|
Size: 14180
Comment:
|
← Revision 83 as of 2013-12-04 08:36:00 ⇥
Size: 15037
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 2: | Line 2: |
| == Connection between Platelets and Immunity == ---------- |
|
| Line 3: | Line 5: |
| = Connection between the Platelets and Immunity = | <<TableOfContents(3)>> === Introduction === ||<tablestyle="float:center; font-size: 0.85em; background: #eeeeee; margin: 0 0 0 0;" style="padding: 0.5em;":> {{attachment:679px-Giant_platelets.JPG}} <<BR>>'''Fig 1.'''<<BR>>''Platelets Amongst Red Blood Cells'' || |
| Line 6: | Line 12: |
| '''Introduction''' | Platelets play a central role in both innate and adaptive immunity. |
| Line 8: | Line 14: |
| Platelets are a unique type of mammalian blood cell. They are anucleate molecules which play an integral role in host homeostasis, immune defence and repair. At sites of infection or injury in a host organism, platelets will be deployed rapidly where they will display their role in defence and signalling. Platelets are activated by agonists, of which there are several. Thrombin, collagen, ADP, platelet activating factor (PAF) and other factors all promote the activation of platelets. There are two types of platelet activation, primary and secondary, both of which lead to platelet aggregation. Primary platelet aggregation occurs when the specialised surface receptors of platelets bind to the negative charges of injured endothelial area (exposed collagen) whilst secondary platelet activation refers to activation of the platelets by their actin and microtubule system as well as by their secretion of coagulation factors. The potency of platelet agonist varies depending on the activation response '''Hemostasis''' Platelets' foremost role is hemostasis. It not only helps the animal from blood loss but it also prevents microorganisms from entering the animal which may cause disease. The role of Platelets in homeostasis would suggest evolution from more primitive multifunctional innate defensive cells as although highly differentiated they have inflammatory and antimicrobial systems and link clotting and immune cascades (A.S Weyrich et al). Hemostasis is crucial to animals, when a blood vessel of theirs becomes damaged or injured. It is the first stage of wound healing. Platelets are present in the blood circulation at a volume of 2-8 x 1011/l, depending on the species. They are unable to move themselves so they passively move with the blood circulation (page 131). After an injury to a blood vessel occurs, vascular constriction is the organisms initial reaction. Platelets are not usually attracted to endothelial surfaces. When an injury of a blood vessel happens, platelets combine with their specialized surface receptors to the negative charges of the endothelial regions. This is primary aggregation (page 200-201). Also, Von Willebrand factor, which is a multimeric glycoprotein released by the endothelial cells plays a key role in the adhesion of platelets to the subendothelium.(M. Hermmann et al). This allows platelet aggregation to increase. Secondary activation follows. The microtubule system which is located beneath the plasma membrane and the actin cytoskeleton system of the platelets activate the reaction (S. Severin et al). This causes white thrombosis to arise. This happens when by filopodia are produced which provides the framework for platelets to attach to each other and also collagen fibres. Endothelial production of prostacyclines and nitrogen monoxyde ceases. Their role is to prevent the production of factors responsible for aggregation. Accordingly, the thrombocytes can now produce their own stimulating factors. These include TXA2, serotonin, adenosine-diphosphates that will stimulate other thrombocytes increasing the aggregation as well as the secretion of coagulation factors (page 201 and 202). This shows how platelets are pivitol in hemostasis. Without them, a relatively innocuous injury to a blood vessel may be fatal. They help heal wounds and by doing this help to prevent harmful microorganisms which could be disease causing from entering the animal. If these disease causing microorganisms were to off got into the body the animal would have to endure processes such as the production of antibodies to fight the infection. These processes are energy consuming and put the animal under stress. Therefore platelet's role in hemostasis can be looked upon as a temporary mechanical barrier between the organism and the outer environment, reducing the threat of disease when an animal is wounded. A.S Weyrich et al The evolving role of platelets in inflammation J.Thromb. Haemost., 1 (2003), pp. 1897-1905) [[http://www.jstor.org/discover/10.2307/30107584?searchUri=/action/doBasicSearch?Query=von+willebrand&Search=Search&gw=jtx&prq=platelets+von+willebrand&hp=25&acc=off&aori=off&wc=on&fc=off&Search=yes&searchText=von&searchText=willebrand&uid=3738216&uid=2129&uid=2134&uid=2&uid=70&uid=4&sid=21102878826017|http://www.jstor.org/discover/10.2307/30107584?searchUri=%2Faction%2FdoBasicSearch%3FQuery%3Dvon%2Bwillebrand%26Search%3DSearch%26gw%3Djtx%26prq%3Dplatelets%2Bvon%2Bwillebrand%26hp%3D25%26acc%3Doff%26aori%3Doff%26wc%3Don%26fc%3Doff&Search=yes&searchText=von&searchText=willebrand&uid=3738216&uid=2129&uid=2134&uid=2&uid=70&uid=4&sid=21102878826017]] http://onlinelibrary.wiley.com/doi/10.1111/jth.12053/pdf |
