|
Size: 17173
Comment:
|
Size: 17294
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 2: | Line 2: |
| '''__Toll-Like Receptors__''' | '''Toll-Like Receptors''' |
| Line 4: | Line 4: |
| ---- | |
| Line 17: | Line 18: |
| ---- | |
| Line 30: | Line 32: |
| {{attachment:TLR.png|text describing image|width="300 height=350"}} Figure 1: TLR, This shows the components of a TLR |
{{attachment:TLR.png|text describing image|width="300 height=350"}} Figure 1: TLR, This shows the components of a TLR |
| Line 34: | Line 35: |
| ==== TLR2 ==== | ---- . '''''TLR2''''' |
| Line 41: | Line 44: |
| ==== TLR 3 ==== | . '''''TLR 3''''' |
| Line 48: | Line 52: |
| ==== TLR 4 ==== | . '''''TLR 4''''' |
| Line 59: | Line 64: |
| ==== TLR 5 ==== | . '''''TLR 5''''' |
| Line 66: | Line 72: |
| ==== TLR 6. ==== | . '''''TLR 6''''' |
| Line 73: | Line 80: |
| ==== TLR 7 ==== | . '''''TLR 7''''' |
| Line 84: | Line 92: |
| ==== Other TLRs ==== | . '''''Other TLRs''''' |
| Line 88: | Line 97: |
| ---- | |
| Line 105: | Line 115: |
| ---- | |
| Line 127: | Line 138: |
| {{attachment:Toll-like receptor pathway.png|text describing image|width="300 height=350"}} Figure 2: This shows the Toll-like receptor pathway |
{{attachment:Toll-like receptor pathways2.png|text describing image|width="300 height=350"}} Figure 2: This shows the Toll-like receptor pathway |
| Line 130: | Line 141: |
| Blasius, A.L. & Beutle, B., 2010. Intracellular Toll-like Receptors.'' Immunity'', 32, pp.305-15. Brightbill, H.D. & Modlin, R.L., 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. ''Immunology'', 101, pp.1-10. Buwitt-Beckmann, U. et al., 2004. Toll-like receptor 6-independent signaling by diacylated liporoteins. ''European Journal of Immunology'', 35(1), pp.282-89. Crespo-Lessmann, , Juárez-Rubio, & Plaza-Moral, , 2009. Role of toll-like receptors in respiratory diseases. ''Archivos de Bronconeumologia'', 46(3), pp.135-42. Harris, , KuoLee, R. & Chen, , 2006. Role of Toll-like receptors in health and diseases of gastrointestinal tract. ''World J Gastroenterol'', 14, pp.2149-60. Hopkins, P.A. & Sriskandan, S., 2005. Mammalian Toll-like receptors: to immunity and beyond. ''Clinical and Experimental Immunology'', 140, pp.395-407. Lachance, C. et al., 2013. Toll-Like Receptor 2-Independent Host Innate Immune Response against an Epidemic Strain of Streptococcus suis That Causes a Toxic Shock-Like Syndrome in Humans. ''PLOS ONE. '' Moresco, E.M.Y., LaVine, D. & Beutler, B., 2011. Toll Like Receptors. ''Current Biology'', 21(13). O'Hare, F., Watson, W.R. & Molloy, E.J., 2013. Toll-like receptors in Neonatal Sepsis. ''Acta Paediatrica'', 102, pp.572-78. Roach, J. et al., 2005. The evolution of vertebrate Toll-like receptors. ''Proceedings of the National Academy of Sciences'', 102(27). Roelofs, M.F. et al., 2008. The Orchestra of Toll-like Receptors and Their Potential Role in Frequently Occurring Rheumatic Conditions. ''Arthritis & Rheumatism,'' 58(2), pp.336-48. Rubio, J., Lessmann, A.C. & Moral, V.P., 2010. Role of toll-like receptors in respiratory diseases. ''Archivos de Bronconeumologia'', pp.135 - 142. Takeda, & Akira, , 2003. Toll receptors and pathogen resistance. ''Cellular Microbiology'', 5(3), pp.143-53. Takeda, K. & Akira, S., 2005. Toll-like receptors in innate immunity. ''International Immunology'', 17(1), pp.1-14. Trude , F.H. et al., 2001. Differential expression of Toll-like receptor 2 in human cells. ''Journal of Leukocyte Biology'', 69(3), pp.474-48. Vercammen, E., Staal, J. & Beyaert, R., 2008. Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. Clinical Microbiology Reviews, 21, pp.13-25. Werling, D., Piercy, J. & Coffey, T.J., 2006. Expression of TOLL-like receptors (TLR) by bovine antigen-presenting cells—Potential role in pathogen discrimination? ''Veterinary Immunology and Immunopathology'', 112(1-2), pp.2-11. Xie, F. et al., 2010. Toll-like receptor signaling and pre-eclampsia. ''American Journal Of Reproductive Immunology'', 63, pp.7-16. Zhang, X. et al., 2006. Toll-like receptor 4 deficiency causes pulmonary emphysema. ''The Journal of Clinical Investigation'', 116(11), pp.3050-59. |
