Written by: Jessica Evert, Cristina Martinez, Rebecca Pisani |
Supervisor: Dr. István Tóth (DVM, PhD) |
University of Veterinary Medicine, Budapest, Hungary |
The metabolic effects of Testosterone Deficiency and Overproduction
Introduction: Importance & function
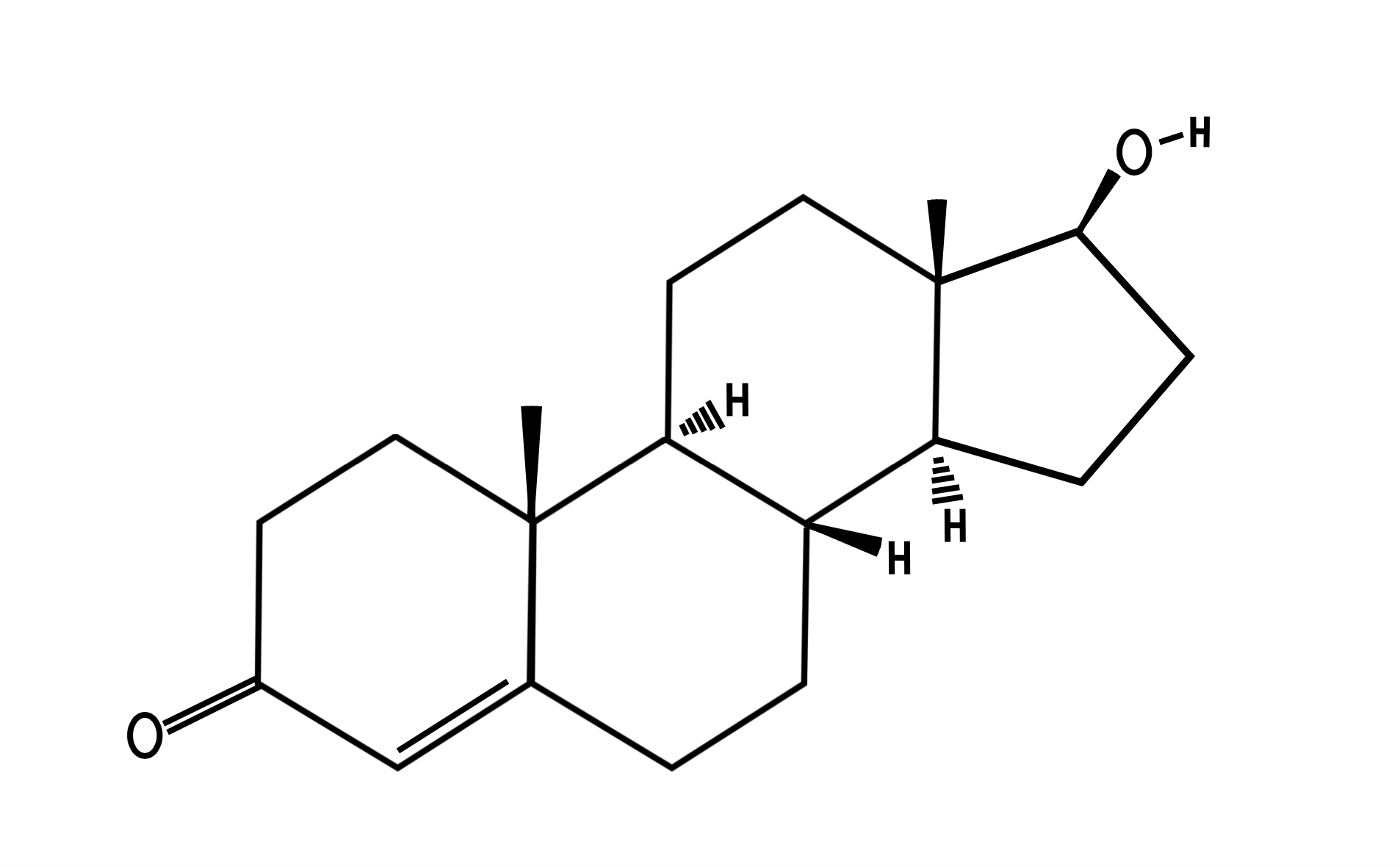
Testosterone (T) (Figure. 1), is a crucial androgen synthesized from cholesterol due to its steroid ring, and acetate (Nieschlag et al, 2012). Leydig cells produce 95% of T of testis while 5% is produced in adrenal glands(Lin and Erinoff, 1990); the adrenal cortex does this by secreting dehydroepiandrosterone (DHEA) and DHEA-sulfate. These are prohormone substrates that aid formation of stronger androgens (T, dihydrotestosterone (DHT)). T plays a vital role in male reproductive organ development and maintenance of sperm production. It affects numerous other cells and organs including signaling erythropoiesis, muscle and bone strength, libido, hair growth and mood alterations(Bhasin and Ravi, 2019). |
|
Figure 1 Structure of Testosterone |
Circulating T consists of 2% free T, 38% bound to albumins and 60% bound to SHBG (sex hormone binding globulin) (Mody et al, 2015). Bioavailable T is the sum of free and albumin bound T. It binds and activates androgen receptors (AR). 5-α-reductase converts T to more potent DHT in androgen sensitive tissues (prostate, epididymis, testes) (Tyagi et al, 2017). Once free T or DHT bind to AR, the hormone-receptor complex binds to DNA, and elicits their biological effects.
The conversion of T to estrogen is catalysed by aromatase - member of the cytochrome P45 enzyme family - in neural tissue, adipose, liver and bone. Estrogen is essential for sperm development and maintenance. In females T and its precursor (androstenedione), are produced in the adrenal cortex and ovary stroma. From the ovary, T is converted to estradiol. Other organs releasing T in females: bones, breast, muscle and fat.
Testosterone Deficiency: symptoms and why it occurs