Platelets are a unique type of mammalian blood cell. They are anucleate molecules which play an integral role in host hemostasis, innate and immune defence and repair. Platelets are present in blood circulation but they are unable to move themselves so they passively move with the blood circulation. At sites of infection or injury in a host organism, platelets will be deployed rapidly where they will display their role in defence and signaling. |
| Line 17: | Line 17: |
| Platelets are rapidly targeted to sites of injury and infection | __''Innate Immunity''__ |
| Line 19: | Line 19: |
| Due to their rapid deployment and activation platelets will display similar surveillance and information transfer abilities as those possessed by dendritic cells, mast cells and macrophages. These cells are pre-positioned in tissues to detect microbial invasion and wounding whilst providing advanced molecular intelligence and directives for subsequent responses of the immune system . Platelets undergo rapid homotypic aggregation in the blood and adhesion to subendothelial matrix proteins and Von Willebrand factor. These responses are crucial in the role of homeostastis . Previous studies ( P.B Maguire, D.J. Fitzgerald) would indicate that platelets also aggregate at sites of bacterial invasion, accumulate in inflammatory exudates, and target tissues subjected to antigen-antibody-mediated injury. This study would also suggest that by aggregating around the bacteria, platelets might promote their clearance from the blood. Further it indicates that adherent platelets are carried by migrating polymorphonuclear leukocytes (neutrophils) to extravascular sites of inflammation and that they might be able to migrate into extravascular tissue. With these abilities the platelets should have the capability of performing rapid targeting mechanism thus localizing platelets to both intravascular compartments and the extravascular milieu in incidents of injury, microbial invasion and inflammation Once activated, platelets interact with and transfer information to other cells of immune defence, such as monocytes or neutrophils. Should platelets accumulate in the vessels or in the extracellular space, they also possess the ability to signal mast cell, macrophages and dendritic cells. Simultaneously these tissue surveillance cells or sentinel cells can also transfer signals to the platelets through the production of substances such as Platelet Activating Fator (PAF). PAF is an inflammatory mediator that is recognized by a receptor on the platelet plasma membrane. Therefore platelets can both supply and respond to signals at early points of control in inflammation and immune progression. |
Innate immunity refers to non specific defence mechanisms that come into play immediately or within hours of antigens appearance in the body. These mechanisms include physical barriers such as skin, chemicals in the blood and immune system cells that attack foreign cells in the body. The innate immune response is activated by chemical properties of the antigen (Henn ''et al'', 2001). |
| Line 23: | Line 21: |
| Cell-Cell interactions involving platelets Activated platelets participate in crucial cell-cell interactions and provide signals in the immune continuum . The following are examples of cellular interactions with the potential to induce, amplify or modify multiple inflammatory or adaptive immune events. |
__''Adaptive Immunity''__ |
| Line 26: | Line 23: |
| A. Activated human platelets adhere to and signal monocytes, forming mixed cellular aggregates which will induce expression of inflammatory gene products. B. Activated platelets synthesize and release Interleukin-1beta, which can then signal endothelial cells and other target cells. A consequence of signalling by this mechanism is the expression of genes that code for adhesion molecules and chemokines that mediate targeting and local activation of Neutrophils and Monocytes. C. Platelets signal maturation and activation of dendritic cells in vitro. This may prove to a key cellular interaction that enables platelets to modify T- and B-Lymphocyte functions in response to experimental viral challenge in vitro. Human Platelets can activate peripheral Blood B Cells and increase production of Immunoglobins Platelets represent a link between hemostasis, inflammation and tissue repair. Their role in immune response and inflammation mainly involves many molecules such as Toll-Like receptors,CD40 and CD154. A study to examine the involvement of platelets in CD154 (as they are the main purveyors of this molecule) dependent immune response was carried out in order to understand the interactions between platelets and peripheral B-Lymphocytes. The results of this experiment revealed that in coculture, platelets and B-lymphocytes were mutually activated, demonstrated by the increase expression of platelet CD62p and B-cell CD86. The Platelet/B-Cell interactions were also accompanied by changes in membrane expression of CD40 and CD154 by both platelets and B-Lymphocytes. IL 12p70 and IL8 gene transcription were also significantly reduced, which was attributable to B-Cells. Conversely there was a significant, platelet-dependent reduction of sCD154 and CCL5 mRNA expression. After a 3-Day incubation period B-Cells increased their production of IgG1, IgG2, IgG3 but not IgG4, IgA or IgM. This data illustrates the potentially important role of platelets in the adaptive immune response. Platelets have an immunoregulatory role that might be applied clinically in multitransfused patients . |