---- 1. Blasius, A.L. & Beutle, B., 2010. Intracellular Toll-like Receptors.'' Immunity'', 32, pp.305-15. 1. Brightbill, H.D. & Modlin, R.L., 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. ''Immunology'', 101, pp.1-10. 1. Buwitt-Beckmann, U. et al., 2004. Toll-like receptor 6-independent signaling by diacylated liporoteins. ''European Journal of Immunology'', 35(1), pp.282-89. 1. Crespo-Lessmann, , Juárez-Rubio, & Plaza-Moral, , 2009. Role of toll-like receptors in respiratory diseases. ''Archivos de Bronconeumologia'', 46(3), pp.135-42. 1. Harris, , KuoLee, R. & Chen, , 2006. Role of Toll-like receptors in health and diseases of gastrointestinal tract. ''World J Gastroenterol'', 14, pp.2149-60. 1. Hopkins, P.A. & Sriskandan, S., 2005. Mammalian Toll-like receptors: to immunity and beyond. ''Clinical and Experimental Immunology'', 140, pp.395-407. 1. Lachance, C. et al., 2013. Toll-Like Receptor 2-Independent Host Innate Immune Response against an Epidemic Strain of Streptococcus suis That Causes a Toxic Shock-Like Syndrome in Humans. ''PLOS ONE.'' 1. Moresco, E.M.Y., LaVine, D. & Beutler, B., 2011. Toll Like Receptors. ''Current Biology'', 21(13). 1. O'Hare, F., Watson, W.R. & Molloy, E.J., 2013. Toll-like receptors in Neonatal Sepsis. ''Acta Paediatrica'', 102, pp.572-78. 1. Roach, J. et al., 2005. The evolution of vertebrate Toll-like receptors. ''Proceedings of the National Academy of Sciences'', 102(27). 1. Roelofs, M.F. et al., 2008. The Orchestra of Toll-like Receptors and Their Potential Role in Frequently Occurring Rheumatic Conditions. ''Arthritis & Rheumatism,'' 58(2), pp.336-48. 1. Rubio, J., Lessmann, A.C. & Moral, V.P., 2010. Role of toll-like receptors in respiratory diseases. ''Archivos de Bronconeumologia'', pp.135 - 142. 1. Takeda, & Akira, , 2003. Toll receptors and pathogen resistance. ''Cellular Microbiology'', 5(3), pp.143-53. 1. Takeda, K. & Akira, S., 2005. Toll-like receptors in innate immunity. ''International Immunology'', 17(1), pp.1-14. 1. Trude , F.H. et al., 2001. Differential expression of Toll-like receptor 2 in human cells. ''Journal of Leukocyte Biology'', 69(3), pp.474-48. 1. Vercammen, E., Staal, J. & Beyaert, R., 2008. Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. Clinical Microbiology Reviews, 21, pp.13-25. 1. Werling, D., Piercy, J. & Coffey, T.J., 2006. Expression of TOLL-like receptors (TLR) by bovine antigen-presenting cells—Potential role in pathogen discrimination? ''Veterinary Immunology and Immunopathology'', 112(1-2), pp.2-11. 1. Xie, F. et al., 2010. Toll-like receptor signaling and pre-eclampsia. ''American Journal Of Reproductive Immunology'', 63, pp.7-16. 1. Zhang, X. et al., 2006. Toll-like receptor 4 deficiency causes pulmonary emphysema. ''The Journal of Clinical Investigation'', 116(11), pp.3050-59. |
| Line 169: | Line 163: |
| ---- | |
| Line 171: | Line 166: |
| Figure 2: Toll-like receptor pathways, Wikimedia Commons | Figure 2: Toll-like receptor pathways revised, Wikimedia Commons |
Toll-Like Receptors
There are several mechanisms which enable organisms to resist and neutralize foreign invading agents. These mechanisms fall under the 2 types of immune responses; innate and adaptive, which are based on the critical distinction of self and non self.
