|
Size: 24192
Comment:
|
Size: 25783
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 2: | Line 2: |
| Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease. Although this particular disease is rare, it is a costly disease too, and has a poor prognosis. Cerebral oedema is a serious, and often fatal, complication of acute liver failure. The exact mechanisms underlying the pathophysiology of this disease are not yet certain, however ammonia and glutamine have both been shown to affect astrocyte function, causing them to swell, and oedema occurs. Although treatment options were limited to liver transplant in the past, knew therapies and drugs are now available. | <<TableOfContents Abstract Liver Anatomy and Physiology Acute Liver Failure Cerebral Oedema Pathophysiology of Cerebral Oedema and Liver Failure Treatments Discussion(3)>> |
| Line 4: | Line 4: |
| The Liver; An Overview of its Morphology and Functions | '''Abstract''' |
| Line 6: | Line 6: |
| The liver is the largest gland in the body and it carries out a number of vital processes. The liver is both an exocrine and endocrine organ, and it produces a wide variety of enzymes and hormones which are of crucial metabolic importance, and help to maintain homeostasis. For instance, the liver is responsible for producing and storing bile, which is a key component of lipid digestion. It is also responsible for producing and storing glycogen, which the organism uses as an energy store. Furthermore, the liver plays a central role in intermediary metabolism, and is responsible for detoxification of metabolites such as urea. The liver is a haematapoeitic organ in the foetus (Drake et al., 2008, Nickel et al., 1954). | Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease. Although this particular disease is rare, it is costly disease and has a poor prognosis. Cerebral oedema is a serious and often fatal complication of acute liver failure. The exact mechanisms underlying the pathophysiology of this disease are not yet certain, however ammonia and glutamine have both been shown to affect astrocyte function, causing them to swell, and oedema occurs. Although treatment options were limited to liver transplant in the past, knew therapies and drugs are now available. |
| Line 8: | Line 8: |
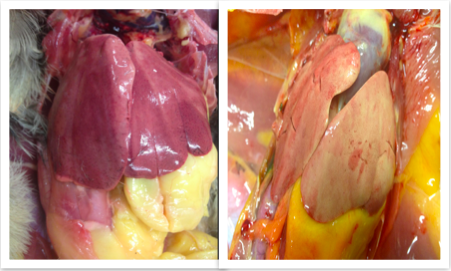
| The colour of the liver depends on factors such as amount of blood it contains, species and the age of animal. The nutritional state of the animal has the most important bearing on the liver’s appearance, as can be seen in Fig 1:1 and 1:2. The liver is normally reddish-brown in colour (Nickel et al., 1979). | __The Liver; An Overview of its Morphology and Functions __ The liver is the largest gland in the body and it carries out a number of vital processes. The liver is both an exocrine and endocrine organ, and it produces a wide variety of enzymes and hormones which are of crucial metabolic importance, and help to maintain homeostasis. For instance, the liver is responsible for producing and storing bile, which is a key component of lipid digestion. It is also responsible for producing and storing glycogen, which the organism uses as an energy store. Furthermore, the liver plays a central role in intermediary metabolism, and is responsible for detoxification of metabolites such as urea. The liver is a haematapoeitic organ in the foetus (''Drake et al., 2008, Nickel et al., 1954''). The colour of the liver depends on factors such as amount of blood it contains, species and the age of animal. The nutritional state of the animal has the most important bearing on the liver’s appearance, as can be seen in Fig 1:1 and 1:2. The liver is normally reddish-brown in colour (''Nickel et al., 1979''). ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Screen Shot 2016-04-24 at 21.37.56.png|pop-up text}} <<BR>>'''Fig 1:1'''<<BR>>''Healthy liver in a domestic chicken (left) and a fatty liver caused by acute liver failure in a domestic chicken (right)'' || |
| Line 12: | Line 17: |
| Figure 1:1; Fatty Liver from a domestic chicken (right) and a healthy liver from a domestic chicken (left). Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2. | |
| Line 14: | Line 18: |
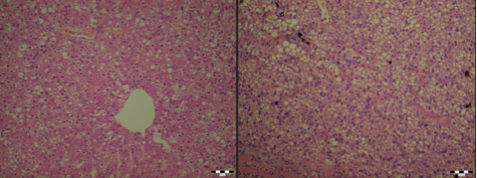
| Several variations occur in terms of liver morphology between the species, mostly regarding the number of lobes and their shapes. Each lobe is covered by the capsule of Glisson. The capsule consists of mesothelium, with a thin connective tissue layer interposed between the mesothelium and the liver’s surface. The capsule consists of sinusoids and plates of parenchymal cells, hepatocytes, radially arranged about a central vein. Portal tracts, which occur at the intersections of three or more lobules each contain one or more branches of a portal vein, hepatic artery, bile ductule and lymphatic vessel. This is also known as the hepatic triad, the functional unit of the liver (Samuelson, 2007). Figure 1;2 below shows the histological differences between a healthy liver and a liver with acute liver failure. | Figure 1:1; . Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2. Several variations occur in terms of liver morphology between the species, mostly regarding the number of lobes and their shapes. Each lobe is covered by the capsule of Glisson. The capsule consists of mesothelium, with a thin connective tissue layer interposed between the mesothelium and the liver’s surface. The capsule consists of sinusoids and plates of parenchymal cells, hepatocytes, radially arranged about a central vein. Portal tracts, which occur at the intersections of three or more lobules each contain one or more branches of a portal vein, hepatic artery, bile ductule and lymphatic vessel. This is also known as the hepatic triad, the functional unit of the liver (''Samuelson, 2007''). Figure 1;2 below shows the histological differences between a healthy liver and a liver with acute liver failure. ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Screen Shot 2016-04-25 at 14.41.51.png|pop-up text}} <<BR>>'''Fig 1:2'''<<BR>>''Histological sections: healthy liver in a domestic chicken (left) and a fatty liver caused by acute liver failure in a domestic chicken (right) '' || |
