|
Size: 23026
Comment:
|
← Revision 36 as of 2018-04-27 06:39:39 ⇥
Size: 23035
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 17: | Line 17: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Pitviper.png|pop-up text|width="300"}} <<BR>>'''Fig. 1'''<<BR>>''Brazilian Pit Viper - The source of the first ever venom derived medicine, Captopril'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Pitviper.png|pop-up text|width="300"}} <<BR>>'''Fig. 1'''<<BR>>''Brazilian Pit Viper - The source of the first ever venom derived medicine, Captopril'' || |
| Line 34: | Line 34: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Crotamin.png|pop-up text|width="200"}} <<BR>>'''Fig. 2'''<<BR>>''Structure of Crotamine'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Crotamin.png|pop-up text|width="200"}} <<BR>>'''Fig. 2'''<<BR>>''Structure of Crotamine'' || |
| Line 47: | Line 47: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Tail.png|pop-up text|width="300"}} <<BR>>'''Fig. 3'''<<BR>>''Tail-flick apparatus used to test analgesic effects'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Tail.png|pop-up text|width="300"}} <<BR>>'''Fig. 3'''<<BR>>''Tail-flick apparatus used to test analgesic effects'' || |
| Line 59: | Line 59: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Thrombin.png|pop-up text|width="600"}} <<BR>>'''Fig. 4'''<<BR>>''The effect of SVTLEs on the formation of the fibrin net'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Thrombin.png|pop-up text|width="600"}} <<BR>>'''Fig. 4'''<<BR>>''The effect of SVTLEs on the formation of the fibrin net'' || |
| Line 80: | Line 80: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Clot.png|pop-up text|width="600"}} <<BR>>'''Fig. 5'''<<BR>>''The effects of Eptifibatide on a heart attack patient. An occlusive blood clot is circled on the left. The right angiograph is taken after Eptifibatide treatment. The previously occluded section is circled'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Clot.png|pop-up text|width="600"}} <<BR>>'''Fig. 5'''<<BR>>''The effects of Eptifibatide on a heart attack patient. An occlusive blood clot is circled on the left. The right angiograph is taken after Eptifibatide treatment. The previously occluded section is circled'' || |
| Line 102: | Line 102: |
| Snake venoms mainly consists of proteins and peptides. They contain many enzymatic and toxicological components which can be used in the production of various drugs and pharmacological tools. The presence of these components allows for the expression of biochemical activities and mechanisms of actions that activate and inhibit important physiological processes. Venoms, and their derivatives, have already been utilized in top-selling, lifesaving medications such as disintegrins and ACE inhibitors. These drugs are widely used for the treatment of acute heart failure and other circulatory pathologies. | Snake venoms mainly consist of proteins and peptides. They contain many enzymatic and toxicological components which can be used in the production of various drugs and pharmacological tools. The presence of these components allows for the expression of biochemical activities and mechanisms of actions that activate and inhibit important physiological processes. Venoms, and their derivatives, have already been utilized in top-selling, lifesaving medications such as disintegrins and ACE inhibitors. These drugs are widely used for the treatment of acute heart failure and other circulatory pathologies. |
Therapeutic Uses of Snake Venom
By: B Melody, E Collins, D Bond
Snakes and their venom have been associated with healing for millennia. In Ancient Greek mythology, Asclepius – the God of Medicine, was said to carry a rod with a sacred snake coiled around it. Venoms have been used throughout history in folk medicine and are now being researched for their potential uses in modern pharmacology.
Contents
Venom Overview:
A - Introduction to Venoms:
Venoms are the specialized toxic secretions of certain animals. Venom is introduced into the body of the venomous animal’s prey by various means including bites, stings or via other sharp appendages. Venoms cause rapid physiological changes that can result in pain or death. (Kenneth et al., 2009). Although there are venomous animals in many phyla, reptiles compose the largest proportion of venomous vertebrates. (Chan et al., 2016). It is believed that the venoms of poisonous snakes have evolved over 120 million years in order to facilitate the survival of these species in specific hostile environments. (Casewell et al., 2013). The composition of venoms varies among snake families but also within the same species. These variations are thought to be a function of age, geographic location, sex and diet (Chippaux et al., 1991).
