|
Size: 39375
Comment:
|
Size: 39601
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 270: | Line 270: |
| The physiological parameters for animals when they feel pain can be altered as an autonomic response to the nociception. However, these parameters are affected by many factors as fear and stress [[#Adolfsson|(Cambridge AJ;Franzini de Souza CC -2000)]]. In the case of animals that suffer chronic pain, these variations tend to be less evident [[#Mckume|(McKune CM)]]. The Biological parameters can include the measurement of chemical mediators and the hormonal concentrations. For example the changes in plasma hormone concentrations like cortisol, β-endorphins and catecholamines, which have been considered as indirect indicators of pain. Only weak correlations of behavior and increases in plasma levels of the mentioned hormones have been determined [[#Guti.2BAOk-rrer|(Gutiérrez BJ-2017)]] more over, it is well-known that the relation among physiological stress, behavioral disorders and pain is complex; therefore endocrine measures can reflect stress responses that may not always be related directly to pain. '' | The physiological parameters for animals when they feel pain can be altered as an autonomic response to the nociception. However, these parameters are affected by many factors as fear and stress [[#Adolfsson|(Cambridge AJ;Franzini de Souza CC -2000)]]. In the case of animals that suffer chronic pain, these variations tend to be less evident [[#Mckume|(McKune CM)]]. The Biological parameters can include the measurement of chemical mediators and the hormonal concentrations. For example the changes in plasma hormone concentrations like cortisol, β-endorphins and catecholamines, which have been considered as indirect indicators of pain. Only weak correlations of behavior and increases in plasma levels of the mentioned hormones have been determined [[#Guti.2BAOk-rrer|(Gutiérrez BJ-2017)]] more over, it is well-known that the relation among physiological stress, behavioral disorders and pain is complex; therefore endocrine measures can reflect stress responses that may not always be related directly to pain. |
| Line 347: | Line 347: |
Claire Bloor. (2017). Pain scoring systems in the canine and feline patient . Available: https://www.theveterinarynurse.com/review/article/pain-scoring-systems-in-the-canine-and-feline-patient. Last accessed 18 April 2020. |
Itt írjon a(z) Pain_physiology-ról/ről
Physiology Of Pain
Project by: Jad El Hawly, Alice Taube, Oscar Joseph Ullomi
Supervisor: Dr. Zoltan Balazs Barany
Physiology Department, University of Veterinary Medicine, Budapest
Contents
What is pain?
Pain is defined by the International Association for the Study of Pain (IASP) as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage". Therefore it holds functions in creating awareness of, and behaviours and reactions to avoid or terminate, stimuli which can cause harm to the organism. The sensory and emotional aspects go to make up the subjective experience of pain, creating a strong and lasting learnt avoidance reaction against similar stimuli that may be encountered in the future. This involves processing of the novel sensory input with memories and established emotional patterns.
The nerve endings sensing painful stimuli are distinct from those which detect other sensations, such as light touch. (Julius, Basbaum 2001). These nerve endings lead to specific primary sensory afferent nerve fibres termed nociceptors, which transmit the stimulus to the dorsal horn of the spinal cord. Here it will synapse to a secondary afferent neuron in the ascending pathway to the brain. The detection of pain itself is termed Nociception, and can be classed as a sense similar to vision or olfaction due to its selectivity for stimuli, in this case those that are harmful not benign (Julius, Basbaum 2001).
There are two types of pain occurring in response to injury. The first is acute, fast pain which is well localised, the second is slow pain, which tends to be dull with a longer duration, and to be less well localised. In response to acute injury there will first be acute first pain indicating the location of injury, and a need to remove or avoid the damaging stimulus. This will be followed by the second pain, which has a function in changing behaviour, such as inducing cleaning behaviours such as licking, and avoiding pressure or impact which could affect the healing and recovery of the injured area.
