|
Size: 24278
Comment:
|
← Revision 40 as of 2016-05-05 19:38:55 ⇥
Size: 24396
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 52: | Line 52: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:IGF_effects_on_reproduction.png|pop-up text|width="500"}} <<BR>>'''Fig 1.'''<<BR>>''IGF-1 effects on reproductive organs.'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:IGF_effects_on_reproduction.png|pop-up text|width="500"}} <<BR>>'''Fig 1.'''<<BR>>''IGF-1 effects on reproductive organs.'' || |
| Line 62: | Line 62: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Kisspeptin_effect_on_pituitary_gland.png|pop-up text|width="500"}} <<BR>>'''Fig 2.'''<<BR>>''Kisspeptin's effect on the pituitary gland.'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Kisspeptin_effect_on_pituitary_gland.png|pop-up text|width="500"}} <<BR>>'''Fig 2.'''<<BR>>''Kisspeptin's effect on the pituitary gland.'' || |
| Line 106: | Line 106: |
| '''Butler WR and Smith RD, '''(1987) Interrelationships between energy balance and postpartum reproductive function in dairy cattle, 768-771 | '''Butler WR and Smith RD '''(1989) Interrelationships between energy balance and postpartum reproductive function in dairy cattle. Journal of Dairy Science 72:3 767-783 |
| Line 120: | Line 120: |
| '''Fenwick MA, Llewellyn S, Fitzpatrick R, Kenny DA, Murphy JJ, Patton J, & Wathes DC.''' (2008). Negative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviduct. ''Reproduction (Cambridge, England)'', ''135''(1), 63–75. http://doi.org/10.1530/REP-07-0243 | '''Fenwick MA, Llewellyn S, Fitzpatrick R, Kenny DA, Murphy JJ, Patton J, & Wathes DC.''' (2008). Negative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviduct. Reproduction (Cambridge, England), 135(1), 63–75. |
| Line 142: | Line 142: |
| '''Marie M. '''Links between nutrition and reproduction in cattle. 9-23 | '''Marie M. '''(1999)''' '''Links between nutrition and Reproduction in Dairy cattle.''' '''Ecole Nationale Superieure d'Agronomie et des Industries Alimentaires, I.N.P.L., Vandoeuvre-les-Nancy, France |
| Line 148: | Line 148: |
| '''Neogrady S. and Dr Matis G'''.,(2016) Pathobiochemical relevance of the metabolism of ruminants, 15-25'''''' | '''Neogrady S. and Dr Matis G'''.,(2016) Pathobiochemical relevance of the metabolism of ruminants, 15-25 |
Contents
Metabolic balance and reproduction in ruminants
Introduction
Reproductive function and metabolic function are closely linked for the simple reason that the former comes at a cost. This is especially true for females, who need to source energy for pregnancy and lactation. Central regulation of both reproduction and metabolic function provides a means whereby sufficient energy or energy deficit can be detected, such that food intake and energy expenditure can be controlled. Our understanding of the central regulation of food intake and energy expenditure has increased due to the identification of a range of neuronal systems within the brain, over the past 30 years. In addition, the somewhat recent identification of neuropeptides that modulate reproductive function have provided an additional layer knowledge. In particular, kisspeptin and gonadotropin inhibitory hormone (GnIH) stand out as key factors in the regulation of reproduction, the main regulators of Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH). Not surprisingly, the neural elements within the brain that control metabolic function and those that control reproduction are inter-connected. All of this and more will be summarized below.
Ruminant metabolism
While there are many external effects on reproduction in ruminants, internal effects and metabolic balance play a major role in the reproduction cycle. During early lactation, the energy requirements for production and maintenance exceed the available energy from feed intake resulting in negative energy balance, NEB (Jorritsma et al, 2002). The production of follicles and release of oocyte require a lot of energy and if a NEB is present the release of an egg is going to be inhibited. There are a number of metabolites than play a role in maintaining correct energy balance.