B - Components of Snake Venom:
Snake Venoms are a complex mixture of various biomolecules, enzymes and inorganic cations. The main component of snake venoms are proteins. Proteins and peptides make up 95% of the dry weight of snake venom. The proteins in snake venom can have enzymatic and toxicological effects. Venoms also contain nucleotides, free amino acids, biogenic amines, lipids and carbohydrates. The inorganic cations found in venom include Na+, K+, Ca+2, Mg+2, Mn+2, Ni+2, Zn+2, Fe+2 and Co+2 (Waheed et al., 2017). These cations can be necessary for the correct functioning of venom enzymes. For example, the activity of acetylcholinesterase found in the venom of sea snakes and cobras is maintained by zinc. Calcium is important for the activation of phospholipase enzymes.
Snake venoms have been found to contain at least 25 different enzymes and between 30-100 different toxins. The combination and strength of these components in each venom vary and no single venom contains them all. The unique composition of a snake’s venom determines the venom type and effect. Enzymes commonly found in snake venom include acetylcholinesterase, L-amino oxidase, serine proteases, phospholipases and fibrogenases (Waheed et al., 2017). The enzymes have various biological effects, for example, L-amino oxidase is the enzyme responsible for giving snake venom it’s yellow colour (Vyas et al., 2013). The bio-activities of other venom enzymes and toxins, as well as their potential biomedical use, will be discussed throughout this wiki page.
|
Venom Effects and Mechanisms of Action:
Snake venoms are known to have neurotoxic, cytotoxic, cardiotoxic, myotoxic and enzyme inhibiting effects (Chan et al., 2016). Venoms of these types have been found to be of potential therapeutic use. Some examples of therapeutically useful venom derived compounds and their mechanisms of action will be outlined in this section.
A - Pharmacological Uses
Venoms that do not necessarily have therapeutic applications can still be useful as pharmacological tools (Chan et al., 2016). The venom of Dendroaspis snakes e.g. Eastern Green Mamba contains muscanaric toxins. These toxins are able to inhibit the ligand binding to muscanaric ATCH receptors. Their high affinity and selectivity allow them to be used as a pharmacological tool to study the distribution and functional role of muscanaric ACTH receptors (Servent et al., 2011). Muscanaric ACTH receptors are of interest in the study of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. (Koh et al., 2006)
B - Anti-Tumor Activity
Venoms with cytotoxic effects are being explored as possible cancer therapies. Cytotoxins comprise as much as 40-70% of cobra venom (Gasanov, 2014). Cytotoxins from cobra venom are three-finger toxins. They are composed of 60 amino acid residues and possess four disulfide linkages (Chan et al., 2016). They are non-enzymatic toxins. Most pathological activities of cytotoxins are due to their ability to bind to cell membranes, forming pores and otherwise causing alterations to the lipid bilayers (Konshina et al., 2011).This induces non-specific lysis, causing cell death (Gasanov et al., 1997).
Cytotoxins further show anti-tumor activity by inducing programmed cell death via lysosomal and mitochondrial mediated pathways (Feofanov et al., 2005). Cobra venom cytotoxins have displayed this form of anti-tumor activity experimentally. The administration of a cytotoxin derived from the venom of the Chinese Cobra extended the life of mice with ascites tumors. The tumor cells exposed to this cytotoxin displayed apoptosis and necrosis, as well as disruption to mitochondrial and lysosomal membranes. (Wu et al., 2013). Similar results were seen using a cytotoxin originating in the venom of the Indian Cobra. Apoptosis was triggered in human leukemia cells which slowed their growth and arrested their cell cycle. This was due to enzymatic disruption and an altering of the mitochondrial membrane potential (Das et al., 2013).