Hypogonadism is the decreased function of the gonads due to reduced reproductive hormone secretion. Primary hypogonadism develops when the testes abnormally respond to Follicle Stimulating Hormone(FSH) or Luteinizing Hormone(LH), leading to insufficient T production. Diminished T levels are not enough to inhibit FSH and LH production via a feedback mechanism, hence their concentrations rise uncontrollably (Hirsch, 2019). Low T and high LH/FSH levels are signs of testicular failure - for which there is currently no effective treatment in veterinary medicine. Other causes of hypogonadism: castration, testicular trauma, chemotherapy, radiation therapy, low Gonadotropin-releasing hormone(GnRH) levels, pituitary tumor (Bain, 2008). It involves numeros symptoms including: anemia, weak bone development and thus higher risk of osteoporosis, reduced hair growth, muscle weakness, low libido and altered behaviours (often depression) (Morales et al, 2015).
Effect on Blood
T elicits erythropoiesis by stimulating production of haematopoietic growth factors in stromal cells of the bone marrow, and by increasing iron bioavailability. Androgens were previously applied in clinical practice to treat anaemia in dialysis patients, as hypogonadism was common in those with chronic kidney disease. However, recombinant human erythropoietin is now used in replacement (Carrero et al, 2011). T deficient patients are often anemic, as insufficient T levels reduce red blood cell (RBC) production.
Effect on Liver
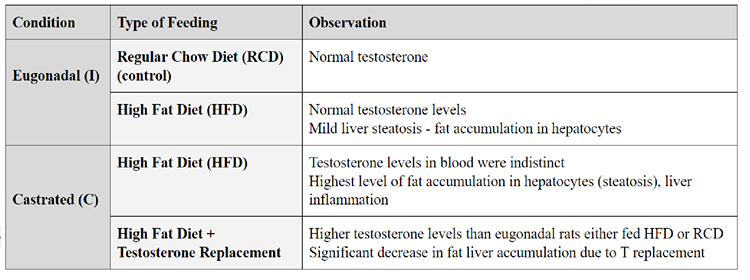
Low T commonly increases visceral adipose tissue (VAT) accumulation and insulin resistance, consequently resulting in metabolic syndrome. Experimental results carried out on young male rats to investigate the connection between gonadal status and diet on liver health are displayed in Table 1. Those castrated and fed High Fat Diet (HFD), developed increased liver fat accumulation, and inflammation. These pathological symptoms decreased when treated with T replacement (C + HFD + T), suggesting a link between T deficiency and hepatocyte adipose accumulation.