| Line 18: | Line 25: |
| Figure 1:2; Liver from a domestic chicken with acute liver failure (right) and a healthy liver (left) from a domestic chicken. Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2. | |
| Line 20: | Line 26: |
| While the liver is capable of regeneration, it is susceptible to a variety of toxins and diseases. Among such diseases is acute liver failure (ALF) (Guyton and Hall, 2006). The present article will examine the pathophysiology of acute liver failure, cerebal oedema and the correlation between the two. | Figure 1:2; Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2. |
| Line 22: | Line 28: |
| Liver Failure | While the liver is capable of regeneration, it is susceptible to a variety of toxins and diseases. Among such diseases is acute liver failure (ALF) (''Guyton and Hall, 2006''). The present article will examine the pathophysiology of acute liver failure, cerebal oedema and the correlation between the two. |
| Line 24: | Line 30: |
| Liver failure is the umbrella term for the condition in which the liver can no longer function properly as a result of the death of a great number of hepatocytes. It can be further subcategorised into chronic liver failure or acute liver failure. Chronic liver failure refers to long term damage and scarring of the liver, brought on by a variety of causes, such as chronic alcohol abuse. Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease (Scott et al., 2013, Mosby Inc., 2013). | __Liver Failure __ |
| Line 26: | Line 32: |
| Acute Liver Failure | Liver failure is the umbrella term for the condition in which the liver can no longer function properly as a result of the death of a great number of hepatocytes. It can be further subcategorised into chronic liver failure or acute liver failure. Chronic liver failure refers to long term damage and scarring of the liver, brought on by a variety of causes, such as chronic alcohol abuse. Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease (''Scott et al., 2013, Mosby Inc., 2013''). |
| Line 28: | Line 34: |
| Acute liver failure is a clinical manifestation of sudden and severe hepatic injury and arises from many causes (Bernal et al., 2010). It is sometimes referred to as fulminant hepatic failure, a term first used by Trey and Davidson, who described a potentially reversible disorder resulting from severe hepatic injury with an onset of encephalopathy (Scott et al., 2013). | __Acute Liver Failure __ |
| Line 30: | Line 36: |
| Although ALF is rare, with approximately one to six cases per million people every year in the United States and Western Europe, the mortality rate and cost of treatment are high (Scott et al., 2013). Young people make up the bulk of new diagnoses (Bower et al., 2007). The two main causes of ALF are paracetamol toxicity and hepatitis A or B. Generally, there is a greater incidence of ALF brought on by drug toxicity in more developed countries, where as in less developed countries ALF is predominantly caused by hepatitis A and B. In Europe and the United States for instance, there is a high incidence of ALF brought on by paracetamol overdose, where as in less developed countries such as Pakistan or South East Asian countries, ALF is predominantly caused by hepatitis (Bernal et al., 2015) | Acute liver failure is a clinical manifestation of sudden and severe hepatic injury and arises from many causes (''Bernal et al., 2010''). It is sometimes referred to as fulminant hepatic failure, a term first used by Trey and Davidson, who described a potentially reversible disorder resulting from severe hepatic injury with an onset of encephalopathy (''Scott et al., 2013''). |
| Line 32: | Line 38: |
| ALF can be further divided into three distinct phenotypes based on the time between the onset of jaundice and the development of encephalopathy. These phenotypes are as follows; hyper acute (within 7 days), acute (8-28 days) and subacute (5-12 weeks) (O'Grady et al., 1993). | Although ALF is rare, with approximately one to six cases per million people every year in the United States and Western Europe, the mortality rate and cost of treatment are high (''Scott et al., 2013''). Young people make up the bulk of new diagnoses (''Bower et al., 2007''). The two main causes of ALF are paracetamol toxicity and hepatitis A or B. Generally, there is a greater incidence of ALF brought on by drug toxicity in more developed countries, where as in less developed countries ALF is predominantly caused by hepatitis A and B. In Europe and the United States for instance, there is a high incidence of ALF brought on by paracetamol overdose, where as in less developed countries such as Pakistan or South East Asian countries, ALF is predominantly caused by hepatitis (''Bernal et al., 2015'') |
| Line 34: | Line 40: |
| The period of active hepatic injury in paracetamol poisoning can be measured in hours, and it is self-limited, in the sense that the toxicity may be resolved spontaneously and without specific treatment. This particular type of hepatotoxicity has a rapid onset and offset and a finite period of necrosis. These patients are characterised as having a “hyperacute” phenotype. In many cases,a rapid recovery follows despite massive multi-organ failure. This type differs greatly from most other forms of ALF, usually termed acute or subacute, wherein the hepatotoxicity evolves over 1-4 weeks, and is not self-limited, but long lasting. Hepatitis B, autoimmune hepatitis and most interdeterminant cases will have a subacute pattern and poorer chances of recovery (Bernal et al., 2015, Bernal et al., 2010). The table below details the symptoms of acute liver failure. | ALF can be further divided into three distinct phenotypes based on the time between the onset of jaundice and the development of encephalopathy. These phenotypes are as follows; hyper acute (within 7 days), acute (8-28 days) and subacute (5-12 weeks) (''O'Grady et al., 1993''). |
