RNA Therapeutics in Cancer Immunotherapy
Introduction
RNA‐based therapeutic technologies represent a rapidly expanding class of therapeutic opportunities with the power to advance cancer immunotherapy in ways we once thought unimaginable. mRNA, microRNAs, small interfering RNAs and antisense oligonucleotides (ASOs) are just some of the therapeutic models being implemented in cancer immunomodulation today. As RNA therapeutics can specifically select targets, there is great hope that cancer treatment will be able to move away from untargeted cytotoxic therapy to selective molecular target therapies (MacLeod and Crooke, 2017). In our essay, we will discuss the delivery of these RNA therapeutics and also the therapeutics advancements that have been made so far in this field of research.
Delivery of RNA therapeutics
Over the past twenty years, RNA- based therapeutics such as miRNA and siRNA have become increasingly popular class of drugs for the treatment and prevention of numerous diseases, including cancer (Yin et al, 2020). Small RNAs, such as microRNAs and small interfering RNAs (siRNA), can downregulate hundreds of target genes. Targeting factors include many site‐specific factors, for example base‐pair complementarity and local context factors (Arvey et al, 2010). RNA-based therapeutics have a large number of promising applications within the field of cancer treatment. They function as either inhibitors (e.g., siRNA and microRNA) or up regulators (e.g., mRNA) of target protein expression (Yin et al, 2020).
siRNA
SiRNA is double stranded in nature and approximately 22 nucleotides in length. It can bind and degrade mRNA through the RISC (RNA-induced silencing complex). These are often used in nanoparticle delivery systems to aid in delivery issues that can arise. SiRNA’s can also be called RNAi inhibitors (MacLeod and Crooke, 2017). The siRNA-RISC complex can bind the targeting site of mRNA, and lead to cleavage by endonuclease Argonaute-2 (AGO2), which therefore decreases protein expression of the target protein (Yin et al, 2020). The in vivo use of siRNAs effectively against cancer depends on the availability of a delivery molecule that can be administered to reach both primary (original) and metastatic tumour cells (cells that have broken off from the original primary tumour, travelled through the lymphatic system or bloodstream and form a new tumour in other organs or tissues), (Pirollo and Chang, 2008). siRNAs provide great encouragement that these RNA therapeutics will ultimately be successfully applied to target traditionally undruggable cancers and therefore significantly impact the treatment of human cancer in the future (MacLeod and Crooke, 2017).
MicroRNA
MicroRNAs or miRNAs are a family of short, between 19-25 nucleotides, non‐protein coding RNAs that play a role in the regulation of gene expression. MiRNAs form sequences on target RNAs by using antisense base pairing, which leads to the inhibition of mRNA translation and degradation of the target mRNA (MacLeod and Crooke, 2017). MiRNA blocks the target gene expression by binding to target sites on the 3’-untranslated regions of protein-coding transcripts (Yin et al, 2020). MicroRNA’s have the ability to target hundreds of mRNA’s but in comparison siRNA has only mRNA specific binding ability, which therefore means each siRNA can only bind to one mRNA target (Yin et al, 2020). microRNA can be used as a gene regulator. These can be used to directly inhibit expressions of immune escape factors and pro-inflammatory cytokines or act as oncogenic factors for facilitating immune system evasion by tumour cells (Yin et al, 2020). MicroRNA drugs are a class of RNA therapeutics that are very impressive as individual miRNAs regulate the expression of multiple important biological pathways. It will be exciting to see what developments are in store for this new class of drugs for use in cancer therapeutics (MacLeod and Crooke, 2017).
ASO’s
The concept of antisense oligonucleotides was first described in 1978, when Stephenson and Zamecnik reported that a chemically modified oligonucleotide, designed to bind to its complementary sequence in Rous sarcoma virus transcript, inhibited gene expression and viral replication (Lundin et al, 2015). ASOs inhibit protein production through a variety of mechanisms e.g. sterically blocking ribosome attachment or eliciting RNase-H activation (Chery, 2016). They can also promote exon skipping, which allows for the deletion of faulty sequences within proteins (Young and Pyle, 2016), and in some cases lead to protein upregulation, which could be used therapeutically in diseases where certain genes are repressed (Liang et al, 2016). It uses in cancer therapy are promising and exciting.