Adaptive immunity refers to antigen specific immune response. The adaptive immune response is more complex than the innate. The antige first must be processed and recognised. Once an antigen has been recognised, the adaptive immune system creates an army of immune cells specifically designed to attack that antigen. Adaptive immunity also includes "memory" that makes future responses against a specific antigen more efficient (Henn ''et al'' , 2001). |
| Line 37: | Line 26: |
| PLATELET MEDIATED MODULATION OF ADAPTIVE IMMUNITY Platelets play a major role in immunity through hemostasis and insuring structural composition of the vessel wall. Recently scientists have discovered platelets have a broader purpose than initially thought in the role of immunity. Platelets are also significantly important in the modulation of inflammation. Inflammation involves the recruitment and activation of neutrophils, monocytes and macrophages that in turn release and provoke endothelial/epithelial cells to release cytokines, chemokines, and vasoactive substances that further enhance inflammation and leukocyte extravasion (movement of leukocyte out of circulatory system towards the site of damaged tissue or infection.) Moser and Loerscher 2001 and Loet al. 1999. A molecule of great influence is CD154 which platelets express upon activation. Through CD154 expression, activated CD4+ T cells provide the signals required for the differentiation of T cell dependent B lymphocyte responses. A process that includes isotype switching. Yang and Wilson 1996, Renshaw et al. 1994, and Caux et al. 1994. CD154 expression on activated CD4+ T cells augment the DC maturation process. A requirement for cross priming and enhancing cytotoxic CD8+ T cells responses through the classical pathway. Ridge et al. 1998, Schoenberger et al. 1998and Bennett et al. 1998. The following experiments prove these theorys and therefore the importance of platelet derived CD154 in immunity. Adoptive transfer studies demonstrate that platelets activated physiologically with adenoviral infection (splenic and hepatic inflammation) are sufficient via CD154 for the induction of isotype switching by B cells and augmentation of the CD8+ T cell response during a viral infection Normal mice depleted of platelets prior to adenovirus infection exhibit decreased efficiency of production of adenovirus specific IgG underscoring the importance of platelets in the generation of an optimal adaptive immune response. Cd154 only present on activated platelets In previous experimental studies (Henn et al 1998, Henn et al. 2001, Ahmad et al. 2001, Diacovoet al 1994, Bombelo et al. 1998, Geba et al. 1996, Collins et al. 1994, Gawaz et al. 2000 and Barry et al. 1998) it was proven that platelet derived CD154 was only present in activated platelets. (platelet activation discussed previously) In the experiment, unactivated platelets and platelets activated with thrombin or collagen were tested for surface CD154. Results show that only activated platelets expressed surface CD 154. CD154 causes Dentritic cell maturation In later experiments, bone marrow derived dentritic cells were generated and incubated with activated platelets. The production of interleukins which are produced naturally by dendritic cells in response to antigenic stimulation was measured. The results show how activated platelets induced the interleukin production by the DC’s in a CD154 dependent mannor. CD154 induces B cell isotype switching The ability of B lymphocytes to switch from the production of IgM/IgD to IgG or other Ig isotypes requires CD40 activation (Renshaw et al. 1994). B cell activation in the absence of CD40 ligation results in hyper IgM syndrome with minimal switching to IgG secretion. As documented by Zhang et al. 2001 and Jaffee et al. 1992 mice injected with andenovirus result in splenic and hepatic inflammation. With this knowledge, experiments were carried out to assess the ability of platelet derived CD154 to induce B lymphocyte isotype switching in mice deficient in CD154 Adenovirus was injected intravenously into a CD154 deficient mouse. Activated platelets from normal mice were injected and then adenovirus specific Ig isotypes were measured. Therefore the transfer of normal activated platelets was sufficient to induce isotype switching Isotype switching was abrogated by anti- CD154. Although adenovirus specific IgM was produced IgG was not, therefore linking CD154 to the isotype switch to IgG. CD154 modulates CD 8+ T cell responses In the experiment, platelets derived from normal an CD154 derived mice were transferred in CD154 derived mice hosts. The mice were then challenged iv with Ad5-mOVA. Ten days later the spleens were isolated and ova-specific lytic activity was measured. Results show that there is adenovirus induced cytolytic T lymphocyte activity in CD154 derived mice , however it show lytic activity equivilant to mice receiving diluents without platelet transfer. Results in mice receiving normal platelets containing CD145 on the surface exhibit enhanced CTL activity. These findings indicate that platelet derived CD154 is sufficient to modulate CD8+ T cell responses. |
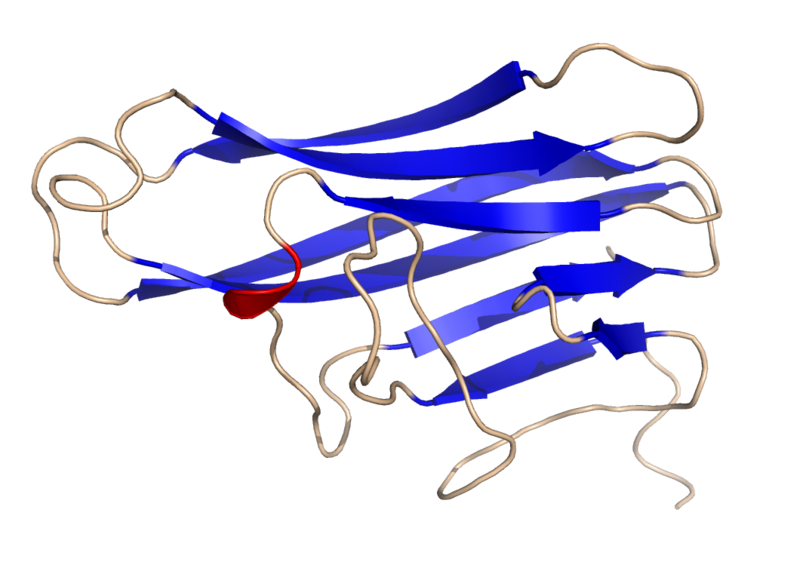