Pain process;
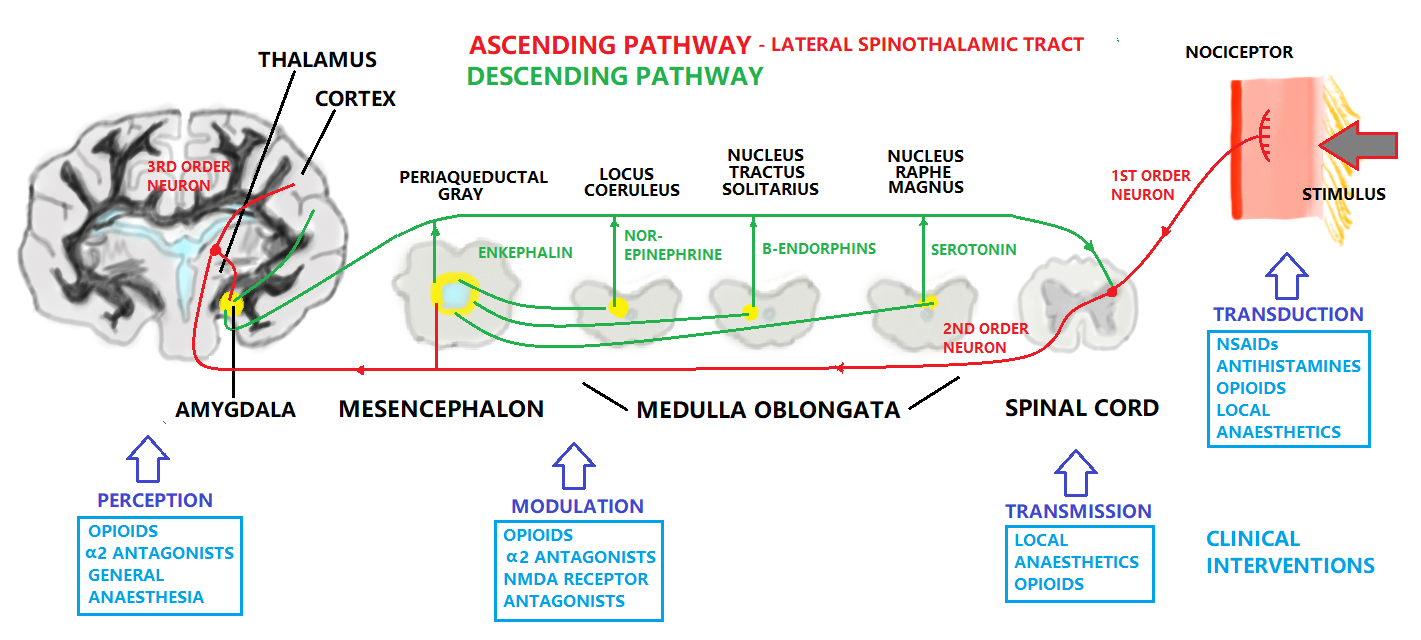
- The pain process is made up of four steps (1) transduction, (2) transmission, (3) modulation, and (4) perception
|
1-Transduction:
Transduction is a process of converting noxious stimuli to a neuronal signal. Receptors for noxious stimuli can be generally categorized as cation channels which de-polarise the peripheral terminals of nociceptors, creating an action potential (Du, Gamper, 2013). Nociceptor terminals may require intense stimulation to generate the action potential, meaning that the threshold potential that must be reached is high. There are several types of noxious stimuli that elicit a pain reaction in the peripheral tissues. These include heat, cold, mechanical, and chemical. Mechanical, heat and cold stimuli are generally short lasting, but chemical stimuli last longer and can also represent the reactions within the tissues to the injury, such as inflammation, contributing to the slow-type second pain. ►Heat ►Mechanical ⇒ ►
⇒ Voltage Gated Sodium Channels ⇒ Voltage Gated Potassium Channels ⇒ Voltage Gated Calcium Channels
The pain Terminology can be classified to:
Physicians & neuroscientists generally classify pain into different ways as the following:
2-Transmission:
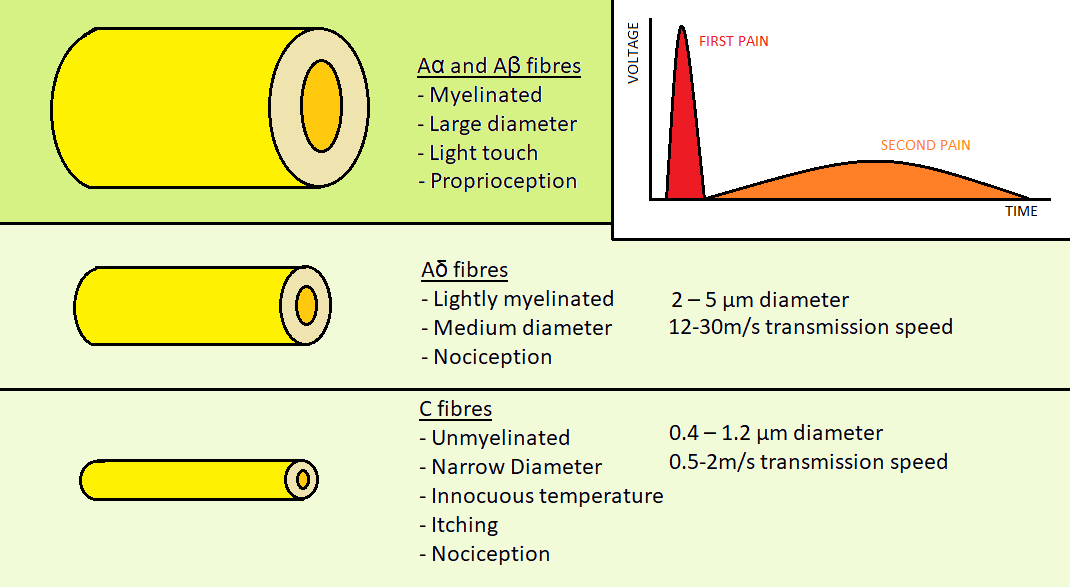
Fig 2.