Metabolites
1) Glucose
Blood glucose is the main energy source throughout the body, as well as beta-OH-butyrate. Miettinen (1990) observed a positive relationship between blood glucose and reproduction performance (onset of ovarian activity, interval to conception). High blood glucose levels at time of first insemination were found to be correlated with higher pregnancy rates, as well as being an energy fuel for the cells of the CNS. These cells can act as a signal for metabolic status on neuronal control of Gonadotropin Releasing Hormone (GnRH) secretion (Marie,1999). GnRH released from the hypothalamus stimulates LH, and FSH.
2) Ketone bodies
Ketone bodies, beta-OH-butyrate and aceto-acetate, are produced in case of elevated oxidation of FFA in the liver as a result of imbalance of energy metabolism. This is particularly common during the fattening of cows during their dry period. As a result of accumulation of ketone bodies in the liver, ketosis occurs. During the biochemical process Ac-CoA is used to produce ketone bodies. (See metabolic diseases, Ketosis.)
3) Cholesterol
It was found that there is a clear association between post-partum cholesterol level and fertility in dairy cows (Kappel et al, 1985). Cholesterol is the main precursor of sexual hormone pregnenolone in the presence of Vitamin A. High cholesterol levels result in better embryo collection in super ovulated cows and are correlated with high number of transferable embryos.
4) Fat metabolism
There is emerging information and research correlating inflammation with energy and fat metabolism. Specifically regarding the interactions among insulin and fat metabolism on inflammation and immune function, (Osborn et al 2012). This is extremely important in the transition period in dairy cattle. Fat has been described as the master regulator in the development of systematic insulin resistance (Osborn et al, 2012). Pre-partum cattle go through a period of substantial insulin resistance that has elements in common with type 1 and type 2 diabetes, (Lucy, 2008). This immune response to fat metabolism is important in reproductive tract disease. Dairy cows go through a period of insulin resistance (IR) as part of nomothetic changes for milk production.
The effect of the hypothalamus
1) Growth Hormone (GH)
Growth hormone (GH) is a key regulator of nutrient partitioning in support of lactation. The mechanisms of partitioning, energy and fat metabolism in dairy cows have been reviewed in detail, (Butler, 2000). In brief, GH increases before calving, causing more gluconeogenesis in the liver and amplified lipolysis. Blood insulin levels are low and the liver, muscles and adipose are insulin resistant, this allows for more glucose to be available to the udder, where it is taken up independently. Blood glucose levels are low despite increased gluconeogenesis because of the massive drain of glucose to the udder. Growth hormone causes the secretion of insulin-like growth factor (IGF)-1 from the liver. A distinct adaptation in dairy cattle is that approximately 2 days before calving, GH receptor expression in the liver is reduced and stays low for around 2 weeks. During this time GH levels are high, favoring lipolysis and characteristic non-essential fatty acid (NEFA) increases (Butler, 2000).
NEFA may be used as an alternative fuel source to glucose in peripheral tissues or incorporated directly into milk fat. Elevated levels of GH and NEFA contribute to insulin resistance, IR, which influences NEB in ruminants.
Glucose, NEFA, insulin (IGF-1 factor) and GnIH all contribute signals to influence secretion of LH, which appears to be a determining factor in progression to the first postpartum ovulation. LH is regulated by GnRH, which is in turn regulated by oestrus phase, season (melatonin) and energy balance. GnRH is released and followed by an LH peak, resulting in ovulation. This LH produces progesterone to prepare the endometrium for possible implantation.