Table 1 Mody et al (2015) carried out an experiment on male mice to prove the relationship between testosterone levels with different diets and the effects on liver fat accumulation (adapted information into own illustration) |
Conversion to estradiol and DHT via 5-α-reductase, reduces fatty acid and cholesterol production. 5-α-reductase inhibition reduces conversion to DHT, thus its low levels contribute to liver steatosis. Low T is also associated with high estradiol in men due to large T conversion to estradiol. Estradiol reduces lipogenesis activity by decreasing fatty acid synthase and phosphorylation of acetyl coenzyme A. Adipose tissue is highly concentrated with aromatase, which aids the conversion to estradiol. Through a feedback mechanism, estradiol inhibits gonadotropin production, thus reducing T levels (Mody et al, 2015). T deficiency enhances VAT build up, causing insulin resistance and increased fatty acid build up in the liver. This increases the risk of coronary artery disease and diabetes. Prescribing T proved to be effective for insulin resistance in hypogonadal patients with type II diabetes.
Effect on Muscle
Sarcopenia is the decrease of muscle mass and strength, often due to ageing. Its numerous causes are: anorexia, inflammation, hypovitaminosis, and hypogonadism. The relationship between androgens and muscle mass is not precisely understood, but several studies hypothesize possibilities for this link.
T has been proven to retain nitrogen, which is important in muscle development. It increases protein synthesis as it re-utilizes amino acids, inducing muscle hypertrophy. It supports mitotic activity of satellite cells, thus increasing myoblast formation which is a vital source of new myonuclei. T also activates the G-Protein which stimulates Calcium increase in myoblasts and therefore expanding cellular growth (Shin et al, 2018). Hypogonadism slows down these mechanisms, resulting in reduced muscle mass, muscle weakness, imparied balance and poor hand grip. T administration has shown to increase muscle ribonucleic acids due to hormone-receptor interaction and muscle nuclei (Griggs et al, 1986). However due to the side effects (high hematocrit, risk of cardiovascular disease) it is not an effective treatment for sarcopenia.
Effect on Bone
Osteoporosis is a common health concern, especially in elderly. It is considered a gender specific disease: lower percentages of men are affected, hypothetically due to stronger bone composition. Aromatase converts T to prohormone estradiol which stimulates estrogen receptors on bone cells, affects bone metabolism. Estrogen is vital in male and female skeletal homeostasis. Therefore, estrogen deficiency or aromatase inhibition indirectly cause bone mass degeneration, despite normal T levels (Nieschlag et al, 2012).
These studies were proven in mouse model experiments; however, the limitation of this research involved the lack of SHBG in rodents, which regulates bioavailable levels of free or albumin bound T and estrogen. SHBG levels are inversely linked to BMD: high levels of SHBG bound to estrogen reduce BMD in both genders. However, AR and aromatase inhibition proved to be a more reliable investigation method.
T directly influences bone metabolism and quality via AR found on osteoblasts (role in bone deposition), osteoclasts (reabsorptive function), osteocytes (maintain homeostasis) and pluripotent mesenchymal stromal cells in bone marrow. T promotes trabecular formation and resorption in osteoblasts and osteocytes via AR signaling, and is essential during osteocyte differentiation. Therefore insufficient T levels reduce AR signaling, which will retard effects of T and diminish bone mineral density (BMD). Inhibiting osteoblast and osteocyte specific AR further prevents T stimulation, which reduces trabecular bone mass formation.