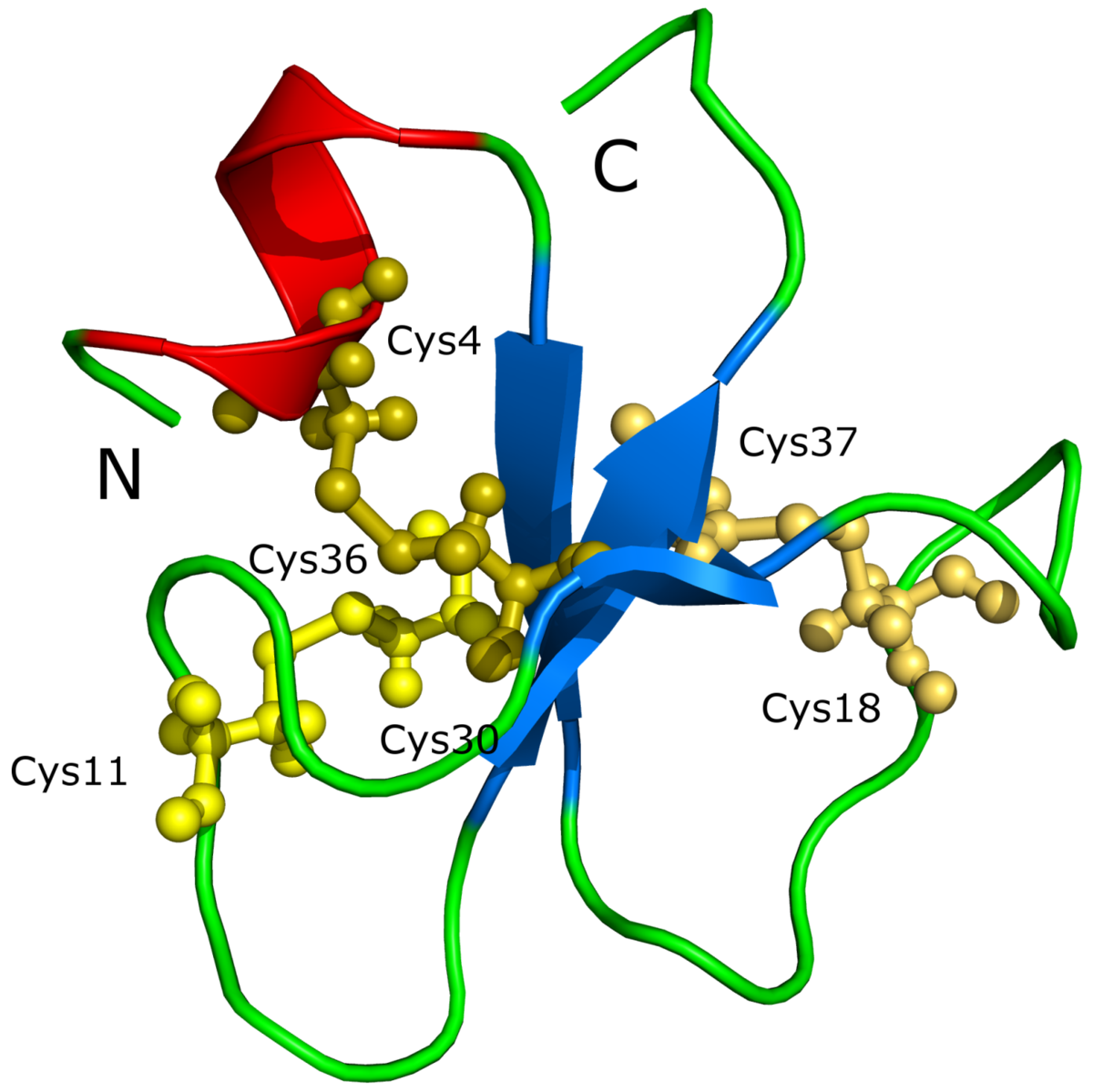
Crotamine, seen in figure 2, is a myotoxin derived from the venom of the South American Rattlesnake. Myotoxins are small, basic peptides found in snake venoms. Myotoxins interfere with voltage-sensitive sodium channels in the sarcolemma of skeletal muscles (Chan et al., 2016). This leads to a sodium influx followed by the formation of an action potential and eventually the contraction of the muscle. This characteristic of crotamine causes quick release of intracellular calcium and a disruption of mitochondrial membrane potential. These effects, along with the disruption of lysozymes and proteolytic enzymes have an overall cytotoxic effect. This allows crotamine to be used to initiate cell death in targeted tumor cells.