2) Insulin-like Growth factor (IGF)
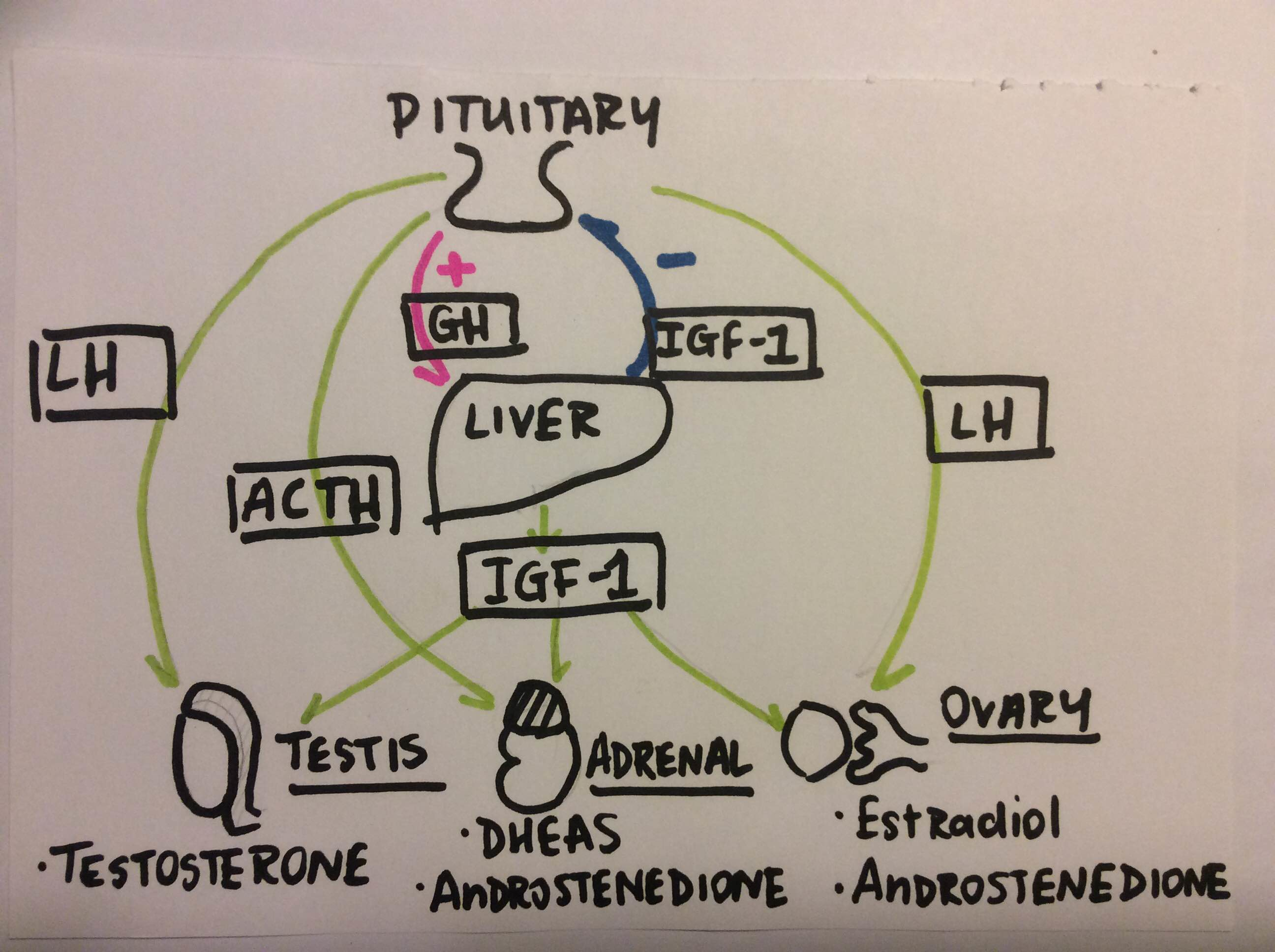
It is nearly certain that insulin lowers GH levels, which can explain the inverse relationship between the two circulating in obese ruminants (and humans) (Comford et al, 2011). The exact relationship between insulin and GH is quite complex due to the fact that insulin like growth factor (IGF) and IGF binding proteins also play a role. one of the impacts of IGF-1 on hormonal secretion can be seen in Fig. 1 below. Insulin-like growth factor-I (IGF-1) is involved in mammary gland development, promoting proliferation and inhibiting apoptosis of mammary epithelial cells (MECs).