T indirectly affects bone metabolism by inhibiting aromatase during estrogen conversion. Aromatase has proven to increase trabecular and cortical BMD, reduce osteoclasts, and prevent epiphyseal closure (Golds et al, 2017). Reduced T and aromatase impairs trabecular bone development, which is essential for load bearing and transferring load from joints (Oftadeh et al, 2015). T deficiency, lack of AR and aromatase, accelerate risk of developing osteoporosis due to declined trabecular and BMD formation; this proves the importance of T on bone development.
Overproduction of Testosterone: symptoms and why it occurs
High levels of T can be induced by a number of factors, ranging from a lack of exercise to the presence of tumors. Having a high level of T affects males and females differently. Vankrieken (1977) proved that high levels of T results in testicular feminization in males and Virilization in females. Unnaturally high T often occurs with abuse of anabolic steroids, T or related hormones which are taken to enhance muscle mass and athletic performance (Harvard Health, 2020).
The most common cause of high T in females is polycystic ovary syndrome (PCOS), where the ovaries contain multiple cysts. PCOS patients can experience the following symptoms; excess or coarse hair, darkened thick skin, increased acne, weight gain, depression and anxiety. One solution available to help treat these symptoms is spironolactone, a diuretic that blocks the action of male sex hormones(Harvard Health, 2020). PCOS is caused by a hormone imbalance of abnormally high LH and insulin; high insulin levels increase T production. T can also be produced by converting insulin to an androstenedione secretion. High levels of T can be linked to obesity, which lowers the androstenedione levels and increases the T:Androstenedione ratio.
Cushing’s disease also causes slight androgen overproduction in pregnant, postmenopausal and androgen-treated patients. Other conditions that present high levels of T, include: acne, ovarian and adrenal tumors, adrenal hyperplasia, and menstrual irregularities (Vankrieken, 1977).
Increased T can overstimulate erythropoiesis, leading to polycythemia. This condition generally occurs when hemoglobin is above 18.5 g/dL or hematocrit is above 52%. High T levels increasingly stimulate erythropoiesis, causing a rise in hemoglobin levels, hematocrit levels and RBC count. Hulisz (2012) proved that this increases blood viscosity, which reduces cerebral blood flow, increasing the risk of developing thrombosis and stroke.
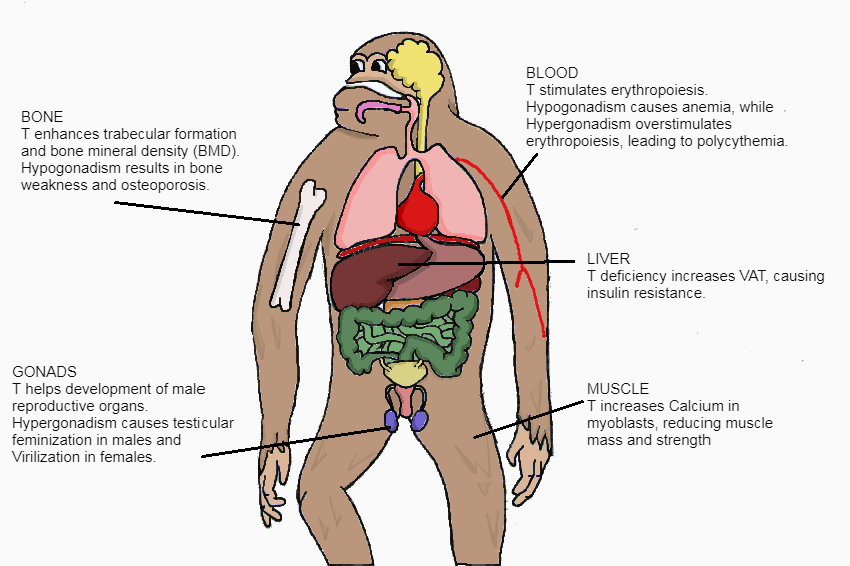
Figure 2 The effects of T illustrated on the body |
Regulation of testosterone
We discuss the regulation of T briefly: T is regulated by the hypothalamic-pituitary-gonadal axis, where the gonads refer to the testes and the ovaries. Much of the T produced in males is bound to Androgen Binding Protein(ABP)-produced by Sertoli cells in the testes(Sherwood, 2010). The gonads rely on regulation by the LH, a gonadotropic hormone produced by the anterior pituitary gland. Together, FSH and LH production is stimulated by the pulsed release of GnRH of the hypothalamus, and are active in both males and females on their respective target organs(Sherwood, 2010). The final serum T concentration remains relatively constant despite the pulsed release of GnRH. In healthy individuals, males produce a higher concentration of T than females even during short intense exercise done to raise these levels(Nassar et al, 2019). Furthermore, there is evidence of a link between adrenal and testicular tissue, since adrenocorticotropic hormone stimulates steroid synthesis from fetal Leydig cells. In these cells, the T can be further metabolized into DHT or estradiol.