|
C - Analgesics
The venoms of certain elapid and viperid snakes are being studied for their potential use as analgesics due to their neurotoxic activity (Rajendra et al., 2004). Venom toxins being studied for their analgesic effects include cobrotoxin from the Chinese Cobra, crotamine from the South American Rattlesnake and mambalgins from the Black Mamba (Chan et al., 2016).
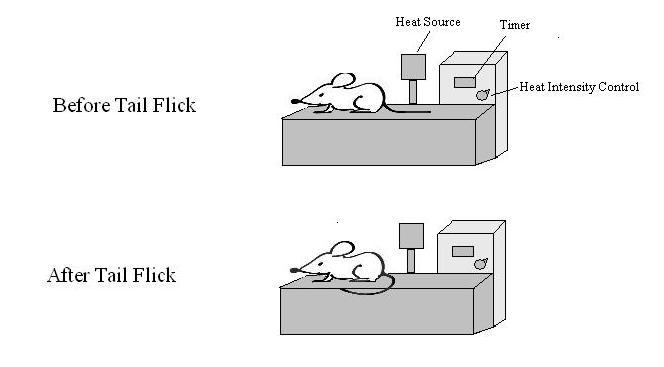
Cobratoxin is a short chain post-synaptic a-neurotoxin (Koh et al., 2006). It was shown to have an analgesic effect on mice as measured by a tail flick test (shown in figure 3). The analgesic response of cobratoxin was experimentally attributed to the central cholinergic neurons (Chen and Robinson, 1990) and was found to be opioid independent (Chen et al., 2006)
Crotamine also demonstrated antinociceptive activity when administered to mice. These effects were shown by hotplate and acetic acid induced writhing tests. When compared with morphine (4mg/kg), crotamine, even in very low doses (133.4 microg/kg) was found to be 30-fold more potent than morphine (w/w) as an analgesic. An opioid action mechanism was suggested, as well as activity in the central and peripheral systems (Mancin et al., 1998).
Mambalgin is a 57 residue polypeptide toxin that has been shown to have an opioid independent analgesic effect on inflammatory and neuropathic pain. (Diochot et al., 2016). The mechanism of action of mambalgin involves the blocking of acid-sensing ion channels (ASICs) in peripheral and central neurons (Chan et al., 2016).
|
D - Anticoagulating Activity
A large proportion of the already developed venom derived pharmaceuticals are anticoagulants. They take the form of platelet aggregators and blood-clotting inhibitors (Koh et al., 2006). Blood coagulation takes place due to the activation of a series of clotting factors which results in the formation of the fibrin net. Coagulation happens as a result of the extrinsic or intrinsic pathways both of which lead to the activation of the common Stuart-Prower (X) factor. The activated X factor (Xa), along with an activation complex, stimulate the prothrombin à thrombin reaction. Thrombin in turn stimulates activation of fibrinogen and factor XIII which forms the fibrin net. There are many snake venom proteins that have been found to interfere with the blood coagulation cascade.
Thrombin is also essential in stopping blood coagulation. Thrombin activates both plasma Protein-C and plasminogen. Protein-C directly prevents the activation of key enzymes of both the extrinsic and intrinsic pathways (V and VII). Plasminogen is a proteolytic enzyme which dissolves the fibrin net. Approximately 100 snake venom toxins have been identified as ‘thrombin like enzymes’, known as SVTLEs (Pirkle, 1998). SVTLEs generally act as fibrinogenase enzymes cleaving one of more fibrinogen chains/fibrinopeptides. They are either serine proteases or metalloproteinases. SVTLEs prevent the formation of the stable fibrin net by inhibiting the activation of factor (XIII) and also break down fibrin rich clots (Koh et al., 2006). The effect of SVTLEs on blood clot formation has been outlined in figure 4. SVTLEs have been isolated from many snakes including the Malayan pit viper, Southern Copperhead and Western Diamondback Rattlesnake.