It seems that insulin and IGF-1 regulate GH synthesis and secretion by different mechanisms (Gahete et al, 2013). The effects of these factors on the reproductive system is important because of the way these hormones regulate metabolic function. Direct actions of GH, growth factors and binding proteins on the reproductive endocrine system are slightly under acknowledged. Wathes (2012) put forward a case for the GH axis impacting upon the reproduction axis in ruminants, arguing that the lowered insulin-like growth factor 1 (IGF-1) levels in early lactation could predispose the animal to infertility. This is a complicated matter involving numerous growth factors and binding proteins, but the effect of these factors could be via the neurons upstream of GnRH cells in the hypothalamus, Kisspeptin neurons for example.
|
3) Kisspeptin
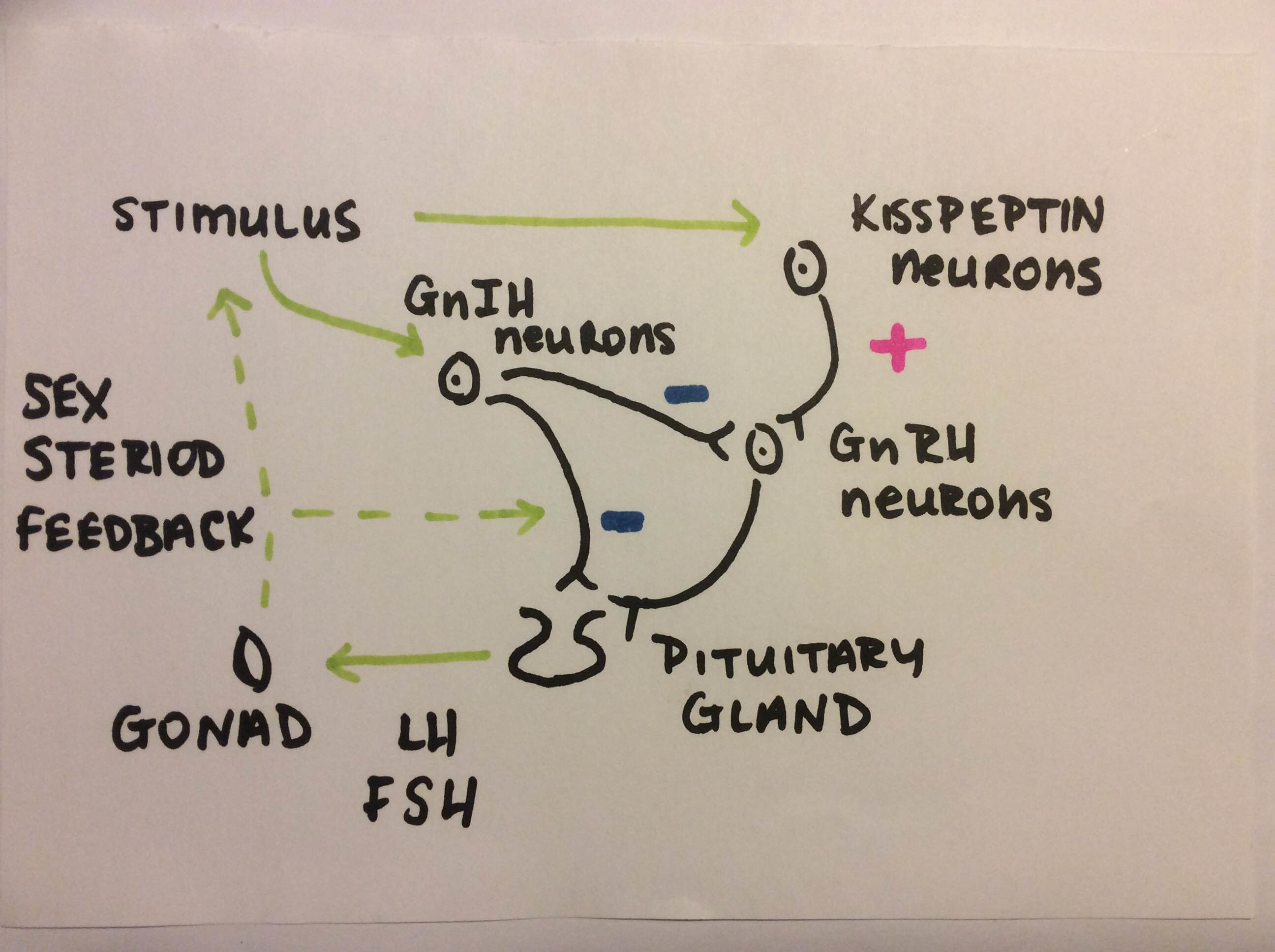
Kisspeptin, formerly know as metastin, is an important protein in reproduction that is encoded with the kiss-1 gene. It plays a role on the hypothalamic level, not on the pituitary gland directly (Daniel et al, 2015). It acts as a stimulus to luteinizing hormone (LH) and follicle stimulating hormone (FSH). However the stimulus is elicited via GnRH and not kisspeptin directly (Tanco et al, 2016) as the kisspeptin stimulus acts further upstream on the brain stem to GnRH (Seminara ,2005). This can be observed in Fig. 2 below. Kisspeptin positive cells are found in the arcuate nucleus, in the ventral medial nucleus of the brain (Alves et al, 2005). In the presence of LH and FSH follicles in the ovaries develop and the oocytes are released in the uterine tubes for fertilization. Kisspeptin trials have been conducted on different flocks of ewes showing its important role in reproduction. Li Q (2012) stated that the GnRH and LH response to Kiss-10 