| Line 36: | Line 42: |
| The period of active hepatic injury in paracetamol poisoning can be measured in hours, and it is self-limited, in the sense that the toxicity may be resolved spontaneously and without specific treatment. This particular type of hepatotoxicity has a rapid onset and offset and a finite period of necrosis. These patients are characterised as having a “hyperacute” phenotype. In many cases,a rapid recovery follows despite massive multi-organ failure. This type differs greatly from most other forms of ALF, usually termed acute or subacute, wherein the hepatotoxicity evolves over 1-4 weeks, and is not self-limited, but long lasting. Hepatitis B, autoimmune hepatitis and most interdeterminant cases will have a subacute pattern and poorer chances of recovery (''Bernal et al., 2015, Bernal et al., 2010''). The table below details the symptoms of acute liver failure. | |
| Line 37: | Line 44: |
| Figure 1;3. The effects of acute liver failure | |
| Line 38: | Line 46: |
| Figure 1;3. The effects of acute liver failure (Bernal et al., 2010). | __Cerebral Oedema __ |
| Line 40: | Line 48: |
| Cerebral Oedema | Disordered brain function resulting in cerebral oedema is a major complication in ALF. This impairment of cerebral function is associated with the development of intracranial hypertension and death. The encephalopathy resulting from ALF rapidly leads to confusion, disorientation and agitation. These symptoms progress to delerium, seizures and eventually coma (''Blei and Larsen, 1999''). Cerebral oedema is a net increase in total brain water content. The rigid skull protecting the brain limits the compliance of the brain and as a consequence a small increase in fluid can cause a significant rise in intracranial pressure (''Scott et al., 2013''). Left untreated, cerebral oedema can rapidly progress to cause herniation of the uncus through the falx cerebri, leading to compression of the brainstem, and ultimately, death (''Aldridge et al., 2015''). Astrocyte foot swelling is the most consistent neuropathological finding postmortem (''Kato et al., 1992''). |
| Line 42: | Line 50: |
| Disordered brain function resulting in cerebral oedema is a major complication in ALF. This impairment of cerebral function is associated with the development of intracranial hypertension and death. The encephalopathy resulting from ALF rapidly leads to confusion, disorientation and agitation. These symptoms progress to delerium, seizures and eventually coma (Blei and Larsen, 1999). Cerebral oedema is a net increase in total brain water content. The rigid skull protecting the brain limits the compliance of the brain and as a consequence a small increase in fluid can cause a significant rise in intracranial pressure (Scott et al., 2013). Left untreated, cerebral oedema can rapidly progress to cause herniation of the uncus through the falx cerebri, leading to compression of the brainstem, and ultimately, death (Aldridge et al., 2015). Astrocyte foot swelling is the most consistent neuropathological finding postmortem (Kato et al., 1992). | It is undisputed that a variety of factors lead to astrocyte foot swelling and cerebral oedema in ALF but ammonia has been shown to have a key role. The effects of oedema include a severe compromise in cerebral metabolism and increased cerebral blood perfusion. Cerebral metabolic rate for oxygen decreases and there is failure of cerebrovascular autoregulation. Aswell as ammonia; glutamine, pro-inflammatory factors and oxidative or nitrosative stress have been implicated in its aetiology. (''Butterworth 2014''). Neuroinflammation is an important etiological factor, the treatment of this disorder will be discussed further (''Scott et al., 2013'') |
| Line 44: | Line 52: |
| It is undisputed that a variety of factors lead to astrocyte foot swelling and cerebral oedema in ALF but ammonia has been shown to have a key role. The effects of oedema include a severe compromise in cerebral metabolism and increased cerebral blood perfusion. Cerebral metabolic rate for oxygen decreases and there is failure of cerebrovascular autoregulation. Aswell as ammonia; glutamine, pro-inflammatory factors and oxidative or nitrosative stress have been implicated in its aetiology. (Butterworth 2014). Neuroinflammation is an important etiological factor, the treatment of this disorder will be discussed further (Scott et al., 2013) | __The Role of Ammonia __ |
| Line 46: | Line 54: |
| The Role of Ammonia | Ammonia is traditionally considered a gut derived nitrogenous toxin produced by bacterial metabolism of amino acids primarily glutamine (''Tapper et al., 2015''). The gut is a critical component in the pathogenesis of cerebral oedema. Dietary nitrogen (particularly glutamine) is converted to ammonia through the action of gut enzymes as well as urease containing bacteria. Glutamine is its self metabolised by glutaminase in the intestinal epithelial cells, which converts more than one third of its nitrogen to ammonia, which diffuses into the portal circulation (''Tapper et al., 2015''). |
| Line 48: | Line 56: |