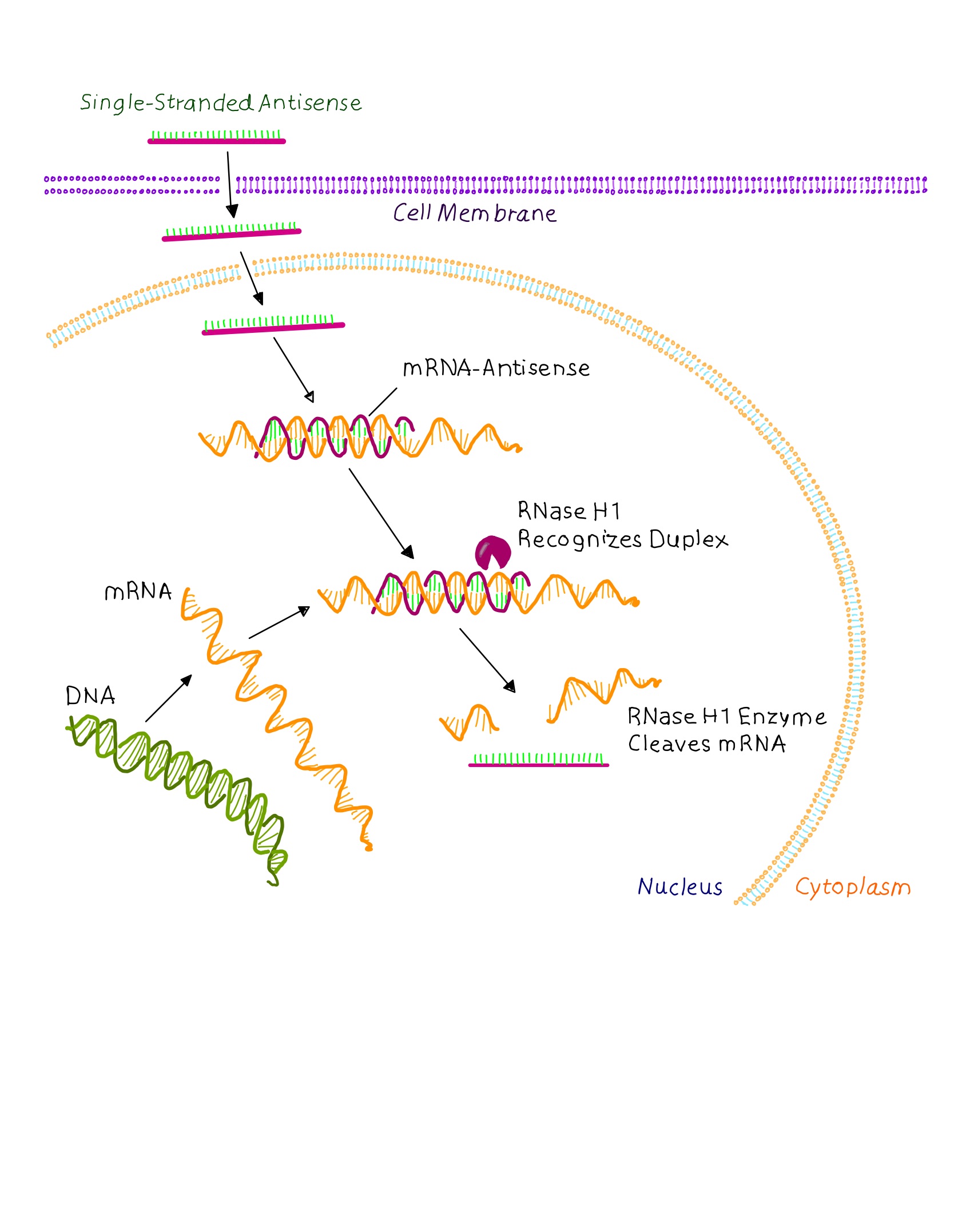
Graphic showing a simplified view of RNase H mechanism. The single stranded DNA- like oligonucleotide enters a cell, interacts with its target RNA in the cytoplasm or nucleus of a cell, and then recruits RNase HI, which mediate the selective cleavage of the target RNA molecule within the DNA:RNA duplex. (MacLeod and Crooke, 2017)
Unlike antibodies and small molecules, ASO-based approaches target the cancer hallmark pathologies at the RNA level by sequence-specific binding of a synthetic ASO to modulate the expression of pro-oncogenic genes (Le et al, 2019). Some known antisense drugs approved by the U.S. Food and Drug Administration (FDA) are: Vitravene for the treatment of cytomegalovirus (CMV) retinitis in immunocompromised patients (Roehr, 1998), Kynamro for the treatment of homozygous familial hypercholesterolemia (Wong and Goldberg, 2014), Exondys51 for the treatment of Duchenne muscular dystrophy (DMD) (Syed, 2016) and Spinraza for the treatment of spinal muscular atrophy (SMA) (Corey, 2017). However, it should be noted that, so far, no drugs were approved for cancer treatments, although several ASO drug candidates have entered different stages of clinical trials (Le et al, 2019).
mRNA
mRNA is the set of instructions by which cells make proteins and send them to various parts of the body. In 2021 the term mRNA became widely used in households all over the world due to its prevalence in the Moderna and Pfizer/BioNTech vaccines against SARS-Co-V-2. While the use of mRNA in virus vaccine technology has been successful, one area that is being further being explored is it use in cancer vaccines.