The Hypothalamus-Anterior pituitary-Leydig cells act on a negative feedback mechanism to control the amount of circulating LH, as well as many other hormones, however we focus on the effect of T unrelated to reproduction. Considering the negative feedback mechanism, T may inhibit LH by either indirectly acting on the Hypothalamus, reducing the amount of GnRH released, subsequently inhibiting both LH and FSH production, or directly by signalling the anterior pituitary gland to inhibit LH production, leaving FSH unaffected. The indirect path is more dominant.(Sherwood, 2010).
Factors increasing production
T, or DHEA in females(Sherwood, 2010)., levels in the body increase significantly during puberty, due to changes in brain activity and neurological input to the hypothalamus(Nassar et al, 2019) Circulating T plays a role in increased Growth hormone(GH) secretion, the apparent reason we see sudden growth spurt in young individuals. Other factors increasing its production are drug use, health conditions such as tumors or PCOS, and hyperandrogenism; whereby the hypothalamus is affected, producing increased levels of GnRH and subsequently increasing gonadotropin levels. This finally leads to increased sex hormone production(Nassar et al, 2019).
At the Olympics, males competitors have an advantage and are often separated into categories therefore athletes are occasionally subjected to testing for various factors/substances that may lead to unfair advantage, such as T that has a significant effect on muscles, endurance, growth and speed. General bodily features can be a cause for suspicion and may lead to tests which include blood, urine and saliva samples(Handelsman et al, 2018).
|
Figure 3 Caster Semenya, female South African athlete has naturally high testosterone. Debates are ongoing, on whether she should be allowed to compete against women. |
Factors decreasing production
Various factors can inhibit T production, including poor sleep, very low or excessive calorie intake over extended periods of time, poor nutrient intake, vitamin and mineral deficiencies, drug use, excess exercise, natural aging, obesity and other metabolic disorders. The overuse of hormonal contraception by women, and mental health problems such as anxiety, stress and depression may also inhibit T production(Nassar et al, 2019). T action may be reduced in obese individuals as the free T becomes trapped in adipose tissue and is unable to reach T sensitive tissues of the body (Lerchbaum et al, 2014).
It is important to note that T is released into the blood, therefore vasectomy will have little to no effect on the amount of T produced in the body, however castration will (Sherwood, 2010). Castration, by removal of the testis reduces production and function of androgens, affecting development of body systems, especially secondary sexual characteristics (body hair, facial hair, larynx enlargement, heavier bone mass, etc.)
Clinical Case
An ex-racehorse mare was examined after showing intermittent oestrus behaviour after being brought to a brood farm. The horse was large, her genitals appeared normal however the speculum reached only a few centimeters, and the opening to the cervix was not present. Upon rectal examination, the reproductive organs; vagina, cervix., uterus and oviducts were absent however gonads were present, expressing the normal ovary shape and structure. T Plasma analysis was conducted and it was concluded that the mare was in fact a male and the ovaries were testes that remained in the abdominal cavity, similar to the cryptorchid condition. Further analysis of the sex chromosomes was done and the horse presented a XY chromosome complement. It is possible that the external genitalia were lacking the necessary AR’s and therefore female external genitalia were expressed instead of male. This lack of sexual differentiation is thought to involve the hypothalamus (Klein, 2013).