| Ammonia is traditionally considered a gut derived nitrogenous toxin produced by bacterial metabolism of amino acids primarily glutamine (Tapper et al., 2015). The gut is a critical component in the pathogenesis of cerebral oedema. Dietary nitrogen (particularly glutamine) is converted to ammonia through the action of gut enzymes as well as urease containing bacteria. Glutamine is its self metabolised by glutaminase in the intestinal epithelial cells, which converts more than one third of its nitrogen to ammonia, which diffuses into the portal circulation (Tapper et al., 2015). | Due to the loss of hepatocyte function in ALF, the liver is unable to carry out its normal processes, specifically detoxification of ammonia using the urea cycle and glutamine synthetase (see Fig 1;5). This increases ammonia concentration in extra hepatic tissues and causes a variety of issues within the brain as ammonia can easily bypass the blood brain barrier (BBB) and enter the organ. The blood brain barrier plays a critical role in establishing and maintaining homeostasis within the brain. It exerts tight control over any exchange of metabolites between the circulating blood and the central nervous system. BBB consists of brain capillary endothelial cells, pericytes and the foot processes of astrocytes. Together they form a neurovascular unit capable of regulating the special composition of the CSF (''Scott et al., 2013, Abbott et al., 2010''). The different hypotheses regaring ammonia’s role are outlined in Fig 1;4 below. |
| Line 50: | Line 58: |
| Due to the loss of hepatocyte function in ALF, the liver is unable to carry out its normal processes, specifically detoxification of ammonia using the urea cycle and glutamine synthetase (see Fig 1;5). This increases ammonia concentration in extra hepatic tissues and causes a variety of issues within the brain as ammonia can easily bypass the blood brain barrier (BBB) and enter the organ. The blood brain barrier plays a critical role in establishing and maintaining homeostasis within the brain. It exerts tight control over any exchange of metabolites between the circulating blood and the central nervous system. BBB consists of brain capillary endothelial cells, pericytes and the foot processes of astrocytes. Together they form a neurovascular unit capable of regulating the special composition of the CSF (Scott et al., 2013, Abbott et al., 2010). The different hypotheses regaring ammonia’s role are outlined in Fig 1;4 below. | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center">{{attachment:Summarisation of astrocyte swelling and oxidative stress.pdf|pop-up text|title="pop-up text"}} <<BR>>'''Fig 1:4'''<<BR>>''Summary of Astrocyte Swelling and Oxidative Stress'' || |
| Line 56: | Line 65: |
| One of the primary functions of astrocytes is the conversion of glutamate to glutamine (a fundamental neurotransmitter) using glutamate synthetase. The reaction uses ammonia as a catalyst while also detoxifying the ammonia (as the brain lacks a urea cycle). However, due to the accumulation of ammonia and therefore the increased conversion of glutamate to glutamine, the increased levels of glutamine cause osmotic stress as water moves into the cell to counter balance its unequal solute gradient, resulting in increased astrocyte volume and cerebral oedema (Aldridge et al., 2015). | One of the primary functions of astrocytes is the conversion of glutamate to glutamine (a fundamental neurotransmitter) using glutamate synthetase. The reaction uses ammonia as a catalyst while also detoxifying the ammonia (as the brain lacks a urea cycle). However, due to the accumulation of ammonia and therefore the increased conversion of glutamate to glutamine, the increased levels of glutamine cause osmotic stress as water moves into the cell to counter balance its unequal solute gradient, resulting in increased astrocyte volume and cerebral oedema (''Aldridge et al., 2015''). ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center">{{attachment:Urea cycle .pdf|pop-up text|title="pop-up text"}} <<BR>>'''Fig 1:5'''<<BR>>''The Urea Cycle'' || |
| Line 60: | Line 71: |
| Figure 1:5. The Urea Cycle | |
| Line 62: | Line 72: |
| Alpha-ketoglutarate is a key metabolite in reducing glutamate levels as it can be used to convert it to glutamine and therefore prevent glutamate’s accumulation. However, in severe cases of ALF where hyperammonia occurs the stores of alpha-ketoglutarate itself will become depleted, having a negative impact on the citric acid cycle where it is a fundamental reactant. Furthermore ammonia may inhibit two rate limiting enzymes; pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase. This substrate depletion and partial enzyme inhibition compromise overall oxidative metabolism and lead to depletion of energy rich phosphate compounds (ATP and GTP) and accumulation of lactate. In addition, ammonia both induces oxidative and nitrosative stress by formation of free radicals which may in turn result in mitochondrial permeability transition (MPT) i.e. a state with imminent power failure of the astrocytes (Aldridge et al., 2015) | Alpha-ketoglutarate is a key metabolite in reducing glutamate levels as it can be used to convert it to glutamine and therefore prevent glutamate’s accumulation. However, in severe cases of ALF where hyperammonia occurs the stores of alpha-ketoglutarate itself will become depleted, having a negative impact on the citric acid cycle where it is a fundamental reactant. Furthermore ammonia may inhibit two rate limiting enzymes; pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase. This substrate depletion and partial enzyme inhibition compromise overall oxidative metabolism and lead to depletion of energy rich phosphate compounds (ATP and GTP) and accumulation of lactate. In addition, ammonia both induces oxidative and nitrosative stress by formation of free radicals which may in turn result in mitochondrial permeability transition (MPT) i.e. a state with imminent power failure of the astrocytes (''Aldridge et al., 2015'') |
| Line 64: | Line 74: |