Figure 2: Aα, Aβ, Aδ & C fibers 3-Modulation:
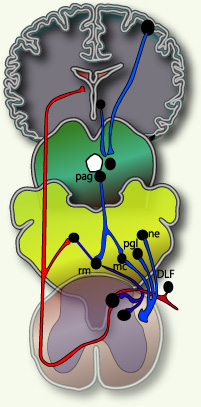
Fig 3.
Figure 3 pain modulation 4-Perception:
The General Pain Pathway
Pain Terminology
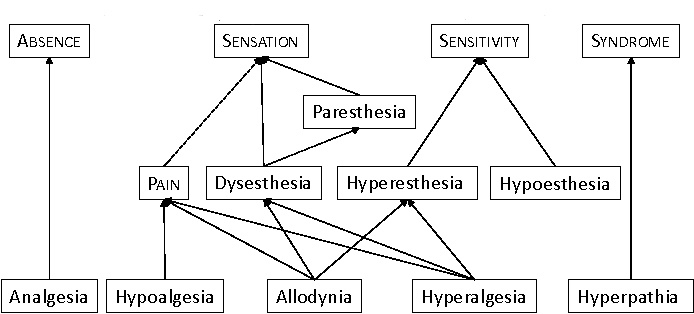
Fig 4.
Figure 4 Pain Terminology Hierachy Types of Pain
►Acute pain
Simple difintion is that acute pain is caused by an injury in the body. Acute pain syndromes have clear etiologies and respond readily to appropriate therapy. The presence of acute pain warns of potential damage that requires action by the brain, and it can develop slowly or quickly and goes away when the injury heals. They are frequently caused by tissue damage such as bone, organs, muscle, for example broken bones / surgery / burns.(Brigitta Brandne 2010) This pain is also often accompanied by acute distress and anxiety. ►Chronic pain
Chronic pain will serves less of a warning function and may become part of the patient's life. This type persists long after the trauma has healed and in many cases, it occurs in the absence of any trauma, it does not warn the body to respond, and it usually lasts longer than acute pain. Some examples that can illustrate chronic pain include: arthritis pain / nerve damage pain / vertebrates pain. Several reasons for the occurrence of chronic pain have been proposed. These include spontaneous inflammatory pain arising from damaged receptors, spinal changes in plasticity due to prolonged pain experiences, and damaged opioid systems (Curatolo et al. 2006). Chronic pain syndromes can be divided into three categories: (1) those associated with clinically significant structural disease (e.g., rheumatoid arthritis, cancer pain); (2) those associated with a history of structural disease but with psychological factors that predispose to physiologic alterations, (e.g. muscle spasm in lower back) ; (3) those with no physiologic or anatomic basis for the patient's complaints, and can be exemplified by the somatoform disorders. Tilll now the time frame for the development of chronic pain is not exact.