Disintegrins are arginylglycylaspartic acid (RGD) containing proteins found in snake venoms. RGD binds to aIIb/b3 Integrin receptors on platelets and blocks glycoprotein IIb/IIIa receptors, preventing platelet aggregation (Koh et al., 2006). Targeting and inhibiting RGD dependent integrins is a major goal of pharmaceutical research for many diseases including thromboembolic disorders. Blockers of platelet integrins could also have application in certain types of cancer treatments as platelets contribute to tumor growth, angiogenesis and metastasis (Trikha and Nakada, 2002). Examples of pharmaceuticals derived from snake toxins effecting the hemostatic system and coagulation cascade will be discussed in a later section.
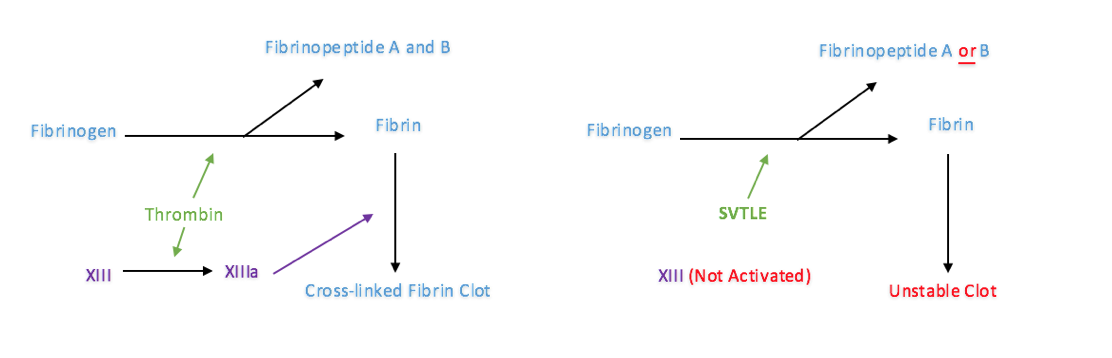
Fig. 4
The effect of SVTLEs on the formation of the fibrin net
E - Ace Inhibitors
Angiotensin Converting Enzyme (ACE) inhibitors are drugs used in the treatment of hypertension and heart failure. The kidney plays a role in the regulation of circulation through the production of angiotensin II. Hypovolemia and reduced Na concentration in the distal tubules stimulates rennin secretion in the kidney. Renin is converted to angiotensin I which is in turn converted to angiotensin II by ACE. Angiotensin II has a pressor effect, increasing blood pressure via salt retention and strong vasoconstriction.
A compound derived from the Brazilian Pit Viper, shown in figure 1, has been shown to have an ACE inhibiting effect. It also potentiates bradykinin, increasing capillary permeability and vasodilation. The discovery of this compound and its effect lead to the production of the first venom derived drug called Captopril.
Currently Available Medicines:
Drugs derived from snake venom toxins can be lifesaving and include some of the top selling medications in the history of medicine (Wexler, 2014). There are already several widely used venom derived medications and many more are currently being researched and clinically trialed. A selection of such drugs have been described in this section.
A - Ancrod
Ancrod is a SVTLE that was derived from the venom of the Malayan Pit Viper. It is a defibrigenating enzyme which shows anticoagulating and clot clearing activity due to the mechanisms outlined above. It had previously been marketed in some European countries but was withdrawn. It is currently under further phase III clinical trials being investigated as a treatment for stroke (Chan et al., 2016).