Despite being a male and high concentration of T, no typical male behaviour was present. This is a classic example of the horse lacking the necessary enzyme that converts T to DHT, and the individual presenting female external genitalia however internally has male reproductive organs.
Conclusion
Therefore we can conclude that T concentration, regulated by a negative feedback mechanism, relies on various hormones, receptors, and/or body conditions. Optimal concentration of T was found to be not only essential for reproduction, masculine appearance and behaviour, but also plays an important role in various organ metabolisms of both genders; affecting bone and muscle development, the heart, erythropoiesis, fat accumulation as well as cause illnesses that affect the lives of both animals and humans alike.
Overproduction of testosterone proves to have little significance in males, however females experience more severe consequences, as seen in PCOS patients, some pregnant and postmenopausal patients and exclusion from sporting events due to unfair advantage.
On the contrary, males tend to suffer more severe consequences from the lack of optimal T concentrations as seen with weakened bones, poor muscle development and features considered to be “feminine”. Both genders therefore rely on optimal T concentrations in order to maintain regular organ metabolism.
Through our investigations, T replacement therapy was proven to be a beneficial treatment option for some cases such as for hypogonadal patients with health concerns such as insulin resistance or heart problems, however proved ineffective in patients with sarcopenia or anemia. On the other hand, abuse of steroids, GH, blood doping and erythropoietin has led to paranoia in the sporting industry; that any athlete expressing unusual characteristics or advantage should be tested for ‘doping’ or overproduction of male hormones, especially in female athletes (Ekmekci, 2016). This brings up concerns with regards to doctor-patient confidentiality and the patients privacy in order to ensure honesty and fair-play. What are your thoughts?
Reference List
Bain, J. (2008): The many faces of testosterone. Clinical Interventions in Aging, 2: 567-576. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2686330/>
Bhasin, S.; Ravi, J. (2019): Reproductive And Nonreproductive Actions Of Testosterone. Encyclopedia Of Endocrine Diseases, 2: 721-734. Elsevier, doi:10.1016/b978-0-12-801238-3.65412-0.
Carrero, J. J.; Bárány P.; Yilmaz I. M.; Qureshi R. A.; Heimbürger O.; Ozgurtas T.; Yenicesu M.; Lindholm B.; Stenvinkel P. (2011): Testosterone Deficiency Is a Cause of Anaemia and Reduced Responsiveness to Erythropoiesis-Stimulating Agents in Men with Chronic Kidney Disease. Nephrology Dialysis Transplantation, 27: (2) 709–715. doi:10.1093/ndt/gfr288.
Ekmekci, P.E. (2016): Physicians' Ethical Dilemma in the Context of Anti-Doping Practices. Amn Sports Med Res. 3: (7).< https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5215887/ >
Golds, G.; Houdek, D.; Arnason, T. (2017): Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. International Journal of Endocrinology, issued: 16 Mar. 2017:1–15., doi:10.1155/2017/4602129.
Griggs, R.; Halliday, D.; Kingston, W.; Moxley, R. (1986): Effect of testosterone on muscle protein synthesis in myotonic dystrophy. Annals of Neurology, 20: (5) 590-596. <https://www.ncbi.nlm.nih.gov/pubmed/2431651>
Handelsman, D.J. et al. (2018): Circulating Testosterone As The Hormonal Basis Of Sex Differences In Athletic Performance. Endocrine Reviews, 39: (5) 803-829. The Endocrine Society, doi:10.1210/er.2018-00020
Hirsch, I.H. (2019): Male Hypogonadism - Genitourinary Disorders - MSD Manual Professional Edition. MSD Manual Professional Edition, https://www.merckmanuals.com/professional/genitourinary-disorders/male-reproductive-endocrinology-and-related-disorders/male-hypogonadism.