=== Hemostasis === Hemostasis is a process which causes bleeding to stop and retention of blood within a damaged blood vessel. Hemostasis is crucial to animals in the case of a damaged blood vessel. It is the first stage of wound heeling. ==== Activation of Platelets ==== There are two types of platelet activation, primary and secondary, both of which lead to platelet aggregation. __''Primary platelet activation''__ Primary platelet activation occurs when the specialised surface receptors of platelets bind to the negative charges of an injured endothelial area (exposed collagen). __''Secondary platelet activation''__ Secondary Platelet activation refers to activation of the platelets by their actin and microtubule system as well as by their secretion of coagulation factors. The potency of platelet agonist varies depending on activation response (Severin ''et al'', 2013). ==== The Process of Activation ==== Platelets are not usually attracted to endothelial surfaces. As endothelial surfaces act to inhibit platelet activation by producing: * Nitric oxide * Endothelial ADPase, which clears away the platelet activator, ADP. * PGI,,2,, __''Primary Activation Process''__ Under physiological conditions, collagen is not exposed to the bloodstream. However, when an injury of a blood vessel occurs, collagen and Von Willebrand Factor (vWF), from the subendthelium is exposed to the bloodstream. vWF is a glycomeric protein produced by endothelial cells, which acts as a cell adhesion ligand enabling endthelial cells to adhere to collagen in the basement membrane. When the platelets come in contact with collagen or vWF they are activated (Hermann ''et al'', 1997). Examples of platelet activating factors: * Thrombin * Collagen * ADP * Platelet activating factor (PAF) __''Secondary Activation Process''__ Following primary activation, the microtubule system which is located below the plasma membrane and the actin cytoskeleton system of the platelets activate the reaction (Severin ''et al'', 2013). This increases aggregation. A thrombus or blood clot is the final product in the blood coagulation set of hemostasis. This happens when filopodia are produced which provide the framework for platelets to attach to each other and also collagen fibres. Endothelial production of prostacyclines and nitrogen monoxide ceases. Their role is to prevent the factors production of factors responsible for aggregation. Accordingly, the thrombocytes can now produce their own stimulating factors. TXA2, serotonin, adenosine Diphosphate that will stimulate other thrombocytes increasing the aggregation as well as the secretion of coagulation factors. === Aggregation at Sites of Bacterial Invasion === Previous studies (Maguire and Fitzgerald, 2003) would indicate that platelets also: * Aggregates at sites of bacterial invasion → by aggregating around the bacteria, platelets might promote their clearance from the blood. * Accumulate in inflammatory exudates → adherent platelets are carried by migrating neutrophils to extravascular sites of inflammation. * Target tissues subjected to antigen-antibody-mediated injury → The adherent platelets are carried by migrating neutrophils into extravasular tissue. With these abilities the platelets should have the capability of performing rapid targeting mechanism thus localizing platelets to both intravascular compartments and the extravascular milieu in incidents of injury, microbial invasion and inflammation. (Wreyrich ''et al'', 2003) === Cell-Cell Interactions Involving Platelets === Once activated, platelets interact with and transfer information to other cells of immune defence, such as monocytes or neutrophils. Should platelets accumulate in the vessels or in the extracellular space, they also posess the ability to signal mast cell, macrophages and dendritic cells. Simultaneously these tissue surveillance cells or sentinel cells can also transfer signals to the platelets through the production of substances such as Platelet Activating Factor (PAF). PAF is an inflammatory mediator that is recognized by a receptor on the platelet plasma membrane. Therefore platelets can both supply and respond to signals at early points of control in inflammation and immune progression. Activated platelets participate in crucial cell-cell interactions and provide signals in the immune continuum. (Wreyrich ''et al'', 2003) The following are examples of cellular interactions with the potential to induce, amplify or modify multiple inflammatory or adaptive immune events. * Activated human platelets adhere to and signal monocytes, forming mixed cellular aggregates which will induce expression of inflammatory gene products. * Activated platelets synthesize and release interleukin-1beta, which can then signal endothelial cells and other target cells. A consequence of signalling by this mechanism is the expression of genes that code for adhesion molecules and chemokines that mediate targeting and local activation of neutrophils and monocytes. * Platelets signal maturation and activation of dendritic cells ''in vitro''. This may prove to be a key cellular interaction that enables platelets to modify T- and B- Lymphocyte functions in response to experimental viral challenge ''in vitro'' === Platelet Expression of CD40L/CD154 in Adaptive Immunity === CD40L is a trimeric, transmembrane protein of the tumour necrosis factor family that was origionally identified on cells of the immune system (activated CD4+ cells, mast cells, basophils, eosinophils