B - Integrilin (Eptifibatide)
Integrilin is a disintegrin, RGD containing drug based on a compound found in the venom of the Southeastern Pygmy Rattlesnake. It was introduced into the market in 1998 and has FDA approval. It blocks glycoprotein IIb/IIIa receptors and shows anti-platelet activity (Chan et al., 2016). Integrilin is used to treat cardiac infarction after acute coronary syndrome (see figure 5). Although it is widely used in human medicine the clinical application of integrin inhibitors remains undetermined in veterinary medicine (Riviere and Papich, 2009)
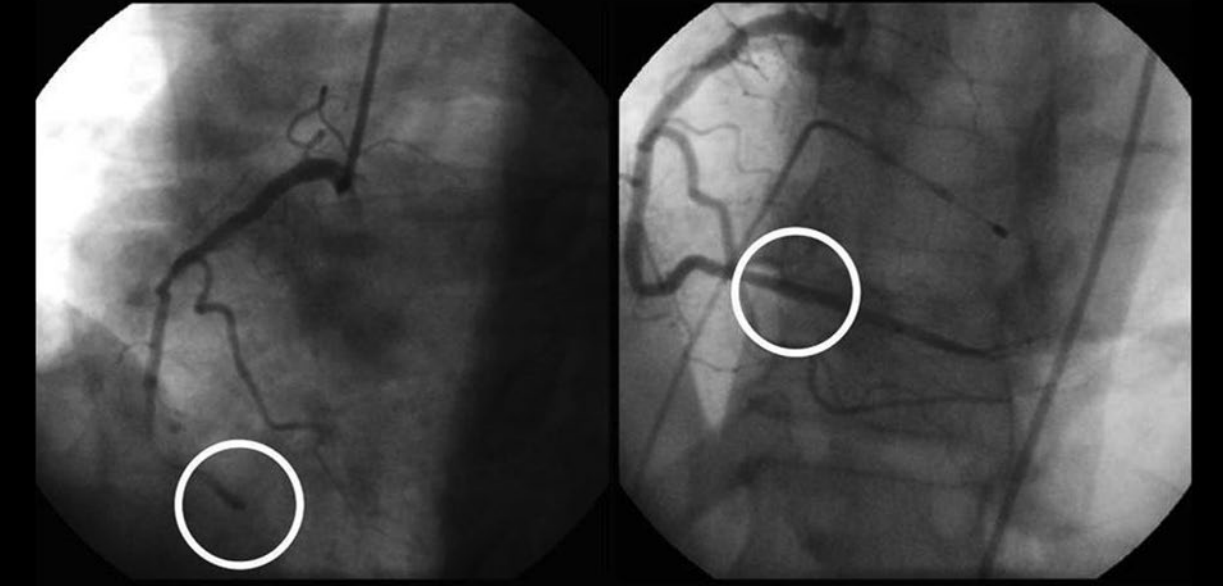
Fig. 5
The effects of Eptifibatide on a heart attack patient. An occlusive blood clot is circled on the left. The right angiograph is taken after Eptifibatide treatment. The previously occluded section is circled
C -Aggrastat (Tirofiban)
- Tirofiban is a disintegrin found in the saw scaled viper. It is a glycoprotein IIb/IIIa inhibitor which gained FDA approval in 1998 (Chan et al., 2016). It is recommended to be used in conjunction with heparin in the treatment of acute coronary disease.
D - Captopril
Captopril is a toxin derived from the venom of the Brazilian Pit Viper. It was discovered in 1975 and studied by Nobel prize winner Sir John Vane, eventually leading to the development of the first orally administered ACE inhibitor. (Koh et al., 2006) The primary use for ACE inhibitors in both human and veterinary medicine is for the treatment of heart failure. ACE inhibitors are also used to treat systemic hypertension in humans but have not been a successful in this regard in other species. There is however a possibility that ACE inhibitors can be useful in treating certain renal conditions in cats and dogs. There is evidence that their use can attenuate the progression of renal failure in cats with significant proteinuria. Captopril is an effective ACE inhibitor with an affinity for ACE approx. 30,000 times greater than that of angiotensin I. Captopril is supplied in tablet form with a 0.5-1.0mg/kg and 0.5-1.5mg/kg dosage recommended in dogs and cats respectively. Although Captopril is generally well tolerated in most patients some side effects like anorexia, vomiting and diarrhea may occur. Its effects generally last less than 4 hours so repeated administration is necessary. For these reasons Captopril is no longer recommended in veterinary practice as more suitable ACE inhibitors are now available. (Maddison et al., 2008)
E - Defibrase (Batroboxin)
Batroboxin is a thrombin-like enzyme isolated from the venom of the Common Lancehead and Brazilian Lancehead. It is a serine protease which functions as a defibrinogenating agent. It is used to treat patients with thrombosis in some countries but does not have FDA approval (Chan et al., 2016).