The immune system can be broadly divided into the phylogenetically older innate immune system and the adaptive immune system. (Moresco et al., 2011) The innate system represents the first line of defence against antigens as its response via general processes is immediate. The adaptive immune system, while providing an extremely powerful and specific response, requires a prolonged period of time until it comes into action.
Toll like receptors (TLRs) form an important part of the innate immune system. These TLRs were initially described in fruit flies of genus Drosophila in 1994. Experiments revealed a gene in Drosophila which encoded receptors responsible for the triggering of mechanisms against fungal infections. (Rubio et al., 2010)
Mammalian TLRs consist of a large family of type 1 transmembrane proteins, made up of at least 11 members which act as sensors by recognizing microbial components on the surface or within extracellular compartments of cells, collectively known as pathogen associated molecular patterns (PAMP).
There is another TLR-independent system, known as the NOD system which is similarly concerned with the intracellular recognition of bacteria. Due to their selective recognition, the TLRs and NOD system are known as pattern recognition receptors (PRR). (Rubio et al., 2010)
Contents
Location of TLRs
In mammals TLRs are widely distributed both in and on the cells of the immune system such as macrophages, neutrophils and dendritic cells, as well as B and T cells. Their function is to provide the first line of defence against a wide variety of microorganisms including protozoa, fungi, bacteria and viruses.
TLRs reside on the cell surface and are an integral part of the membrane.
They are made up of two components:
- an extracellular part which contains many leucine rich repeats (LRRs) and
- one Toll/IL-1R domain (TIR) within the cytoplasm, a feature they share with the family of Interleukin 1 receptors.
It is via the LRRs that the TLRs recognise the various ligands, which is why the extracellular domain is so variable. (Brightbill & Modlin, 2000) The function of the TLR is to bind and activate adaptor proteins necessary in order to alert the innate immune system.
The locations of expression also vary between receptors. Besides the cell surface, TLRs also reside in the intracellular compartments. These include TLR3, TLR7, TLR8 and TLR9 which are found in intracellular compartments such as endosomes.
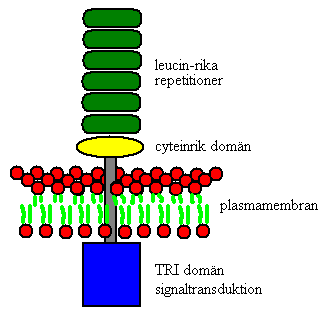 Figure 1: TLR, This shows the components of a TLR
Figure 1: TLR, This shows the components of a TLR
Function of individual TLRs
TLR2
TLR2 can recognize and signal the presence of a wide variety of bacterial products. These structural building blocks include peptidoglycans (part of the cell wall of bacteria), lipoteichoic acid (a component of outer membrane of gram-positive bacteria which can cause septic shock).
It can also recognize lipoarabinomannan which is a glycoprotein associated with mycobacteria including mycobacteria tuberculosis. This recognition occurs through functional cooperation with several proteins. TlR2 can also recognize bacteria including mycoplasma and spirochetes. (Trude et al., 2001)
TLR 1 and 6 are also required for TLR 2’s activation.
TLR 3
TLR 3 is found both on the cell surface and on the endosome compartment. The receptor recognises the polyinosinic-polycytidylic acid (poly I: C) analogue, which is a synthetic form of double stranded RNA (dsRNA), associated with retroviruses. For this reason, TLR3 takes part in the defence against various viral infections including the Influenza virus.
At times, TLR3 itself is the cause of various pathologies through a number of mechanisms. Indeed, overproduction of TLR3 can result in lupus nephritis and auto-immune liver disease. (Vercammen et al., 2008)
Endogenous cellular mRNA also activates TLR3.
TLR 4
This receptor acts on the cellular surface and is expressed in the spleen, peripheral blood leukocytes, monocytes, macrophages and granulocytes.
TLR 4 plays a crucial role in the initial organisms’ defence against Gram negative bacteria as it recognises liposaccharides (LPS), found on the surface of such bacteria. Takeda and Akira (2003) confirmed that TLR4 is crucial for LPS signalling by using genetically engineered mice in which the gene encoding for the TLR4 had been inactivated. Due to the lack of receptors required to transfer the signals of lipopolysaccharides, the degree of response was very low.