|
|||
Type |
Duration |
Characteristics |
Management |
Acute Pain |
<3 months |
Severe, but usually manageable such pain from injuries |
by anesthesiologist pain, + opioids |
Chronic Pain |
>3-6 months |
Difficult to treat, drug-seeking |
by analgesics, antidepressants + anticonvulsants |
Table 1 Comparison between Acute & Chronic pain |
|||
Type Characteristics Mechanism Pharmacologic Treatments Radicular Pain localized & diffuse pain Associated with localized inflammation NSAID, steroid, opioids Somatic Pain Superficial or deep pain Mechanical, thermal, or chemical stimuli NSAID, antispasmodics Visceral Pain Constant & poorly localized Visceral distension NSAID, steroid Table 2 Comparison between Nociceptive pain types
Transition from Acute to Chronic Pain
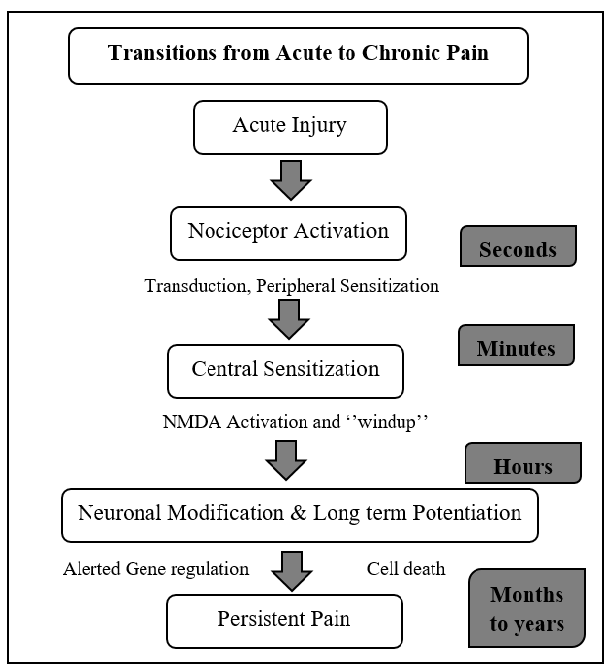
Fig 5.
Figure 5 Transition from Acute to Chronic Pain ►Nociceptive pain
This is the pain detected by nociceptors around the body. There are three types of nociceptive pain:
Radicular pain occurs only when the nerve roots are irritated. It goes on forlimb or hindlimb through a nerve that comes from the the spinal cord.
►Neuropathic pain
After disappearance of inflammation and complete healing of tissue damage, the brain may become hypersensitive to the slightest pain signals. Nociceptive pain is often associated with tissue damage where as neuropathic pain is one that involves the nervous system. Neuropathic pain involves pain pathways that have become ingrained and reinforced for a long time. This pain results from damage to a nerve or some other part of the central nervous system, and it is not frequently diagnosed in veterinary medicine, mainly because animals cannot communicate a problem such as a tingling sensation.(Kimberly Holland) ►Cancer pain
Or it can be named as malignant this pain is associated with malignant tumors. Cancer pain can also be associated with invasive procedures or treatments and note that some neuroscientists classify cancer pain with chronic pain .(Curatolo et al. 2006) ►Referred pain
Referred pain is pain that is felt on other areas of the body from where the signal originates, usually a peripheral pain sensation indicating a visceral origin. These tend to be consistently corresponding defined areas known as "head zones". Referred pain can be misleading but with understanding of the different head zones can be utilised in the diagnoses of illnesses, a common example would be pain in the chest and left arm, indicating pathologies of the heart in humans. There are four physiological mechanisms have been proposed to explain referred pain (M Osterweis, 1987):
Activity in sympathetic nerves - Sympathetic nerve projections can sensitize peripheral nociceptor terminals as part of the sympathetic response. Clinical aspects of Pain, and Therapeutic Methods
|
|||
Pharmacological interventons |
Methods of Interventions |
Non-pharmacological interventions |
Rehabilitation |
NSAIDs, Opioids |
Local anaesthesia, injections |
Acupuncture, massage, music therapy |
Physical therapy, Music therapy |
Table 3. Clinical interventions for pain |
|||
Pain Scoring
In human medicine, pain determination is based on self-reporting. Veterinary patients however are non-verbal and in order for the practitioner to determine the presence and severity of pain there are some scoring systems used in practice. There is not currently a universally accepted scoring system, as it is difficult to achieve complete objectivity. One type of scoring system commonly implemented is the Simple Scoring System. This attributes a numerical value to a range which spans from the absence of pain to extremely severe debilitating pain. A similar type of scoring system is the Visual Analogue Scale which is non-numerical, but allows the practitioner to assign a degree on a scale of severity. The scoring will be assigned to the state of the animal based on the presence or absence of several parameters which vary according to the species (Bloor, 2017).