Nucleic acid vaccines are non-infectious, free of protein or virus-derived contaminations during production and are thus considered well tolerated for both prophylactic and therapeutic applications (Faghfuri et al, 2021). The rationale behind mRNA as an appealing cancer vaccination platform is to deliver the transcript of interest(s), encoding one or more TAAs or TSAs, into the host cell (typically APCs) cytoplasm, to be expressed into the targeted antigen(s) (Miao et al, 2021). The expressed TAAs (tumour associated antigens) and TSAs (tumour specific antigens) can be presented to the surface of antigen presenting cells by major histocompatibility complexes (MHCs) to activate anti-tumour immunity. Advantages of mRNA over DNA as cancer vaccine strategy include the fact mRNAs can be translated in both dividing and non-dividing cells, where RNA only needs to be internalised into the cytoplasm, followed by a one-step translation into the antigen (s) of interest. It is also to be noted that the rate and magnitude of protein expression of mRNA is also typically higher than DNA vaccines (Liu, 2019).
Many patients’ cancer responds only partially or does not respond at all to anti-cancer therapies. Pharmaceutical companies like Moderna and Merck are taking the approach to administer cancer vaccine that encodes for peptides containing mutations found in the patients’ specific cancer. Essentially creating a personalised cancer immunotherapy composed of neoantigens unique to a patient’s tumour. Moderna are currently working with Merck in the development of an mRNA-based approach to advance a cancer vaccine that encodes for the four most common KRAS mutations. KRAS is a frequently mutated oncogene in epithelial cancers, primarily in non-small cell lung cancer (NSCLC), colorectal, and pancreatic cancers (Waters and Der, 2018). mRNA is a robust and versatile cancer vaccine platform and such developments in major pharmaceutical companies will only strengthen our ability to combat cancers.
Therapeutic Advances with RNA therapeutics in Oncology
Cancer mRNA therapeutics has undergone major development and clinical improvements in recent years. For nearly 20 years, scientific research has overcome considerable challenges; including intracellular delivery, stability and immune response activation (Kaczmarek et al, 2017). Ever since the FDA (food and drug administration) approved the use of the first siRNA therapeutics in 2018 (Akinc et al, 2019), the use of this therapy has grown exponentially.In previous years, pharmaceutical companies depended on small-molecule therapeutics to make drugs, these act in an antagonistic way, targeting proteins in the body, in doing this, they hinder some biological processes (Gurevich et Gurevich, 2014).
In 2006, it was discovered that phi29 pRNA could be used as a carrier for the manufacture of RNA nanoparticles to transmit therapeutic RNAs, for example, siRNAs and ribosomes to specific cancer cells (Guo et al, 2006). This detection was picked up by the National Cancer Institution (NCI) nanotechnology program leaders. This was the beginning of the NCI’s promotion of RNA therapeutics for cancer therapy (Shu et al, 2014).
There has been a considerable number of barriers for clinical application of RNA therapeutics, these include; the chemical and thermodynamic instability of RNA, the low yield and the expensive production (Shu et al, 2014). The discovery of Patisaran (also known as Onpattro), a double-stranded siRNA helped to overcome these blockades. It is used to treat transthytetin-mediated amyloidosis (Akinc et al, 2019). The discovery of Onpattro led to the FDA approval.
With traditional chemotherapy there are many side effects, such as hair loss, nausea, tiredness, anaemia, and the list continues on. It is hoped that with the advancements in these technologies we can move away from this treatment and proceed with targeted molecular therapies (MacLeod and Crooke, 2017). However, due to the level of difficulty and also cost this has yet to reach its full potential. This is mainly due to the fact that many proteins involved in cancer have been known to be difficult “to drug”, therefore, these proteins remain “undrugged” at this moment in time (MacLeod and Crooke, 2017).
Conclusion
The considerable potential of RNA therapeutics in cancer immunotherapy is indisputable. Since approval of the first therapeutics by the FDA, extensive advancements have been made. It comes to no one’s surprise the share of difficulties such as cost and level of complexity that encompass this highly specific technology. However, it is hoped that in the near future these obstacles will be overcome, and we will be able to reap the great benefits that these RNA therapeutics withhold.