is higher during non-breeding season as well as being a regulator of sheep reproduction. The reason behind this is that with rising progesterone levels, for example during pregnancy, cells expressing Kiss-10 in sheep decreases (Sabet et al, 2007). Ewes are seasonal breeders, the come into heat when the day light hours shorten in autumn time. De Bond et al (2013) stated that Kisspeptin is necessary for ewes to respond when the ram is introduced due to the higher levels of FSH and higher LH pulse in the body. Ovulation can be stimulated outside of the breeding season after the infusion of Kisspeptin, intra venous to the ewe causing a LH surge in the anoestrus ewe (Sebert et al, 2010).
4) Gonadotropin-inhibitory hormone (GnIH)
GnIH is a potent inhibitor of reproductive function in ruminants and also stimulates food intake (Clarke et al., 2012). GnIH cells project to GnRH cells as well as to the appetite regulation peptides cells throughout the ruminant hypothalamus (Qi et al., 2009), providing a neuronal substrate for dual effect on reproduction and regulation of food intake. GnIH also stimulates food intake in the rat (Johnson et al., 2007) and the non-human primate (Clarke et al., 2012). It has been indication that there is an inverse relationship between reproductive function and appetite that is driven by GnIH. It was shown that GnIH expression and input to GnRH cells are lower during the breeding season in ewes (during declining day length), when appetite drive is highest (Smith et al., 2008). The effect of altered body weight on GnIH cell activity in sheep is, however, not known. Accordingly, a role for GnIH in the photoperiodic changes in body weight is not clearly apparent. GnIH does not seem to regulate energy expenditure, in terms of thermogenesis, at least in the sheep (Clarke et al., 2012).