| MPT usually develops in response to an increase in mitochondrial calcium levels and results in a sudden opening of the permeability transition pore, a large non-selective permeability pore in the inner mitochondrial membrane. This makes the inner mitochondrial membrane more permeable to protons, ions, and other small solutes. As a result, the inner mitochondrial dysfunction leads to spilling of the mitochondrial matrix, defective oxidative phosphorylation and adenosine triphosphate (ATP) production and generation of reactive oxygen species (Aldridge et al., 2015). | MPT usually develops in response to an increase in mitochondrial calcium levels and results in a sudden opening of the permeability transition pore, a large non-selective permeability pore in the inner mitochondrial membrane. This makes the inner mitochondrial membrane more permeable to protons, ions, and other small solutes. As a result, the inner mitochondrial dysfunction leads to spilling of the mitochondrial matrix, defective oxidative phosphorylation and adenosine triphosphate (ATP) production and generation of reactive oxygen species (''Aldridge et al., 2015''). |
| Line 66: | Line 76: |
| The “Trojan horse” hypothesis has recently been proposed as an alternative theory to explain the development of astrocyte swelling, and implicates an important role for both ammonia and glutamine. The excess glutamine synthesised within astrocytes is transported into the mitochondria where it is metabolised by phosphate-activated glutaminase (PAG) to ammonia and glutamate. Glutamate (the trojan horse) thereby acts as a carrier of ammonia into the mitochondria, where its accumulation can lead to oxidative stress and ultimately astrocyte swelling. Ammonia interferes with mitochondrial energy metabolism resulting in high levels of pyruvate and lactate and similar effects described under the prior hypothesis (Aldridge et al., 2015). | The “Trojan horse” hypothesis has recently been proposed as an alternative theory to explain the development of astrocyte swelling, and implicates an important role for both ammonia and glutamine. The excess glutamine synthesised within astrocytes is transported into the mitochondria where it is metabolised by phosphate-activated glutaminase (PAG) to ammonia and glutamate. Glutamate (the trojan horse) thereby acts as a carrier of ammonia into the mitochondria, where its accumulation can lead to oxidative stress and ultimately astrocyte swelling. Ammonia interferes with mitochondrial energy metabolism resulting in high levels of pyruvate and lactate and similar effects described under the prior hypothesis (''Aldridge et al., 2015''). |
| Line 68: | Line 78: |
| However, while it is widely accepted that ammonia has a fundamental role in cerebral oedema the exact role and mechanism is still not yet fully understood as blood ammonia concentrations was not predictive or consistent with severity in HE. This finding has been replicated with one such study showing the 69% of individuals with no overt signs of HE had elevated blood ammonia levels, whilst a number of patients with more significant grade 3 or 4 HE had either normal or only slightly elevated levels of ammonia in their blood (Aldridge et al., 2015). | However, while it is widely accepted that ammonia has a fundamental role in cerebral oedema the exact role and mechanism is still not yet fully understood as blood ammonia concentrations was not predictive or consistent with severity in HE. This finding has been replicated with one such study showing the 69% of individuals with no overt signs of HE had elevated blood ammonia levels, whilst a number of patients with more significant grade 3 or 4 HE had either normal or only slightly elevated levels of ammonia in their blood (''Aldridge et al., 2015''). |
| Line 70: | Line 80: |
| Bernal and colleagues demonstrated ammonia to be an independent risk factor for the development of both HE and ICH, with ICH occurring in 55% of patients with blood ammonia concentration >200 micromol/L. Blood ammonia levels >(or equal to) 150 micromol/L are indicitive of a greater likelihood of cerebral herniation and death in patients with ALF (Aldridge et al., 2015). The pathogensis of cerebral oedema, as well as some treatments, are outlined in Fig 1;6. | Bernal and colleagues demonstrated ammonia to be an independent risk factor for the development of both HE and ICH, with ICH occurring in 55% of patients with blood ammonia concentration >200 micromol/L. Blood ammonia levels >(or equal to) 150 micromol/L are indicitive of a greater likelihood of cerebral herniation and death in patients with ALF (''Aldridge et al., 2015''). The pathogensis of cerebral oedema, as well as some treatments, are outlined in Fig 1;6. |
| Line 72: | Line 82: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:Screen Shot 2016-04-24 at 21.37.56.png|pop-up text}} <<BR>>'''Fig 1:6'''<<BR>>''This Illustrates the Pathogenesis of Cerebral Oedema, and its Treatment Options'' || | |
| Line 73: | Line 84: |
Figure 1:6; This illustrates The Pathogenesis of Cerebral Oedema, and its treatment options. |
|
| Line 111: | Line 120: |
<<TableOfContents Abstract Liver Anatomy and Physiology Acute Liver Failure Cerebral Oedema Pathophysiology of Cerebral Oedema and Liver Failure Treatments Discussion(3)>>
Abstract
Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease. Although this particular disease is rare, it is costly disease and has a poor prognosis. Cerebral oedema is a serious and often fatal complication of acute liver failure. The exact mechanisms underlying the pathophysiology of this disease are not yet certain, however ammonia and glutamine have both been shown to affect astrocyte function, causing them to swell, and oedema occurs. Although treatment options were limited to liver transplant in the past, knew therapies and drugs are now available.