Hulisz, D. (2012): Polycythemia From Testosterone Therapy: To Treat Or Not? Medscape, https://www.medscape.com/viewarticle/773465.
Klein, B.G. (2013): Cunningham's Textbook Of Veterinary Physiology. 5th Edition, Elsevier Saunders, p. 434.
Lin, C.G.; Erinoff, L. (1990): Anabolic Steroid Abuse. National Institute On Drug Abuse, 12: 113, 114.
Lerchbaum, E.; Schwetz, V.; Rabe, T.; Giuliani, A.; Obermayer-Pietsch, B. (2014): Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype. PLoS ONE 9: (10) e108263. doi:10.1371/journal.pone.0108263
Mody A.; White D.; Kanwal F.; Garcia M. J. (2015): Relevance Of Low Testosterone To Nonalcoholic Fatty Liver Disease. Cardiovascular Endocrinology, 4:(3) 83-89. Ovid Technologies (Wolters Kluwer Health), doi:10.1097/xce.0000000000000057.
Morales, A.; Bebb, R.; Manjoo, P.; Assimakopoulos, P.; Axler, J.; Collier, C.; Elliott, S.; Goldenberg, L.; Gottesman, I.; Grober, E.; Guyatt, G.; Holmes, D.; Lee, J. (2015): Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Canadian Medical Association Journal,187: (18) 1369-1377.
Nassar G.N.; Raudales F.; Leslie S.W. (2020): Physiology, Testosterone. [Updated 2019 Nov 24]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; Available from: https://www.ncbi.nlm.nih.gov/books/NBK526128/
Nieschlag E.; Hemran M. B; Susan N. (2012): Testosterone: Action, Deficiency, Substitution. 4th edition. Cambridge University Press
Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.; Vaziri, A.; Nazarian, A. (2015): Biomechanics and Mechanobiology of Trabecular Bone: A Review. Journal of Biomechanical Engineering, 137: (1). <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5101038/>
Publishing, Harvard. (2020): Testosterone — What It Does And Doesn't Do - Harvard Health. Harvard Health, ,https://www.health.harvard.edu/drugs-and-medications/testosterone--what-it-does-and-doesnt-do?fbclid=IwAR3-maQprYikOCmeeJYp-vIgNaCRtjKTLMVl54Dz7peva61WYsi80xrUwAA.
Sherwood, L. (2010): Introduction to human physiology. 8th Edition p.734, 783-789
Shin, M.; Jeon, Y.; Kim, I. (2018): Testosterone and Sarcopenia. The World Journal of Men's Health, [online] 36: (3) 192. Available at: <https://www.ncbi.nlm.nih.gov/pmcarticles/PMC6119844/>
Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. (2017): Revisiting the Role of Testosterone: Are We Missing Something?. Reviews in Urology, 19:16–24. doi:10.3909/riu0716.
Vankrieken, L. (1977): Testosterone And The Free Androgen Index. Diagnostic Products Corporation. Available at: http://www.dpcweb.com/documents/news&views/spring00/techreports/zb158-a.pdf.
Figures List
Figure 1 was redrawn from https://pubchem.ncbi.nlm.nih.gov/compound/Testosterone#section=Information-Sources
Figure 2 was self drawn, using the knowledge collected in writing this essay and imagination.
Figure 3 was taken directly from free to use Wikimedia Commons. No changes were made to the original content. https://commons.wikimedia.org/wiki/File:Caster_Semenya_London_2012_(cropped).jpg
Table 1 information was adapted into our own illustration:
Mody A.; White D.; Kanwal F.; Garcia M. J. (2015): Relevance Of Low Testosterone To Nonalcoholic Fatty Liver Disease. Cardiovascular Endocrinology, 4:(3) 83-89. Ovid Technologies (Wolters Kluwer Health), doi:10.1097/xce.0000000000000057.