|
Metabolic Diseases and their effect on reproduction
1) Ketosis
Primary ketosis is seen in high yielding dairy cows. It occurs when ruminants are in NEB, as increase in lactose production and fatty acid synthesis demands more glucose than is supplied. In this case, oxalo-acetate, OAC, must maintain the blood glucose level (2-3 mmol/L). This prevents Ac-CoA from entering the Citrate Cycle, reducing energy available. Ketone bodies are produced as a result of the breakdown of Ac-CoA. The increase in ketone bodies leads to a condition known as ketosis. Ketosis can be detected in the blood (ketoanemia), urine (ketouria) and milk (ketolactia) (Neogrady et al, 2016). In a large experiment on 474 herds, ketosis, estimated by milk acetone, was associated with the lengthening of interval from calving to first service, a decrease in fertility and an increase or ovarian cysts (Anderson et al, 1991). Pregnancy toxicosis is a similar metabolic disease, which occurs in sheep expecting twins or triplets.
2) Lipid mobilisation
Also known as “Fatty liver disease” or “Fat cow syndrome”. It prevails among dairy cows with high milk production during the first trimester of lactation (Neogrady et al, 2016). High milk yield potential and fatness at calving appear to be influencing factors (Fronk et al, 1980). Energy deficiency in early lactation triggers lipid mobilisation, this causes an increase in free fatty acids, FFA, in the blood which are synthesized to tri-glycerol fatty acids and stored in the liver. An increase in metabolic, infectious, digestive and reproductive disorders in cows increases significantly with a body condition score of four or greater (Morrow, 1976). A seperate experiment demonstrated that sixty-two percent of a 600 cow herd retained fetal membranes. Metritis, slow uterine involution and delayed rebreeding were also reported in these cows (MacCormack, 1987).
Infection and inflammation of the uterus and cervix affect approximately one in three dairy cows, with substantial impacts on the probability and timing on pregnancy. There is considerable overlap among the regulation of fat metabolism and uterine immune function and inflammation.
Consequences of under nutrition on reproduction
Under nutrition is the main reason for delayed first ovulation postpartum. Suckling, start of milk production and lack of essential nutrients prolong suppression of Luteinizing Hormone (LH) pulses. (Grimard et al, 1992) observed that one of the main factors effecting first cycling was body condition score, BCS, more so than suckling.
Chilliard et al (1998) defined under nutrition as not only a limitation in food supply but also a physiological increase in nutrients use e.g. during early lactation. Under nutrition in the first year will cause a delay in puberty (Ebling, 1990), suppressed ovulation (Gunn, 1983), postpartum ovulation in female adults (Bocquier ,1993) and/or increases embryo mortality (Gunn, 1983). The postpartum interval to first ovulation ranges from 17 to 42 days, some studies have found a positive correlation between milk yield and first ovulation (Butler et al, 1987). However, the same study concluded that this correlation only becomes significant after day 40 of lactation. Differences in stage of lactation may have an important bearing on fertility (Butler et al 1981), therefore energy balance must be considered in the postpartum interval to first ovulation. Butler et al (1981) showed first ovulation occurred, on average, approximately 10 days after maximal negative energy balance and near peak lactation.
The impact of Negative Energy Balance, NEB
Negative energy balance (NEB) during early lactation in dairy cows leads to an altered metabolic state that has major effects on the production of IGF family members. This has a severe impact on fertiliy. Modern high-yielding dairy cows enter a state of NEB around calving when the energy demand for maintenance and lactation exceeds that of dietary energy intake (Bauman et al, 1980). NEB may influence oviductal recovery and function postpartum by disrupting the balance of local IGF availability.
NEB reduces both circulating IGF-I and the local expression of IGF binding protein within the oviduct. It is possible that the predicted increased signalling by IGF-2 may perturb embryo development, contributing to the high rates of embryonic mortality in dairy cows (Fenwick et al. 2008). In conclusion, the IGF system is relevant to many aspects of reproductive physiology and the involvement of these growth factors in the oviduct provides another link between nutritional status and fertility. Embryos from cows in NEB experience altered IGF signalling within the oviduct, which likewise has adverse effects on their development, contributing to the high rates of embryo mortality seen in high-yielding dairy cows (Fenwick et al. 2008).