The Liver; An Overview of its Morphology and Functions
The liver is the largest gland in the body and it carries out a number of vital processes. The liver is both an exocrine and endocrine organ, and it produces a wide variety of enzymes and hormones which are of crucial metabolic importance, and help to maintain homeostasis. For instance, the liver is responsible for producing and storing bile, which is a key component of lipid digestion. It is also responsible for producing and storing glycogen, which the organism uses as an energy store. Furthermore, the liver plays a central role in intermediary metabolism, and is responsible for detoxification of metabolites such as urea. The liver is a haematapoeitic organ in the foetus (Drake et al., 2008, Nickel et al., 1954).
The colour of the liver depends on factors such as amount of blood it contains, species and the age of animal. The nutritional state of the animal has the most important bearing on the liver’s appearance, as can be seen in Fig 1:1 and 1:2. The liver is normally reddish-brown in colour (Nickel et al., 1979).
|
Figure 1:1; . Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2.
Several variations occur in terms of liver morphology between the species, mostly regarding the number of lobes and their shapes. Each lobe is covered by the capsule of Glisson. The capsule consists of mesothelium, with a thin connective tissue layer interposed between the mesothelium and the liver’s surface. The capsule consists of sinusoids and plates of parenchymal cells, hepatocytes, radially arranged about a central vein. Portal tracts, which occur at the intersections of three or more lobules each contain one or more branches of a portal vein, hepatic artery, bile ductule and lymphatic vessel. This is also known as the hepatic triad, the functional unit of the liver (Samuelson, 2007). Figure 1;2 below shows the histological differences between a healthy liver and a liver with acute liver failure.
|
Figure 1:2; Image courtesy of Dr. János Gál PhD, Dipl. ECZM Head of department Department of Exotic Animal and Wildlife Medicine H-1078, Hungary, Budapest, Istvám str. 2.
While the liver is capable of regeneration, it is susceptible to a variety of toxins and diseases. Among such diseases is acute liver failure (ALF) (Guyton and Hall, 2006). The present article will examine the pathophysiology of acute liver failure, cerebal oedema and the correlation between the two.
Liver Failure
Liver failure is the umbrella term for the condition in which the liver can no longer function properly as a result of the death of a great number of hepatocytes. It can be further subcategorised into chronic liver failure or acute liver failure. Chronic liver failure refers to long term damage and scarring of the liver, brought on by a variety of causes, such as chronic alcohol abuse. Acute liver failure is a complex clinical syndrome, that results from a rapid and severe loss of hepatocyte function in a patient without pre-existing liver disease (Scott et al., 2013, Mosby Inc., 2013).
Acute Liver Failure
Acute liver failure is a clinical manifestation of sudden and severe hepatic injury and arises from many causes (Bernal et al., 2010). It is sometimes referred to as fulminant hepatic failure, a term first used by Trey and Davidson, who described a potentially reversible disorder resulting from severe hepatic injury with an onset of encephalopathy (Scott et al., 2013).
Although ALF is rare, with approximately one to six cases per million people every year in the United States and Western Europe, the mortality rate and cost of treatment are high (Scott et al., 2013). Young people make up the bulk of new diagnoses (Bower et al., 2007). The two main causes of ALF are paracetamol toxicity and hepatitis A or B. Generally, there is a greater incidence of ALF brought on by drug toxicity in more developed countries, where as in less developed countries ALF is predominantly caused by hepatitis A and B. In Europe and the United States for instance, there is a high incidence of ALF brought on by paracetamol overdose, where as in less developed countries such as Pakistan or South East Asian countries, ALF is predominantly caused by hepatitis (Bernal et al., 2015)
ALF can be further divided into three distinct phenotypes based on the time between the onset of jaundice and the development of encephalopathy. These phenotypes are as follows; hyper acute (within 7 days), acute (8-28 days) and subacute (5-12 weeks) (O'Grady et al., 1993).
The period of active hepatic injury in paracetamol poisoning can be measured in hours, and it is self-limited, in the sense that the toxicity may be resolved spontaneously and without specific treatment. This particular type of hepatotoxicity has a rapid onset and offset and a finite period of necrosis. These patients are characterised as having a “hyperacute” phenotype. In many cases,a rapid recovery follows despite massive multi-organ failure. This type differs greatly from most other forms of ALF, usually termed acute or subacute, wherein the hepatotoxicity evolves over 1-4 weeks, and is not self-limited, but long lasting. Hepatitis B, autoimmune hepatitis and most interdeterminant cases will have a subacute pattern and poorer chances of recovery (Bernal et al., 2015, Bernal et al., 2010). The table below details the symptoms of acute liver failure.
Figure 1;3. The effects of acute liver failure
Cerebral Oedema
Disordered brain function resulting in cerebral oedema is a major complication in ALF. This impairment of cerebral function is associated with the development of intracranial hypertension and death. The encephalopathy resulting from ALF rapidly leads to confusion, disorientation and agitation. These symptoms progress to delerium, seizures and eventually coma (Blei and Larsen, 1999). Cerebral oedema is a net increase in total brain water content. The rigid skull protecting the brain limits the compliance of the brain and as a consequence a small increase in fluid can cause a significant rise in intracranial pressure (Scott et al., 2013). Left untreated, cerebral oedema can rapidly progress to cause herniation of the uncus through the falx cerebri, leading to compression of the brainstem, and ultimately, death (Aldridge et al., 2015). Astrocyte foot swelling is the most consistent neuropathological finding postmortem (Kato et al., 1992).