and natural killer cells) subsequently on several cells of the vasculature such as endothelial cells, smooth muscal cells, macrophages and monocytes. The role of CD40L in the immune response involves binding to its receptor on B cells, CD40, to induce B- cell proliferation, generate memory B cells, block B- apoptosis, and mediate antibody class switching. Additionallz, the pioneering work of Henn and collaborators showed that CD40L and CD40 also exist in platelets. CD40L is cryptic in unactivated platelets but is rapidly presented on the platelet surface after platelet activation. (Henn ''et al'', 2001) Studies on the cellular distribution of CD40L indicate that >95% of the circulating CD40L exists in platelets. This suggests that platelet activation events must be considered in the biological and pathological context of CD40L function. The following experiments prove the importance of platelet derived CD154 in immunity. '''''CD154 only present on activated platelets''''' In previous experimental studies (Henn ''et al'', 1998, Henn ''et al'', 2001) It was proven that platelet derived CD154 was only present in activated platelets. (Platelet activation discussed previously.) In this experiment, unactivated platelets and platelets activated with thrombin or collagen were tested for surface CD154. Results show that only activated platelets expressed surface CD154. '''''CD154 causes dendritic cell maturation''''' In later experiments bone marrow derived dendritic cells were generated and incubated with activated platelets. The production of interleukins which are produced naturally by dendritic cells in response to antigenic stimulation was measured. The results show how activated platelets induced the interleukin production by the DC's in a CD154 dependent mannor. '''''CD154 induces B cell isotype switching''''' The ability of B Lymphocytes to switch from the production of IgM/IgD to IgG or other Ig isotypes requires CD40 activation (Renshaw ''et al'', 1994). B cell activation in the absence of CD40 ligation results in hyper IgM syndrome with minimal switching to IgG secretion. Mice injected with adenovirus results in splenic and hepatic inflammation. (Jaffee ''et al'', 1992) With this knowledge, experiments were carried out to assess the ability of platelet derived CD154 to induce B lymphocyte isotype switching in mice deficient in CD154. Adenovirus was injected intravenously into a CD154 deficient mouse. Activated platelets from normal mice were injected and then adenovirus specific Ig isotypes were measured. The transfer of normal activated platelets was sufficient to induce isotype switching. Isotype switching was abrogated by anti-CD154. Although adenovirus specific IgM was produced, IgG was not. This therefore links CD154 to the isotype switch to IgG. '''''CD154 Modulates CD8+ T cell responses''''' In this experiment, platelets derived from normal CD154 presenting mice were transfered into CD154 derived mice hosts. The mice were then challenged intravenously with Ad5-mOVA. Ten days later the spleens were isolated and ova-specific lytic activity was measured. Results show that there is adenovirus induced cytolytic T lymphocyte activity in CD154 deprived mice, however it shows lytic activity equivilant to mice recieving diluents without platelet transfer. Results in mice recieving normal platelets containing CD154 on the surface exhibit enhanced cytolytic activity. These findings indicate that platelet derived CD154 is sufficient to modulate CD8+ T cell responses. ||<tablestyle="float:center; font-size: 0.85em; background: #eeeeee; margin: 0 0 0 0;" style="padding: 0.5em;":> {{attachment:800px-Human_CD40L_Crystal_Structure.rsh.png}} <<BR>>'''Fig 2.'''<<BR>>''Crystal Structure of CD154'' || === Experiment to Demonstrate the Clinical Application of Platelet Expressed CD154 in Immunity === __''Aim''__ : A study to examine the involvement of platelets in CD154 dependent immune response was carried out in order to understand the interactions between platelets and peripheral B-Lymphocytes. __''Method''__: The capacity of platelets to bind non stimulated B-Cells, phenotypic changes by flow cytometry and confocal scanning laser microscopy modulation of cytokines/chemokines and total levels of immunoglobin (Ig) A, IgG, IgM and IgG subclasses in supernatants coculture, platelets, and B-Lymphocytes was performed by sandwich determined reverse transcriptase polymerase chain reaction. __''Result''__ : The results of this experiment revealed that in Co-culture, platelets and B-Lymphocytes were mutually activated, demonstrated by the increased expression of platelet CD62p and B-Cell CD86. The Platelet/B-cell interactions were also accompanied by changes in membrane expression of CD40 and CD154 by both platelets and B-Lymphocytes. IL 12p70 and IL8 gene transcription were also significantly reduced, which was attributable to B-Cells. Conversely there was a significant, platelet-dependent reduction of sCD154 and CCL5 mRNA expression. After a 3 day incubation period B-Cells increased their production of IgG1, IgG2, IgG3 but not IgG4, IgA or IgM. __''Conclucion''__:This data illustrate platelets have an immunoregulatory role that might be applied clinically in multitransfused patients. (Cognasse ''et al'', 2007) === References === 1. Cognasse Fabrice, Hind Hamezh-Cognasse, Sandrine Lafarge, Patricia Chavarin, Michel Cogne, Yolande Richard