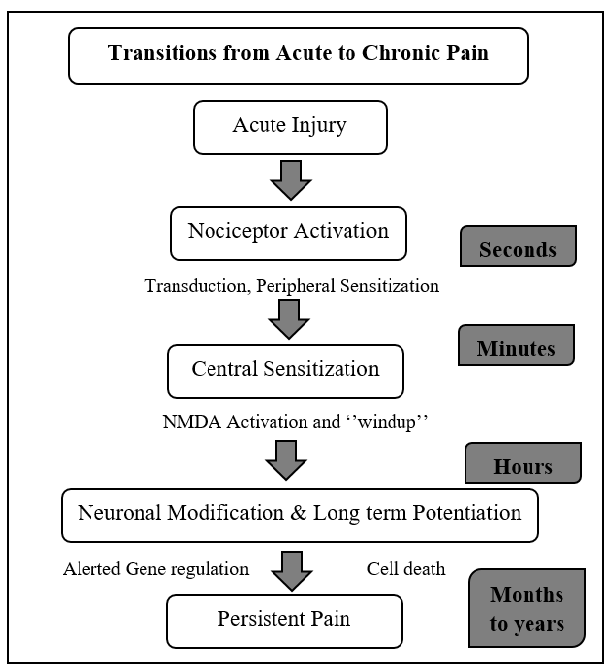
This type persists long after the trauma has healed and in many cases, it occurs in the absence of any trauma, it does not warn the body to respond, and it usually lasts longer than acute pain. Some common examples of chronic pain include: nerve damage pain / vertebrates pain / arthritis pain … Several reasons for the occurrence of chronic pain have been proposed. These include spontaneous inflammatory pain arising from damaged receptors, spinal changes in plasticity due to prolonged pain experiences, and damaged opioid systems (Curatolo et al. 2006)
|
|||
Type |
Duration |
Characteristics |
Management |
Acute Pain |
<3 months |
Severe, but usually manageable such pain from injuries |
by anesthesiologist pain, + opioids |
Chronic Pain |
>3-6 months |
Difficult to treat, drug-seeking |
by analgesics, antidepressants + anticonvulsants |
Table 1 Comparison between Acute & Chronic pain |
|||
Transmission from Acute to Chronic Pain
|
►Cancer pain
Or it can be named as malignant this pain is associated with malignant tumors. Tumors invade healthy tissues and exert pressure on nerves or blood vessels, producing pain. Cancer pain can also be associated with invasive procedures or treatments and note that some neuroscientists classify cancer pain with chronic pain.(Curatolo et al. 2006)
►Nociceptive pain
This is the pain detected by nociceptors around the body. There are three types of nociceptive pain (AE Dubin - 2010) :
Radicular pain occurs when the nerve roots are irritated. It goes on forlimb or hindlimb through a nerve that comes from the the spinal cord.(AE Dubin - 2010)
Somatic pain occurs when any of the pain receptors in tissues, such as muscles, bone, or skin, are activated. This type of pain is often stimulated by movement.(AE Dubin - 2010)
Visceral pain occur in internal organs, such as involuntary muscles in the heart it usually described as aching. (AE Dubin - 2010). It utilises similar nociceptors to second pain, and as such tends to be dull and poorly localized.
|
|||
Type |
Characteristics |
Mechanism |
Pharmacologic Treatments |
Radicular Pain |
localized & diffuse pain |
Associated with localized inflammation |
NSAID, steroid, opioids |
Somatic Pain |
Superficial or deep pain |
Mechanical, thermal, or chemical stimuli |
NSAID, antispasmodics |
Visceral Pain |
Constant & poorly localized |
Visceral distension |
NSAID, steroid |
Table 2 Comparison between Nociceptive pain types |
|||
►Neuropathic pain
This pain results from damage to a nerve or some other part of the central nervous system, and its not frequently diagnosed in veterinary medicine, mainly because animals cannot communicate a problem such as a tingling sensation.(Kimberly Holland)
References:
Articles:
https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698
https://www.physio-pedia.com/Pain_Mechanisms
https://teachmephysiology.com/nervous-system/sensory-system/pain-pathways/
https://www.merckvetmanual.com/SearchResults?query=pain
https://www.healthline.com/health/nociceptive-pain
https://www.healthline.com/health/somatic-vs-visceral-pain#overview1
https://www.healthline.com/health/pain-relief/oxycodone-vs-hydrocodone
http://www.allcare.org/CancerPain-and-SymptomManagement/comfort/cfm2/cfm2_cont.htm#perception
https://www.ncbi.nlm.nih.gov/pubmed/8275913
https://teachmephysiology.com/nervous-system/sensory-system/pain-pathways/
https://www.webmd.com/pain-management/glossary-pain-management
David Julius, Allan I. Basbaum. (2001). Molecular mechanisms of nociception. NATURE. 413, 203-210.
Allan I. Basbaum, Diana M. Bautista, Grégory Scherrer, David Julius. (2009). Cellular and Molecular Mechanisms of Pain. CELL. 139 (2), 267–284.
Michael H. Ossipov, Gregory O. Dussor, Frank Porreca. (2010). Central modulation of pain. The Journal of Clinical Investigation. 120 (11), 3779-3787.
Michele Curatolo, Lars Arendt-Nielsen, Steen Petersen-Felix. (2006). Central Hypersensitivity in Chronic Pain: Mechanisms and Clinical Implications. Physical Medicine Rehabilitation Clinic of North America. 17, 287–302.
Casey, L., Minoshima, S., Morrow, T. J., & Koeppe, R. A. (1996). Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. Journal of Neurophysiology, 76, 571-581.
W. Zieglgänsberger. (2019). Substance P and pain chronicity. Cell and Tissue Research. 375 (1), 227–241.
Chong Hyun Lee, Peter C. Ruben. (2008). Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels. 2 (6), 407-412.
John F Park, Z David Luo. (2010). Calcium channel functions in pain processing. Channels. 4 (6), 510-517.
Xiaona Du, Nikita Gamper. (2013). Potassium Channels in Peripheral Pain Pathways: Expression, Function and Therapeutic Potential. Current Neuropharmacology. 11 (6), 621–640.
IASP Newsletter, 3, 3-5. Jones, A. K. P. (1997). Pain, its perception, and pain imaging.
https://Fields, H. L., & Basbaum, A. L. (1994). Central nervous system mechanisms of pain modulation. In M. R. Wall PD (Ed.), Textbook of pain (3rd ed. ed., pp. 243-257). New York: Churchill Livingstone
Figures:
♦Figure 1: Hand drawn figure
♦Figure 2:from Fields, H. L. Pain (McGraw-Hill, New York, 1987) as it was used in many review articles eg Molecular mechanisms of nociception by David Julius
♦Figure3: from 2001 D.J. Wilkie Book (pain)