Conclusion
There are many factors, both exogenous and endogenous which can affect the reproduction rate of ruminants. For maximum productivity one must consider the role of metabolism, its integration with the reproduction system and the hormones it impacts on. Metabolism and reproduction work synergistically and this is evident by the above information regarding glucose, lipids, cholesterol and hormone synthesis. What is also a notable feature is the intense integration of the two metabolic roles in the hypothalamus. They are intertwined within the arcuate and ventral medial nuclei of the brain, with feeding and appetite control having a vast impact on reproductive and lactative status. Consequences of poor metabolism have a substantial impact on reproduction rate both through, delayed or lack of ovulation, or/and infection. This interaction of metabolism and reproduction must be closely monitored in the future to ensure maximum and sustainable reproduction rates in ruminants.
References
Alves BR, Cardoso RC, Prezotto LD, Thorson JF, Bedenbaugh M, Sharpton SM, Caraty. A, Keisler DH, Tedeschi LO, Williams GL, Amstalden M. (2015). Elevated body weight gain during the juvenile period alters neuropeptide Y-gonadotropin-releasinghormone circuitry in prepubertal heifers. Biology of Reproduction 92:46, 1–10 DOI 10.1095/biolreprod.114.124636
Andersson L, Gustafsson AH, Emanuelson U, (1991), Effect of hyperketonemia and feeding on fertility in dairy cows, theriogenology. 521-536
Barbcock DK, (1985) Relationship between fertility and glucose and cholesterol concentrations in Holstein cows, Am. J. Vet. 2607-2612
Bauman DE, Currie WB. (1980) Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. Journal of Dairy Science. ;63:1514–1529.
Bocquier F et al, (1993) Effects of body composition variations on the duration of the postpartum an ovulatory period of milked ewes submitted to two different photoperiods, Reprod. Nutr. Dev. 33 395-403
Brandebourg D, Chad D Foradori, (2016) Distribution and regulation of gonadotropin-releasing hormone, Kisspeptin, RF-amide related peptide-3, and dynorphin in the bovine hypothalamus, PeerJ 4:e1833; DOI 10.7717/peerj.1833
Butler WR, (2000): Nutritional interactions with reproductive performance in dairy cattle. Anim Reprod Sci 60–61, 449–457
Butler WR and Smith RD (1989) Interrelationships between energy balance and postpartum reproductive function in dairy cattle. Journal of Dairy Science 72:3 767-783
Butler WR, RW Everett and CE Coppock (1981). The relationships between energy balance, milk production and ovulation in post-partum Holstein cows, J. Anim. Sci 50:919
Castano JP, Christian H, Luque RM, Kineman RD, (2013). Insulin and IGF-I inhibit GH synthesis and release in vitro and in vivo by separate mechanisms. Endocrinology 154, 2410–2420
Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS, (2012)a. Gonadotropin inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 95, 305–316.
Cornford AS, Barkan AL, Horowitz JF. (2011). Rapid suppression of growth hormone concentration by overeating: potential mediation by hyperinsulinemia. J. Clin Endocrinol. Metab. 96, 824–830
De Bond JP, Li Q, Millar RP, Clarke IJ, Smith JT.( 2013) Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PLoS One.;8:1–11.
Ebling FPJ. et al, (1990) Metabolic interfaces between growth and reproduction. III- Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb, Endocrinology 126 2719-2727
Fenwick MA, Llewellyn S, Fitzpatrick R, Kenny DA, Murphy JJ, Patton J, & Wathes DC. (2008). Negative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviduct. Reproduction (Cambridge, England), 135(1), 63–75.