and Olivier Garraud (2007): Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Experimental Haemotology 35:(9) pp.1376-1387 2. Henn V., Slupsky J.R., Grafe M., Anagnostopoulos I., Forster R., Muller-Berghaus G., Kroczek R.A. (1998): CD40 Ligand on activated platelets triggers an inflammation reaction of endothelial cells. Nature 391: pp.591-594 3. Henn V., Steinbach S. , Buchnar K. ''et al'' (2001): The Inflammatory action of CD40L (CD154) Expressed on Activated Human Platelets is temporarily limited by co-expressed CD40. Blood 98: pp.1047-1054 [[http://www.biology.arizona.edu/immunology/tutorials/immunology/page3.html]] 4. Hermann M., Hartleib J, Kehrel B., Montgomery R.R, Sixmajj and Peters G. (1997): Interaction of Von Willebrand Factor with Staphylococcus Aureus. The Journal of Infectious Diseases 176:(4) PP.984-991 5. Jaffe H.A., Danel C., Longenacker G., Metzger M., Setoguchi Y., Rosenfeld M.A., Gant T.W., Thorgeirsson S.S., Stratford-Perricaudet L.D., Perricaudet M. (1992): Adenovirus-mediated ''in vivo'' gene transfer and expression in normal rat liver. Nat.Genet. 1: pp.372-378 === Other References === 6. Maguire P.B., Fitzgerald D.J. (2003): Platelet proteomics. J. Thromb. Haemost. 1: pp.1593-1601 7. Renshaw B.R., Fansaow III W.C., Armitage R.J., Campbell K.A., Liggitt D., Wright B., Davison B.L., Maliszewski C.R. (1994): Humeral Immune Responses in CD40 Ligand-deficient Mice. Journal of Experimental Medecine 180: pp.1889-1900 8. Severin S., Gaits-Iacovoni F., Allart S., Gratacap M.P., Payrastre B. (2013), Confocal-based morphometric analysis shows functional cross talk between the actin filament system and microtubules in thrombin-stimulated platelets. J. Thromb. Haemost. 11: pp. 183-186 9. Weyrich A.S., Zimmerman G.A (2003): The evolving role of platelets in inflammation. J. Thromb. Haemost. 1: pp.1897-1905 === Figure References === Figure 1. Platelets Amongst Red Blood Cells, Wikimedia Commons Figure 2. Crystal Structure of CD154, Wikimedia Commons |
Connection between Platelets and Immunity
Contents
-
Connection between Platelets and Immunity
- Introduction
- Hemostasis
- Aggregation at Sites of Bacterial Invasion
- Cell-Cell Interactions Involving Platelets
- Platelet Expression of CD40L/CD154 in Adaptive Immunity
- Experiment to Demonstrate the Clinical Application of Platelet Expressed CD154 in Immunity
- References
- Other References
- Figure References
Introduction
|
Platelets play a central role in both innate and adaptive immunity.
Platelets are a unique type of mammalian blood cell. They are anucleate molecules which play an integral role in host hemostasis, innate and immune defence and repair. Platelets are present in blood circulation but they are unable to move themselves so they passively move with the blood circulation. At sites of infection or injury in a host organism, platelets will be deployed rapidly where they will display their role in defence and signaling.
Innate Immunity
Innate immunity refers to non specific defence mechanisms that come into play immediately or within hours of antigens appearance in the body. These mechanisms include physical barriers such as skin, chemicals in the blood and immune system cells that attack foreign cells in the body. The innate immune response is activated by chemical properties of the antigen (Henn et al, 2001).
Adaptive Immunity
Adaptive immunity refers to antigen specific immune response. The adaptive immune response is more complex than the innate. The antige first must be processed and recognised. Once an antigen has been recognised, the adaptive immune system creates an army of immune cells specifically designed to attack that antigen. Adaptive immunity also includes "memory" that makes future responses against a specific antigen more efficient (Henn et al , 2001).
Hemostasis
Hemostasis is a process which causes bleeding to stop and retention of blood within a damaged blood vessel. Hemostasis is crucial to animals in the case of a damaged blood vessel. It is the first stage of wound heeling.
Activation of Platelets
There are two types of platelet activation, primary and secondary, both of which lead to platelet aggregation.
Primary platelet activation
Primary platelet activation occurs when the specialised surface receptors of platelets bind to the negative charges of an injured endothelial area (exposed collagen).
Secondary platelet activation
Secondary Platelet activation refers to activation of the platelets by their actin and microtubule system as well as by their secretion of coagulation factors. The potency of platelet agonist varies depending on activation response (Severin et al, 2013).
The Process of Activation
Platelets are not usually attracted to endothelial surfaces. As endothelial surfaces act to inhibit platelet activation by producing:
- Nitric oxide
- Endothelial ADPase, which clears away the platelet activator, ADP.
PGI2
Primary Activation Process
Under physiological conditions, collagen is not exposed to the bloodstream. However, when an injury of a blood vessel occurs, collagen and Von Willebrand Factor (vWF), from the subendthelium is exposed to the bloodstream. vWF is a glycomeric protein produced by endothelial cells, which acts as a cell adhesion ligand enabling endthelial cells to adhere to collagen in the basement membrane. When the platelets come in contact with collagen or vWF they are activated (Hermann et al, 1997).