There can be inferred a motor response to pain, causing complex behaviors that are different in each species. For this reason, behavioral changes have been recognized as indicators of pain in all the animals, where this unpleasant emotional and sensory experience generates changes in the behavior, showing location and severity of where the pain is .(León-Olea M-2002). Some examples of behavioral changes that must be taken in consideration for animals are self multilation, aggression, vocalizations, restlesssness, changes in social interaction, sleep alteration. A good knowledge of the species normal behaviour is required in order to assign the pain score, and in some cases a behavioral history of the patient will also need to be considered. Some species hide pain extremely well until it becomes critical, for example rabbits and ferrets.
Some examples of the parameters indicating pain are:
⇒ Facial expression, such as squeezed eyes and open mouth. Facial “grimace” scales are often used in research.
⇒ Body movement and posture, such as tucking of abdomen, clenching of digits, limping.
⇒ Response to pressure on different parts of the body, conducted by palpitation.
⇒ Vocalisation, degree and type such as crying and hissing.
⇒ Behaviour, such as restlessness, excessive sleeping, irritability.
⇒ Physiological markers, such as heart rate, blood pressure, breathing, pulillary diameter.
⇒ Biological markers, such as cortisol and adrenaline in body secretions. (Bloor, 2017)
The physiological parameters for animals when they feel pain can be altered as an autonomic response to the nociception. However, these parameters are affected by many factors as fear and stress (Cambridge AJ;Franzini de Souza CC -2000). In the case of animals that suffer chronic pain, these variations tend to be less evident (McKune CM). The Biological parameters can include the measurement of chemical mediators and the hormonal concentrations. For example the changes in plasma hormone concentrations like cortisol, β-endorphins and catecholamines, which have been considered as indirect indicators of pain. Only weak correlations of behavior and increases in plasma levels of the mentioned hormones have been determined (Gutiérrez BJ-2017) more over, it is well-known that the relation among physiological stress, behavioral disorders and pain is complex; therefore endocrine measures can reflect stress responses that may not always be related directly to pain.
Conclusion
It is the professional obligation of the veterinarian to alleviate suffering in their patients that may come from injury or illness. Pain is also sometimes an unavoidable consequence of necessary veterinary interventions, such as surgeries. It's important in a clinical setting to have a good appreciation of pain physiology, in order to understand the causes and select appropriate therapies for pain management. Reducing pain improves patient outcomes, quality of life, and enhances the relationship between the practitioner, client and patient (AAHA, 2015).
References:
Articles:
Allan I. Basbaum, Diana M. Bautista, Grégory Scherrer, David Julius. (2009). Cellular and Molecular Mechanisms of Pain. CELL. 139 (2), 267–284.
Casey, L., Minoshima, S., Morrow, T. J., & Koeppe, R. A. (1996). Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. Journal of Neurophysiology, 76, 571-581.
Bertrand Coste, Jayanti Mathur, Manuela Schmidt, Taryn J. Earley, Sanjeev Ranade, Matt J. Petrus, Adrienne E. Dubin, Ardem Patapoutian. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically-activated cation channels. Science. 330 (6000), 55–60.
Brody H. The lie that heals: the ethics of giving placebos. Ann Intern Med. 1982;97:112–18.
Michele Curatolo, Lars Arendt-Nielsen, Steen Petersen-Felix. (2006). Central Hypersensitivity in Chronic Pain: Mechanisms and Clinical Implications. Physical Medicine Rehabilitation Clinic of North America. 17, 287–302.
Xiaona Du, Nikita Gamper. (2013). Potassium Channels in Peripheral Pain Pathways: Expression, Function and Therapeutic Potential. Current Neuropharmacology. 11 (6), 621–640.
Fields HL, Levine JD. Pain: a clinical approach based on physiological principles. In: Isselbacher KJ, et al., eds. Harrison's principles of internal medicine. Update II. New York: McGraw-Hill 1982;205–20.
David Julius, Allan I. Basbaum. (2001). Molecular mechanisms of nociception. NATURE. 413, 203-210.
Katon W, Kleinman A, Rosen G. Depression and somatization: a review. Am J Med. 1982;72:127–35. , 241–347.
Chong Hyun Lee, Peter C. Ruben. (2008). Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels. 2 (6), 407-412.
Levine J. Pain and analgesia: the outlook for more rational treatment. Ann Intern Med. 1984;100:269–76.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–79.