Fronk TJ, LH Schultz and AR Hardie. (1980). Effect of dry period over conditioning on subsequent metabolic disorders and performance of dairy cows. J. Dairy Sci. 63:1080
Gahete MD, Cordoba-Chacon J, Lin Q, Bruning JC, Kahn CR, Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ, (2008). Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology 149, 1951–1959.
Grimard, B, Humblot P, Parez V, Mialot JP, Thibier M, (1992) Synchronisation de l’oestrus chez la vache Charolaise : Facteurs de variation de la cyclicite pretraitement, du taux d’ovulation après traitement et du taux de fertilite a l’oestrus induit Elev. Insemin. 250 5-17
Gunn RG, (1983) The influence of nutrition on the reproductive performances of ewes in: Haresign W. (Ed.), Sheep Production, Butterworths, London, 99-110
Johnson MA, Tsutsui K, Fraley GS, (2007). Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 51, 171–180
Joseph A Daniel, Chad D Foradori, Brian K Whitlock, James L Sartin, (2015) Reproduction and Beyond, kisspeptin in ruminants, journal of Animal Science and Biotechnology 6:23 DOI 10.1186/s40104-015-0021-4
Kappel LC, Ingraham RH, Morgan EB, Zeringue L, Wilson D, Osborn O, Olefsky JM, (2012): The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18, 363–374.
Li Q, Roa A, Clarke IJ, Smith JT. (2012) Seasonal variation in the gonadotropinreleasing hormone response to kisspeptin in sheep: possible kisspeptin regulation of the kisspeptin receptor. Neuroendocrinology.;96:212–21
Lucy MC, (2008): Nutrient partitioning andreproductive performance in dairy cows. Proceedings Intermountain Nutrition Conference, 139–150.
MacCormack J. (1987). Fat cow syndrome and its complications Vet. Med. Small Anim. Clin. 73:1057
Marie M. (1999) Links between nutrition and Reproduction in Dairy cattle. Ecole Nationale Superieure d'Agronomie et des Industries Alimentaires, I.N.P.L., Vandoeuvre-les-Nancy, France
Miettinen PV. (1990) Metabolic balance and reproductive performance in finish dairy cows, Zentralbl. Veterinaymed. 417-424
Morrow DA, (1976). The fat cow syndrome. J. Dairy Sci. 59:1635
Neogrady S. and Dr Matis G.,(2016) Pathobiochemical relevance of the metabolism of ruminants, 15-25
Qi Y, Oldfield BJ, Clarke IJ, (2009). Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J. Neuroendocrinol. 21, 690–697.
Ruurd Jorritsma, Theo Wensing, Theo AM Kruip, Peter LAM Vos, Jos PTM Noordhuizen. (2002) Metabolic changes in early lactation and impaired reproductive performance in dairy cows.
S, Rahmanifar F, Jafarzadeh Shirazi MR, Tanideh N, Moghadam A, Niazi A. (2014). Expression of RFamide-related peptide-3 (RFRP-3) mRNA in dorsomedial hypothalamic nucleus and KiSS-1 mRNA in arcuate nucleus of rat during pregnancy. International Journal of Fertility and Sterility 8:333–340
Sébert ME, Lomet D, Saïd SB, Monget P, Briant C, Scaramuzzi RJ, et al. (2010) Insights into the mechanism by which kisspeptin stimulates a preovulatory LH surge and ovulation in seasonally acyclic ewes: potential role of estradiol. Domest Anim Endocrinol.;38:289–98
Seminara SB. (2005). We all remember our first kiss: kisspeptin and the male gonadal axis. Journal of Clinical Endocrinology and Metabolism 90:6738–6740 DOI 10.1210/jc.2005-2246
Wathes DC, (2012). Mechanisms linking metabolic status and disease with reproductive outcome in the dairy cow. Reprod. Domest. Anim. 47 (Suppl. 4), 304–312.
Yves Chillard, Francois Bocquier, Michel Doreau, (1998) Digestive and metabolic adaptions of ruminants to undernutrition, and conquences on reproduction., 131- 152