F - a-Cobratoxin
Cobratoxin is a neurotoxin derived from Chinese Cobra venom. It is being evaluated for its use as an analgesic and has completed phase I clinical trials in China (Harvey, 2014).
Conclusion:
Snake venoms mainly consist of proteins and peptides. They contain many enzymatic and toxicological components which can be used in the production of various drugs and pharmacological tools. The presence of these components allows for the expression of biochemical activities and mechanisms of actions that activate and inhibit important physiological processes. Venoms, and their derivatives, have already been utilized in top-selling, lifesaving medications such as disintegrins and ACE inhibitors. These drugs are widely used for the treatment of acute heart failure and other circulatory pathologies.
Snake venoms have proven to be a rich source of bioactive molecules and inspiration for the pharmaceutical industry and research into their potential use is ongoing. Current research is dependent on finding ways to stabilize the complex peptides found in venoms or creating peptidomimetic compounds which can mimic their actions. Although relatively few venom derived drugs have been successful in making it to market, there are many more currently being researched and clinically trialed. The compounds being researched now may hold the key to potential advances into the treatment of many ailments including Alzheimer’s, Parkinson’s, hypertension, various kinds of cancer and chronic pain.
References:
Casewell, N., Wüster, W., Vonk, F., Harrison, R. and Fry, B. (2013). Complex cocktails: the evolutionary novelty of venoms. Trends in Ecology & Evolution, 28(4), pp.219-229.
Chan, Y., Cheung, R., Xia, L., Wong, J., Ng, T. and Chan, W. (2016). Snake venom toxins: toxicity and medicinal applications. Applied Microbiology and Biotechnology, 100(14), pp.6165-6181.
Chen, R. and Robinson, S. (1990). The effect of cholinergic manipulations on the analgesic response to cobrotoxin in mice. Life Sciences, 47(21), pp.1949-1954.
Chen, Z., Zhang, H., Gu, Z., Chen, B., Han, R., Reid, P., Raymond, L. and Qin, Z. (2006). A long-form alpha-neurotoxin from cobra venom produces potent opioid-independent analgesia1. Acta Pharmacologica Sinica, 27(4), pp.402-408.
Chippaux, J., Williams, V. and White, J. (1991). Snake venom variability: methods of study, results and interpretation. Toxicon, 29(11), pp.1279-1303.
Das, T., Bhattacharya, S., Biswas, A., Gupta, S., Gomes, A. and Gomes, A. (2013). Inhibition of leukemic U937 cell growth by induction of apoptosis, cell cycle arrest and suppression of VEGF, MMP-2 and MMP-9 activities by cytotoxin protein NN-32 purified from Indian spectacled cobra (Naja naja) venom. Toxicon, 65, pp.1-4.
Diochot, S., Alloui, A., Rodrigues, P., Dauvois, M., Friend, V., Aissouni, Y., Eschalier, A., Lingueglia, E. and Baron, A. (2016). Analgesic effects of mambalgin peptide inhibitors of acid-sensing ion channels in inflammatory and neuropathic pain. PAIN, 157(3), pp.552-559.
Feofanov, A., Sharonov, G., Astapova, M., Rodionov, D., Utkin, Y. and Arseniev, A. (2005). Cancer cell injury by cytotoxins from cobra venom is mediated through lysosomal damage. Biochemical Journal, 390(1), pp.11-18.
Gasanov, S. (2014). Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. Journal of Clinical Toxicology, 4(1).