It is undisputed that a variety of factors lead to astrocyte foot swelling and cerebral oedema in ALF but ammonia has been shown to have a key role. The effects of oedema include a severe compromise in cerebral metabolism and increased cerebral blood perfusion. Cerebral metabolic rate for oxygen decreases and there is failure of cerebrovascular autoregulation. Aswell as ammonia; glutamine, pro-inflammatory factors and oxidative or nitrosative stress have been implicated in its aetiology. (Butterworth 2014). Neuroinflammation is an important etiological factor, the treatment of this disorder will be discussed further (Scott et al., 2013)
The Role of Ammonia
Ammonia is traditionally considered a gut derived nitrogenous toxin produced by bacterial metabolism of amino acids primarily glutamine (Tapper et al., 2015). The gut is a critical component in the pathogenesis of cerebral oedema. Dietary nitrogen (particularly glutamine) is converted to ammonia through the action of gut enzymes as well as urease containing bacteria. Glutamine is its self metabolised by glutaminase in the intestinal epithelial cells, which converts more than one third of its nitrogen to ammonia, which diffuses into the portal circulation (Tapper et al., 2015).
Due to the loss of hepatocyte function in ALF, the liver is unable to carry out its normal processes, specifically detoxification of ammonia using the urea cycle and glutamine synthetase (see Fig 1;5). This increases ammonia concentration in extra hepatic tissues and causes a variety of issues within the brain as ammonia can easily bypass the blood brain barrier (BBB) and enter the organ. The blood brain barrier plays a critical role in establishing and maintaining homeostasis within the brain. It exerts tight control over any exchange of metabolites between the circulating blood and the central nervous system. BBB consists of brain capillary endothelial cells, pericytes and the foot processes of astrocytes. Together they form a neurovascular unit capable of regulating the special composition of the CSF (Scott et al., 2013, Abbott et al., 2010). The different hypotheses regaring ammonia’s role are outlined in Fig 1;4 below.
|
Figure 1:4; Summary of astrocyte swelling and oxidative stress
One of the primary functions of astrocytes is the conversion of glutamate to glutamine (a fundamental neurotransmitter) using glutamate synthetase. The reaction uses ammonia as a catalyst while also detoxifying the ammonia (as the brain lacks a urea cycle). However, due to the accumulation of ammonia and therefore the increased conversion of glutamate to glutamine, the increased levels of glutamine cause osmotic stress as water moves into the cell to counter balance its unequal solute gradient, resulting in increased astrocyte volume and cerebral oedema (Aldridge et al., 2015).
|
Alpha-ketoglutarate is a key metabolite in reducing glutamate levels as it can be used to convert it to glutamine and therefore prevent glutamate’s accumulation. However, in severe cases of ALF where hyperammonia occurs the stores of alpha-ketoglutarate itself will become depleted, having a negative impact on the citric acid cycle where it is a fundamental reactant. Furthermore ammonia may inhibit two rate limiting enzymes; pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase. This substrate depletion and partial enzyme inhibition compromise overall oxidative metabolism and lead to depletion of energy rich phosphate compounds (ATP and GTP) and accumulation of lactate. In addition, ammonia both induces oxidative and nitrosative stress by formation of free radicals which may in turn result in mitochondrial permeability transition (MPT) i.e. a state with imminent power failure of the astrocytes (Aldridge et al., 2015)
MPT usually develops in response to an increase in mitochondrial calcium levels and results in a sudden opening of the permeability transition pore, a large non-selective permeability pore in the inner mitochondrial membrane. This makes the inner mitochondrial membrane more permeable to protons, ions, and other small solutes. As a result, the inner mitochondrial dysfunction leads to spilling of the mitochondrial matrix, defective oxidative phosphorylation and adenosine triphosphate (ATP) production and generation of reactive oxygen species (Aldridge et al., 2015).
The “Trojan horse” hypothesis has recently been proposed as an alternative theory to explain the development of astrocyte swelling, and implicates an important role for both ammonia and glutamine. The excess glutamine synthesised within astrocytes is transported into the mitochondria where it is metabolised by phosphate-activated glutaminase (PAG) to ammonia and glutamate. Glutamate (the trojan horse) thereby acts as a carrier of ammonia into the mitochondria, where its accumulation can lead to oxidative stress and ultimately astrocyte swelling. Ammonia interferes with mitochondrial energy metabolism resulting in high levels of pyruvate and lactate and similar effects described under the prior hypothesis (Aldridge et al., 2015).
However, while it is widely accepted that ammonia has a fundamental role in cerebral oedema the exact role and mechanism is still not yet fully understood as blood ammonia concentrations was not predictive or consistent with severity in HE. This finding has been replicated with one such study showing the 69% of individuals with no overt signs of HE had elevated blood ammonia levels, whilst a number of patients with more significant grade 3 or 4 HE had either normal or only slightly elevated levels of ammonia in their blood (Aldridge et al., 2015).