New York: Wiley Osterweis, M., Kleinman, A., & Mechanic, D. (Eds.) (1987).
Michael H. Ossipov, Gregory O. Dussor, Frank Porreca. (2010). Central modulation of pain. The Journal of Clinical Investigation. 120 (11), 3779-3787.
John F Park, Z David Luo. (2010). Calcium channel functions in pain processing. Channels. 4 (6), 510-517.
W. Zieglgänsberger. (2019). Substance P and pain chronicity. Cell and Tissue Research. 375 (1), 227–241.
Cambridge AJ, Tobias KM, Newberry RC, et al. Subjective and objective measurements of postoperative pain in cats. JAVMA. (2000) [PubMed]
Franzini de Souza CC, Dias DPM, Souza RN, et al. Use of behavioural and physiological responses for scoring sound sensitivity in dogs. Plos One. (2000)
Gutiérrez BJ. Evaluación de la eficacia del dexketoprofeno en el control del dolor intra y postoperatorio en perros sometidos a cirugía ortopédica [Doctoral dissertation] España: Universidad de Córdoba; (2017)
León-Olea M. Evolución filogenética del dolor. Elementos. (2002)
Dubin AE, Patapoutian ANociceptors: the sensors of the pain pathway. J Clin Invest 120:3760-3772
Acute Pain OPML Editors L Bromley, B Brandner Oxford University Press 2010, ISBN 978-0-19-923472
Books:
McKune CM, Murrel JC, Nolan AM. Nociception and pain In: Grimm KA, Lamont LA, Tranquili WJ, et al, editors. Lumb and Jones Veterinary anesthesia and analgesia,
Multidisciplinary Pain Management for Pediatric Patients with Acute and Chronic Pain: A Foundational Treatment Approach When Prescribing Opioids DeGowin EL, DeGowin RL. Bedside diagnostic examination. New York: Macmillan, 1981.
Fields H. L., & Basbaum, A. L. (1994). Central nervous system mechanisms of pain modulation. In M. R. Wall PD (Ed.), Textbook of pain (3rd ed. ed., pp. 243-257). New York: Churchill Livingstone
Posner JB. Disorders of sensation. In: Wyngaarden JB, Smith LH, eds. Cecil textbook of medicine. 17th ed. Philadelphia: W.B. Saunders, 1985.
Other Publications:
IASP Newsletter, 3, 3-5. Jones, A. K. P. (1997). Pain, its perception, and pain imaging.
Osterweis M, Kleinman A, Mechanic D, (editors) (1987). Institute of Medicine (US) Committee on Pain, Disability, and Chronic Illness Behavior. Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives. Washington (DC): National Academies Press (US).
Mark Epstein, Ilona Rodan,Gregg Griffenhagen, Jamie Kadrlik, Michael Petty, Sheilah Robertson. (2015). 2015 AAHA/AAFP Pain Management Guidelinesfor Dogs and Cats. American Animal Hospital Association, Practice guidelines.
Websites:
Claire Bloor. (2017). Pain scoring systems in the canine and feline patient . Available: https://www.theveterinarynurse.com/review/article/pain-scoring-systems-in-the-canine-and-feline-patient. Last accessed 18 April 2020.
https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698
https://www.physio-pedia.com/Pain_Mechanisms
https://teachmephysiology.com/nervous-system/sensory-system/pain-pathways/
https://www.merckvetmanual.com/SearchResults?query=pain
https://www.healthline.com/health/nociceptive-pain
https://www.healthline.com/health/somatic-vs-visceral-pain#overview1
https://www.healthline.com/health/pain-relief/oxycodone-vs-hydrocodone
http://www.allcare.org/CancerPain-and-SymptomManagement/comfort/cfm2/cfm2_cont.htm#perception
https://www.ncbi.nlm.nih.gov/pubmed/8275913
https://teachmephysiology.com/nervous-system/sensory-system/pain-pathways/
https://global.oup.com/academic/product/acute-pain-9780199234721?cc=us&lang=en&
https://www.webmd.com/pain-management/glossary-pain-management
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2964977/
Figures:
♦Figure 1: Hand drawn figure by us
♦Figure 2: Hand draw figure by us {from Fields, H. L. Pain (McGraw-Hill, New York, 1987) }
♦Figure3: Taken from 2001 D.J. Wilkie Book Pain
♦Figure 4: Schematic drawing
♦Figure 5: Schematic drawing