Gasanov, S., Alsarraj, M., Gasanov, N. and Rael, E. (1997). Cobra Venom Cytotoxin Free of Phospholipase A 2 and Its Effect on Model Membranes and T Leukemia Cells. Journal of Membrane Biology, 155(2), pp.133-142.
Harvey, A. (2014). Toxins and drug discovery. Toxicon, 92, pp.193-200.
Koh, D., Armugam, A. and Jeyaseelan, K. (2006). Snake venom components and their applications in biomedicine. Cellular and Molecular Life Sciences, 63(24), pp.3030-3041.
Konshina, A., Boldyrev, I., Utkin, Y., Omel'kov, A. and Efremov, R. (2011). Snake Cytotoxins Bind to Membranes via Interactions with Phosphatidylserine Head Groups of Lipids. PLoS ONE, 6(4), p.e19064.
Mancin, A., Soares, A., Andrião-Escarso, S., Faça, V., Greene, L., Zuccolotto, S., Pelá, I. and Giglio, J. (1998). The analgesic activity of crotamine, a neurotoxin from Crotalus durissus terrificus (South American rattlesnake) venom: A biochemical and pharmacological study. Toxicon, 36(12), pp.1927-1937.
Pirkle, H. (1998). Thrombin-like Enzymes from Snake Venoms: An Updated Inventory. Thrombosis and Haemostasis, 79(03), pp.675-683.
Rajendra, W., Armugam, A. and Jeyaseelan, K. (2004). Toxins in anti-nociception and anti-inflammation. Toxicon, 44(1), pp.1-17.
Servent, D., Blanchet, G., Mourier, G., Marquer, C., Marcon, E. and Fruchart-Gaillard, C. (2011). Muscarinic toxins. Toxicon, 58(6-7), pp.455-463.
Trikha, M. and Nakada, M. (2002). Platelets and Cancer: Implications for Antiangiogenic Therapy. Seminars in Thrombosis and Hemostasis, 28(1), pp.39-44.
Vyas, V., Brahmbhatt, K., Bhatt, H. and Parmar, U. (2013). Therapeutic potential of snake venom in cancer therapy: current perspectives. Asian Pacific Journal of Tropical Biomedicine, 3(2), pp.156-162.
Waheed, H., Moin, S. and Choudhary, M. (2017). Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Current Medicinal Chemistry, 24(17).
Wu, M., Ming, W., Tang, Y., Zhou, S., Kong, T. and Dong, W. (2013). The Anticancer Effect of Cytotoxin 1 from Naja atra Cantor Venom is Mediated by a Lysosomal Cell Death Pathway Involving Lysosomal Membrane Permeabilization and Cathepsin B Release. The American Journal of Chinese Medicine, 41(03), pp.643-663.
Bibliography:
Kenneth VK, Scott AW, Tamara LS (2009) Reptile venom glands. In: Mackessy SP (ed) Handbook of venoms and toxins of reptiles. Boca Raton: CRC, pp. 65–66.
Maddison, J., Page, S. and Church, D. (2008). Small animal clinical pharmacology. Edinburgh: Saunders Elsevier, p. 418.
Riviere, J. and Papich, M. (2009). Veterinary pharmacology and therapeutics. Ames, Iowa: Wiley-Blackwell, p. 688.
Wexler, P. (2014). Encyclopedia of toxicology. 3rd ed. Amsterdam: Elsevier, pp.252-259.
Figures:
Figure 1:
https://commons.wikimedia.org/wiki/File:Jararaca_-_Bothrops_jararaca_-_Sibilando.jpg
Figure 2:
https://commons.wikimedia.org/wiki/Category:Crotamine#/media/File:Crotamin_1H5O.png
Figure 3:
https://commons.wikimedia.org/wiki/File:Tail_Flick_Test_Apparatus.jpg
Figure 4:
Prepared by Breda Melody
Figure 5:
http://zoltantakacs.com/zt/ma/dl/Takacs_Zoltan_2014_Animal_venoms_medicine.pdf