Examples of platelet activating factors:
- Thrombin
- Collagen
- ADP
- Platelet activating factor (PAF)
Secondary Activation Process
Following primary activation, the microtubule system which is located below the plasma membrane and the actin cytoskeleton system of the platelets activate the reaction (Severin et al, 2013). This increases aggregation.
A thrombus or blood clot is the final product in the blood coagulation set of hemostasis. This happens when filopodia are produced which provide the framework for platelets to attach to each other and also collagen fibres. Endothelial production of prostacyclines and nitrogen monoxide ceases. Their role is to prevent the factors production of factors responsible for aggregation. Accordingly, the thrombocytes can now produce their own stimulating factors. TXA2, serotonin, adenosine Diphosphate that will stimulate other thrombocytes increasing the aggregation as well as the secretion of coagulation factors.
Aggregation at Sites of Bacterial Invasion
Previous studies (Maguire and Fitzgerald, 2003) would indicate that platelets also:
- Aggregates at sites of bacterial invasion → by aggregating around the bacteria, platelets might promote their clearance from the blood.
- Accumulate in inflammatory exudates → adherent platelets are carried by migrating neutrophils to extravascular sites of inflammation.
- Target tissues subjected to antigen-antibody-mediated injury → The adherent platelets are carried by migrating neutrophils into extravasular tissue.
With these abilities the platelets should have the capability of performing rapid targeting mechanism thus localizing platelets to both intravascular compartments and the extravascular milieu in incidents of injury, microbial invasion and inflammation. (Wreyrich et al, 2003)
Cell-Cell Interactions Involving Platelets
Once activated, platelets interact with and transfer information to other cells of immune defence, such as monocytes or neutrophils. Should platelets accumulate in the vessels or in the extracellular space, they also posess the ability to signal mast cell, macrophages and dendritic cells. Simultaneously these tissue surveillance cells or sentinel cells can also transfer signals to the platelets through the production of substances such as Platelet Activating Factor (PAF). PAF is an inflammatory mediator that is recognized by a receptor on the platelet plasma membrane. Therefore platelets can both supply and respond to signals at early points of control in inflammation and immune progression.
Activated platelets participate in crucial cell-cell interactions and provide signals in the immune continuum. (Wreyrich et al, 2003) The following are examples of cellular interactions with the potential to induce, amplify or modify multiple inflammatory or adaptive immune events.
- Activated human platelets adhere to and signal monocytes, forming mixed cellular aggregates which will induce expression of inflammatory gene products.
- Activated platelets synthesize and release interleukin-1beta, which can then signal endothelial cells and other target cells. A consequence of signalling by this mechanism is the expression of genes that code for adhesion molecules and chemokines that mediate targeting and local activation of neutrophils and monocytes.
Platelets signal maturation and activation of dendritic cells in vitro. This may prove to be a key cellular interaction that enables platelets to modify T- and B- Lymphocyte functions in response to experimental viral challenge in vitro
Platelet Expression of CD40L/CD154 in Adaptive Immunity
CD40L is a trimeric, transmembrane protein of the tumour necrosis factor family that was origionally identified on cells of the immune system (activated CD4+ cells, mast cells, basophils, eosinophils and natural killer cells) subsequently on several cells of the vasculature such as endothelial cells, smooth muscal cells, macrophages and monocytes. The role of CD40L in the immune response involves binding to its receptor on B cells, CD40, to induce B- cell proliferation, generate memory B cells, block B- apoptosis, and mediate antibody class switching. Additionallz, the pioneering work of Henn and collaborators showed that CD40L and CD40 also exist in platelets. CD40L is cryptic in unactivated platelets but is rapidly presented on the platelet surface after platelet activation. (Henn et al, 2001) Studies on the cellular distribution of CD40L indicate that >95% of the circulating CD40L exists in platelets. This suggests that platelet activation events must be considered in the biological and pathological context of CD40L function.
The following experiments prove the importance of platelet derived CD154 in immunity.
CD154 only present on activated platelets
In previous experimental studies (Henn et al, 1998, Henn et al, 2001) It was proven that platelet derived CD154 was only present in activated platelets. (Platelet activation discussed previously.)
In this experiment, unactivated platelets and platelets activated with thrombin or collagen were tested for surface CD154. Results show that only activated platelets expressed surface CD154.
CD154 causes dendritic cell maturation
In later experiments bone marrow derived dendritic cells were generated and incubated with activated platelets. The production of interleukins which are produced naturally by dendritic cells in response to antigenic stimulation was measured. The results show how activated platelets induced the interleukin production by the DC's in a CD154 dependent mannor.
CD154 induces B cell isotype switching
The ability of B Lymphocytes to switch from the production of IgM/IgD to IgG or other Ig isotypes requires CD40 activation (Renshaw et al, 1994). B cell activation in the absence of CD40 ligation results in hyper IgM syndrome with minimal switching to IgG secretion.