References
1. Akinc, A., Maier, M. A., Manoharan, M., Fitzgerald, K., Jayaraman, M., Barros, S., Ansell, S., Du, X., Hope, M. J., Madden, T. D., Mui, B. L., Semple, S. C., Tam, Y. K., Ciufolini, M., Witzigmann, D., Kulkarni, J. A., van der Meel, R., & Cullis, P. R. (2019). The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nature nanotechnology, 14(12), 1084–1087. https://doi.org/10.1038/s41565-019-0591-y
2. Arvey, A., Larsson, E., Sander, C., Leslie, C. S., & Marks, D. S. (2010). Target mRNA abundance dilutes microRNA and siRNA activity. Molecular systems biology, 6, 363. https://doi.org/10.1038/msb.2010.24
3. Chery J. (2016). RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc journal: a journal of postdoctoral research and postdoctoral affairs, 4(7), 35–50. https://doi.org/10.14304/surya.jpr.v4n7.5
4. Corey D. R. (2017). Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nature neuroscience, 20(4), 497–499. https://doi.org/10.1038/nn.4508
5. Faghfuri, E., Pourfarzi, F., Faghfouri, A. H., Abdoli Shadbad, M., Hajiasgharzadeh, K., & Baradaran, B. (2021). Recent developments of RNA-based vaccines in cancer immunotherapy. Expert opinion on biological therapy, 21(2), 201–218. https://doi.org/10.1080/14712598.2020.1815704
6. Guo, S., Huang, F., & Guo, P. (2006). Construction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells. Gene therapy, 13(10), 814–820. https://doi.org/10.1038/sj.gt.3302716
7. Gurevich, E. V., & Gurevich, V. V. (2014). Therapeutic potential of small molecules and engineered proteins. Handbook of experimental pharmacology, 219, 1–12. https://doi.org/10.1007/978-3-642-41199-1_1
8. Kaczmarek, J.C., Kowalski, P.S. & Anderson, D.G. (2017). Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med 9, 60 https://doi.org/10.1186/s13073-017-0450-0
9. Le, B. T., Raguraman, P., Kosbar, T. R., Fletcher, S., Wilton, S. D., & Veedu, R. N. (2019). Antisense Oligonucleotides Targeting Angiogenic Factors as Potential Cancer Therapeutics. Molecular therapy. Nucleic acids, 14, 142–157. https://doi.org/10.1016/j.omtn.2018.11.007
10. Liang, X. H., Shen, W., Sun, H., Migawa, M. T., Vickers, T. A., & Crooke, S. T. (2016). Translation efficiency of mRNAs is increased by antisense oligonucleotides targeting upstream open reading frames. Nature biotechnology, 34(8), 875–880. https://doi.org/10.1038/nbt.3589
11. Yin, Y. X., Wang, Y., Blake, S., Yu, M., Mei, L., Wang, H., & Shi, J. (2020). RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics, 10(1), 281–299. https://doi.org/10.7150/thno.35568
12. Liu M. A. (2019). A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines, 7(2), 37. https://doi.org/10.3390/vaccines7020037
13. Lundin, K. E., Gissberg, O., & Smith, C. I. (2015). Oligonucleotide Therapies: The Past and the Present. Human gene therapy, 26(8), 475–485. https://doi.org/10.1089/hum.2015.070
14. Macleod, A., & Crooke, S. (2017). RNA Therapeutics in Oncology: Advances, Challenges, and Future Directions. The Journal of Clinical Pharmacology, 57
15. Miao, L., Zhang, Y. & Huang, L. (2021). mRNA vaccine for cancer immunotherapy. Mol Cancer 20, 41. https://doi.org/10.1186/s12943-021-01335-5
16. Pirollo, K. F., & Chang, E. H. (2008). Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer research, 68(5), 1247–1250. https://doi.org/10.1158/0008-5472.CAN-07-5810
17. Roehr B. (1998). Fomivirsen approved for CMV retinitis. Journal of the International Association of Physicians in AIDS Care, 4(10), 14–16.
18. Shu, Y., Pi, F., Sharma, A., Rajabi, M., Haque, F., Shu, D., Leggas, M., Evers, B. M., & Guo, P. (2014). Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Advanced drug delivery reviews, 66, 74–89. https://doi.org/10.1016/j.addr.2013.11.006
19. Syed Y. Y. (2016). Eteplirsen: First Global Approval. Drugs, 76(17), 1699–1704. https://doi.org/10.1007/s40265-016-0657-1
20. Waters, A. M., & Der, C. J. (2018). KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harbor perspectives in medicine, 8(9), a031435. https://doi.org/10.1101/cshperspect.a031435
21. Wong, E., & Goldberg, T. (2014). Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P & T: a peer-reviewed journal for formulary management, 39(2), 119–122.
22. Young, C. S., & Pyle, A. D. (2016). Exon Skipping Therapy. Cell, 167(5), 1144. https://doi.org/10.1016/j.cell.2016.10.050
Other References