Structural cells in the lungs such as the endothelial cells and fibroblasts also express TLR4, as its presence is required for the lungs’ structural integrity. (Crespo-Lessmann et al., 2009). The absence of the receptor has been associated with an increased level of increased NADPH oxidase (Nox) which causes emphysema to develop. Nox3 brings about an increased oxidation reactions and the breakdown of the elastin. These processes lead to oxidative stress. (Zhang et al., 2006)
When present in adequately high concentrations, endogenous ligands like heat shock proteins and the oligosaccharide chains present on fibrinogen or hyaluronic acid are also recognised by TLR4. (Takeda & Akira, 2005)
TLR4 is also used as a vaccine adjuvant in both hepatitis B virus vaccines and a papillomavirus vaccine.
TLR 5
Studies prove that flagellated bacteria activate TLR5, confirming flagellin as a specific ligand for TLR5. Like TLR4, this receptor acts on the cellular surface and it has been found in the ovary, the prostate and peripheral blood leukocytes. This type of receptor also has a role in lung and gut mucosal activity. It is particularly expressed on the gastro intestinal mucosa, where it can be found exclusively on the basolateral surface of the epithelial cells.
In this environment, TLR5 is exposed to a number of flagella-bearing pathogens, including but not limited to Salmonella and Shigella. These encounters elicit an inflammatory response. (Takeda & Akira, 2003)
It is the only protein-binding TLR that has been conserved in vertebrates‘ lineage during their evolution from fish to mammals. Fish have a slightly shorter form of the gene which expresses the TLR 5. (Roach et al., 2005) While the role of TLR5 in the defence of these organisms is undisputed, overproduction can result in a number of diseases including inflammatory bowel disease including Chron’s disease. (Harris et al., 2006)
TLR 6
This receptor is also located on the cellular surface and it is expressed by organs like the thymus, spleen and the lung.
This toll like receptor is associated with oral cavity cancer and invasive aspergillosis, ie the fungal infectious diseases caused by the Aspergillus.
TLR2 needs to interact with other TLRs in order to recognise its ligand. TLR 6 is required because of its ability to distinguish different lipopeptides. Recognition of triacylated lipoproteins is independent of TLR6, requiring solely the TLR2-TLR1 heterodimer formation. On the other hand, a TLR2-TLR6 heterodimer is required to induce a signalling pathway via diacyl lipoprotein. Accordingly, the absence of TLR6 in mice resulted in their macrophages’ inability to produce cytokines upon exposure to diacyl lipoproteins. (Takeda, Akira et al., 2003) (Buwitt-Beckmann et al., 2004)
TLR 7
TLR 7 is an intracellular TLR like TLR3, 8, & 9. The aforementioned are all capable of recognizing nucleic acids. They are therefore critical in the body’s defence against viruses. Although they are mainly directed against specific molecules, they are flexible and can recognize other molecules.
The receptors reside within the endosomal compartment. Viruses and other pathogens are transported to this compartment after endocytosis or phagocytosis.
TLR 7 recognizes single stranded RNA (ssRNA). This has a clinically important implication because it is able to recognize HIV and influenza viruses besides Borrelia burgdorferi (Lyme disease).
TLR 7 has been implicated in systemic lupus erythematosus and as the target of imiquomod which is used in the treatment of genital warts and squamous cell carcinoma. (Blasius & Beutle, 2010)
TLR 9 TLR 9 recognises CpG DNA and unmethylated DNA.
Other TLRs
The ligands for TLRs 8 and 10 are as yet, unknown.
Mode of action
TLRs occur as dimers. TLR2 heterodimerizes with TLR6 or TRL 1, thus being able to recognize bacterial diacylated and triacylated lipopeptides respectively. TLRs 3, 4, 5, 8 and 9 homodimerize.
After the recognition of pathogens, the TLRS form dimers which trigger inflammatory processes as the primary response. Signalling pathways are activated and cytokines including TNF-a, IL-6 and IL-12 are released.
The binding of the specific ligand to its receptor activates the extracellular domain of the TLR, and this causes the recruitment of the adaptor protein molecules. There are 5 types of such adaptors. These include:
Myeloid differentiation primary response gene (88) (MyD88)
MyD88-adaptor-like (MAL, also known as TIRAP)
- TIR-domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM1),
- TRIF-related adaptor molecule (TRAM; also known as TICAM2) and
- Sterile α- and armadillo-motif containing protein (SARM)
MyD88 is critical in the protection against bacterial infection in humans and other mammals It is essential for signalling mechanism in all TLRs with the exception of TLR3.