Mice injected with adenovirus results in splenic and hepatic inflammation. (Jaffee et al, 1992)
With this knowledge, experiments were carried out to assess the ability of platelet derived CD154 to induce B lymphocyte isotype switching in mice deficient in CD154.
Adenovirus was injected intravenously into a CD154 deficient mouse. Activated platelets from normal mice were injected and then adenovirus specific Ig isotypes were measured.
The transfer of normal activated platelets was sufficient to induce isotype switching.
Isotype switching was abrogated by anti-CD154. Although adenovirus specific IgM was produced, IgG was not. This therefore links CD154 to the isotype switch to IgG.
CD154 Modulates CD8+ T cell responses
In this experiment, platelets derived from normal CD154 presenting mice were transfered into CD154 derived mice hosts. The mice were then challenged intravenously with Ad5-mOVA. Ten days later the spleens were isolated and ova-specific lytic activity was measured.
Results show that there is adenovirus induced cytolytic T lymphocyte activity in CD154 deprived mice, however it shows lytic activity equivilant to mice recieving diluents without platelet transfer.
Results in mice recieving normal platelets containing CD154 on the surface exhibit enhanced cytolytic activity. These findings indicate that platelet derived CD154 is sufficient to modulate CD8+ T cell responses.
|
Experiment to Demonstrate the Clinical Application of Platelet Expressed CD154 in Immunity
Aim : A study to examine the involvement of platelets in CD154 dependent immune response was carried out in order to understand the interactions between platelets and peripheral B-Lymphocytes.
Method: The capacity of platelets to bind non stimulated B-Cells, phenotypic changes by flow cytometry and confocal scanning laser microscopy modulation of cytokines/chemokines and total levels of immunoglobin (Ig) A, IgG, IgM and IgG subclasses in supernatants coculture, platelets, and B-Lymphocytes was performed by sandwich determined reverse transcriptase polymerase chain reaction.
Result : The results of this experiment revealed that in Co-culture, platelets and B-Lymphocytes were mutually activated, demonstrated by the increased expression of platelet CD62p and B-Cell CD86. The Platelet/B-cell interactions were also accompanied by changes in membrane expression of CD40 and CD154 by both platelets and B-Lymphocytes. IL 12p70 and IL8 gene transcription were also significantly reduced, which was attributable to B-Cells. Conversely there was a significant, platelet-dependent reduction of sCD154 and CCL5 mRNA expression. After a 3 day incubation period B-Cells increased their production of IgG1, IgG2, IgG3 but not IgG4, IgA or IgM.
Conclucion:This data illustrate platelets have an immunoregulatory role that might be applied clinically in multitransfused patients. (Cognasse et al, 2007)
References
1. Cognasse Fabrice, Hind Hamezh-Cognasse, Sandrine Lafarge, Patricia Chavarin, Michel Cogne, Yolande Richard and Olivier Garraud (2007): Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Experimental Haemotology 35:(9) pp.1376-1387
2. Henn V., Slupsky J.R., Grafe M., Anagnostopoulos I., Forster R., Muller-Berghaus G., Kroczek R.A. (1998): CD40 Ligand on activated platelets triggers an inflammation reaction of endothelial cells. Nature 391: pp.591-594
3. Henn V., Steinbach S. , Buchnar K. et al (2001): The Inflammatory action of CD40L (CD154) Expressed on Activated Human Platelets is temporarily limited by co-expressed CD40. Blood 98: pp.1047-1054 http://www.biology.arizona.edu/immunology/tutorials/immunology/page3.html
4. Hermann M., Hartleib J, Kehrel B., Montgomery R.R, Sixmajj and Peters G. (1997): Interaction of Von Willebrand Factor with Staphylococcus Aureus. The Journal of Infectious Diseases 176:(4) PP.984-991
5. Jaffe H.A., Danel C., Longenacker G., Metzger M., Setoguchi Y., Rosenfeld M.A., Gant T.W., Thorgeirsson S.S., Stratford-Perricaudet L.D., Perricaudet M. (1992): Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat.Genet. 1: pp.372-378
Other References
6. Maguire P.B., Fitzgerald D.J. (2003): Platelet proteomics. J. Thromb. Haemost. 1: pp.1593-1601
7. Renshaw B.R., Fansaow III W.C., Armitage R.J., Campbell K.A., Liggitt D., Wright B., Davison B.L., Maliszewski C.R. (1994): Humeral Immune Responses in CD40 Ligand-deficient Mice. Journal of Experimental Medecine 180: pp.1889-1900
8. Severin S., Gaits-Iacovoni F., Allart S., Gratacap M.P., Payrastre B. (2013), Confocal-based morphometric analysis shows functional cross talk between the actin filament system and microtubules in thrombin-stimulated platelets. J. Thromb. Haemost. 11: pp. 183-186
9. Weyrich A.S., Zimmerman G.A (2003): The evolving role of platelets in inflammation. J. Thromb. Haemost. 1: pp.1897-1905
Figure References
Figure 1. Platelets Amongst Red Blood Cells, Wikimedia Commons
Figure 2. Crystal Structure of CD154, Wikimedia Commons