Once the ligand binds a conformational change occurs that brings the two TIR domains on the cytosolic face in close proximity which builds up a new signalling complex. Following activation of TLRs, MyD88 binds to the TLR via its Toll-Interleukin-1-receptor (TIR). It then recruits interleukin-1-receptor-associated kinase (IRAK)-4 to the receptor. This brings about the activation of various transcription factors including NF-Kb, Ap-1, IRF5 and IRF7.
Importance for diseases
TLRs are powerful immuno-stimulators. Nature has provided tight control downstream to protect against TLRs over activation which can result in inflammatory diseases.
TLRs are important in a number of disorders ranging from atherosclerosis to systemic lupus erythematosus (SLE) to respiratory diseases.
A substantial amount of evidence is available which indicate that TLRs play a significant role in chronic inflammatory conditions. Thus the number of Human TLRs 2, 3, 4 and 7 are found in increased amounts in rheumatoid arthritis synovial tissue. (Roelofs et al., 2008)
TLRs play an important role in the development and progression pre-eclampsia, a condition which occurs during pregnancy. It is characterized by oedema and hypertension occurring in pregnancy. In fact, the interstitial trophoblasts in such patients show an amplified expression of TLR4. (Xie et al., 2010)
Animal studies have shown that TLR2 and TLR 4 expression and TLR 4 protein levels are increased soon after onset of sepsis. TLR 4 levels controls the magnitude of the host’s response to lipopolysaccharides from gram-negative bacteria. While TLR4 expression is effective in helping the host fight off the invading bacteria, overexpression of TLR 4 is ultimately harmful towards the host as it results in cell damage through a systemic inflammatory response. If the latter cannot be controlled it will cause hypotension and shock.
Some pathogens are able to escape the organism’s response to infection. Such an example would be Mycobacteria bovis, a gram positive bacterium that binds to TLR2 but has evolved the ability to survive the resulting pro-inflammatory response. As a matter of fact M. tuberculosis is able to inhibit and interfere with the MyD88 signalling pathways as well as the endosomal pathways. These mechanisms have thus enabled the strain to survive the host’s initial response to infection. (Werling et al., 2006)
TLR2 plays an important role in infections caused by Streptococcus suis serotype 2 which is a major swine pathogen of high economic importance to swine breeders.
It is also of importance to humans. In Western countries the disease is mainly restricted to workers in close contact with pigs and pork by-products, while in China and neighbouring countries it occurs in people who have not been in direct contact with swine, thus causing an epidemic of adult meningitis of which the bacterium is a prime cause.
In the recent fatal human outbreaks a streptococcal toxic shock-like syndrome occurred. This results from an exacerbated inflammatory reaction which contributes to the disease itself. While the body defends itself by binding through TLR 2 to stop the infection, the exacerbated inflammatory reaction results in shock which can be fatal. (Lachance et al., 2013)
Further studies and direction include the use of TLRs in their use as vaccine adjuvants and in the treatment of viral infection. (Rubio et al., 2010)
TLR interactions Various drugs have been used to test their interaction with TLRs. One such drug is a lipid A analogue known as eritoran (E5564). Eritoron antagonizes the TLR4 mechanism by preventing the activation of the transcription factor NF-Kb, hence suppressing the cytokine production which normally ensues. TLR 4 expression and action increases in magnitude in response to sepsis, leading to tissue damage in the organism. Clinical trials have shown that Eritoron is beneficial in the management of such septic patients. (O'Hare et al., 2013)
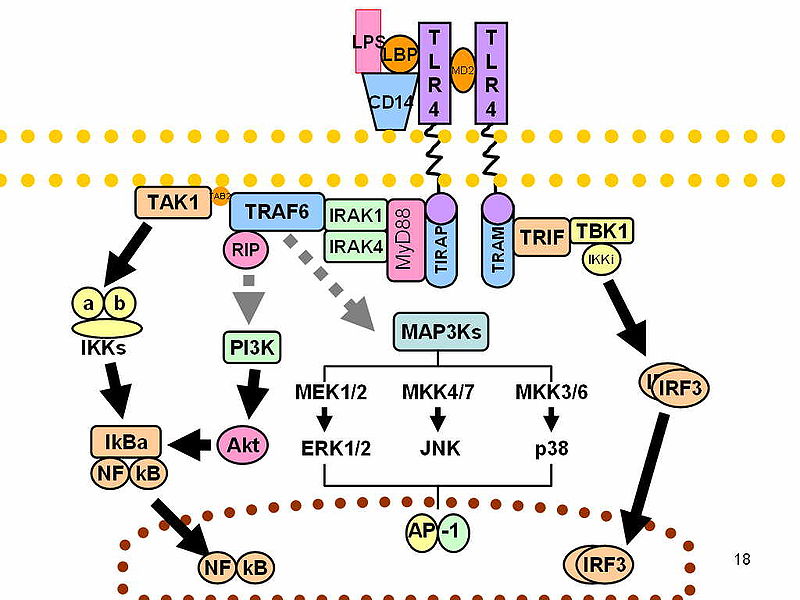 Figure 2: This shows the Toll-like receptor pathway
Figure 2: This shows the Toll-like receptor pathway
References
Blasius, A.L. & Beutle, B., 2010. Intracellular Toll-like Receptors. Immunity, 32, pp.305-15.
Brightbill, H.D. & Modlin, R.L., 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology, 101, pp.1-10.
Buwitt-Beckmann, U. et al., 2004. Toll-like receptor 6-independent signaling by diacylated liporoteins. European Journal of Immunology, 35(1), pp.282-89.
Crespo-Lessmann, , Juárez-Rubio, & Plaza-Moral, , 2009. Role of toll-like receptors in respiratory diseases. Archivos de Bronconeumologia, 46(3), pp.135-42.
Harris, , KuoLee, R. & Chen, , 2006. Role of Toll-like receptors in health and diseases of gastrointestinal tract. World J Gastroenterol, 14, pp.2149-60.
Hopkins, P.A. & Sriskandan, S., 2005. Mammalian Toll-like receptors: to immunity and beyond. Clinical and Experimental Immunology, 140, pp.395-407.
Lachance, C. et al., 2013. Toll-Like Receptor 2-Independent Host Innate Immune Response against an Epidemic Strain of Streptococcus suis That Causes a Toxic Shock-Like Syndrome in Humans. PLOS ONE.
Moresco, E.M.Y., LaVine, D. & Beutler, B., 2011. Toll Like Receptors. Current Biology, 21(13).
O'Hare, F., Watson, W.R. & Molloy, E.J., 2013. Toll-like receptors in Neonatal Sepsis. Acta Paediatrica, 102, pp.572-78.
Roach, J. et al., 2005. The evolution of vertebrate Toll-like receptors. Proceedings of the National Academy of Sciences, 102(27).
Roelofs, M.F. et al., 2008. The Orchestra of Toll-like Receptors and Their Potential Role in Frequently Occurring Rheumatic Conditions. Arthritis & Rheumatism, 58(2), pp.336-48.
Rubio, J., Lessmann, A.C. & Moral, V.P., 2010. Role of toll-like receptors in respiratory diseases. Archivos de Bronconeumologia, pp.135 - 142.
Takeda, & Akira, , 2003. Toll receptors and pathogen resistance. Cellular Microbiology, 5(3), pp.143-53.
Takeda, K. & Akira, S., 2005. Toll-like receptors in innate immunity. International Immunology, 17(1), pp.1-14.
Trude , F.H. et al., 2001. Differential expression of Toll-like receptor 2 in human cells. Journal of Leukocyte Biology, 69(3), pp.474-48.
Vercammen, E., Staal, J. & Beyaert, R., 2008. Sensing of Viral Infection and Activation of Innate Immunity by Toll-Like Receptor 3. Clinical Microbiology Reviews, 21, pp.13-25.
Werling, D., Piercy, J. & Coffey, T.J., 2006. Expression of TOLL-like receptors (TLR) by bovine antigen-presenting cells—Potential role in pathogen discrimination? Veterinary Immunology and Immunopathology, 112(1-2), pp.2-11.
Xie, F. et al., 2010. Toll-like receptor signaling and pre-eclampsia. American Journal Of Reproductive Immunology, 63, pp.7-16.
Zhang, X. et al., 2006. Toll-like receptor 4 deficiency causes pulmonary emphysema. The Journal of Clinical Investigation, 116(11), pp.3050-59.
Pictures
Figure 1: TLR, Wikimedia Commons
Figure 2: Toll-like receptor pathways revised, Wikimedia Commons