Bernal and colleagues demonstrated ammonia to be an independent risk factor for the development of both HE and ICH, with ICH occurring in 55% of patients with blood ammonia concentration >200 micromol/L. Blood ammonia levels >(or equal to) 150 micromol/L are indicitive of a greater likelihood of cerebral herniation and death in patients with ALF (Aldridge et al., 2015). The pathogensis of cerebral oedema, as well as some treatments, are outlined in Fig 1;6.
|
Treatment
Emergency Liver Transplantation
Emergency liver transplants are probably the most successful and commonly used therapeutic methods today in patients with a very poor prognosis. Elective surgeries however have a much higher outcome than emergency transplants. This is mainly due to multi-organ failure and sepsis. Despite this, surgical methods have shown increasing improvement with 1 year survival exceeding 80% (Bernal et al., 2010).
While liver transplants are not common within the veterinary field, there is current research into potential surgical methods and ethical debates as to whether this could be a future therapeutic model (see A Method for Modeling Growth of Organs and Transplants Based on the General Growth Law: Application to the Liver in Dogs and Humans (Liu et al., 2013, Shestopaloff and Sbalzarini, 2014)
The reasoning behind targeting cerebral oedema during treatment is to mainly prevent infection and manage inflammation and hypo-osmolality. For intracranial hypertension within humans, intracranial pressure should be less than 20 mmHg while cerebral perfusion pressure should read above 70 mmHg, although in practice this is hard to achieve. Other considerations:
- intubation and ventilation may be required in patents that have grade 3 encephalopathy
- short acting agents are effective in easing mechanical ventilation and reducing seizures
- hyperventilation may be imperative for patients displays imminent signs of brain herniation i.e. pupillary dilatation and extensor posturing (the induction of hypocapnia causes pre capillary vasoconstriction and thus reduces cerebral blood flow)
- fluid resuscitation
- hyponatraemia should be reverted via isotonic saline solution (Scott et al., 2013).
Broad spectrum antibiotics and antifungals can be used to prevent the onset of sepsis which causes a rapid degeneration to severe encephalopathy. Other more specific treatments are discussed below (Scott et al., 2013).
N-Acetylcysteine
Now considered as standard treatment, intravenous N-acetylcysteine is used to treat acetaminophen induced and non-acetaminophen induced ALF as it acts as both an antioxidant and an anti-inflammatory (Aldridge et al., 2015).
Novel Treatments
Minocycline
Minocycline is a broad spectrum antibiotic which attenuated neuroinflammation in an experimental model of ALF (Henry et al., 2008) Jiang et al showed that hepatic encephalopathy and brain oedema were delayed in onset as the tetracycline antibiotic is able to exhibit a potent inhibitory effect on microglial activation (Jiang et al., 2009).
NMDA receptor antagonists
One of the more problematic effects of cerebral oedema is the development of oxidative stress. NMDA receptor antagonists have proven to block oxidative stress induced with hyperammonemia most probably because in ammonia toxicity the reactive oxygen species are mediated by NMDA-receptor activation (Kosenko et al., 1999, Marcaida et al., 1992).
Endotoxin removal
This therapeutic method is still in early clinical testing and requires further scientific evaluation before its actual biochemical and physiological effects are tested and reliable however the process involves an albumin replacement system with an endotoxin ligation which was tested in a pig model and proved to be successful (Zwirner et al., 2010).
Novel Anti-inflammatory Agents
Systemic inflammation is regularly seen in patients with ALF which sensitises the brain to the effects of ammonia. If this mechanism is removed then potentially the development of cerebral oedema can be prevented. However this is problematic as the acute rise in anti-inflammatory agents are fundamental to the liver’s repair and regeneration. Despite this, there are methods under review that target the different immune systems (innate and adaptive) in ALF (Scott et al., 2013).
Plasmapheresis
Despite the increase in cerebral blood flow, high volume plasmapheresis can alleviate brain oedema. It may also have a positive effect on reducing the systemic immune problems and endothelial dysfunction that is commonly seen. A randomised clinical trial of high volume plasmapheresis showed results which suggest improvement in the survival in patients unsuitable for liver transplantation (Larsen et al., 1996, Scott et al., 2013).
Mild hypothermia
Studies on animal models demonstrated that a decrease in temperature by 1 or 2 degrees can not only extend survival time but altogether prevent cerebra oedema. The mechanism is thought to be multifactorial but include the following effects; reduction of blood-brain ammonia transfer and decrease brain lactate synthesis which improves the brain's energy status. Coupled with the above effects, mild hypothermia also has anti-inflammatory properties and thus can reduce microglial activation in the brain alongside reduction of brain pro-inflammatory cytokines. Mild hypothermia is most commonly used during the transportation of patients to tertiary centres to await for liver transplantation (Butterworth, 2015).
Discussion
Acute liver failure, although rare, is expensive to treat and has a high mortality rate. As shown in Figure 1.4 while the causes of cerebral oedema in relation to acute liver failure have been hypothesised and are widely accepted (specifically ammonia’s role), their exact mechanisms are still under scrutiny as seen by the contradicting “Trojan-Horse” and “Ammonia-Glutamine” hypothesis. The multifaceted nature of the disease is reflected in the wide variety of treatment options. These each target different aspects of the pathology in order to alleviate specific systems e.g. antioxidants are given to prevent oxidative stress caused by the accumulation of glutamate. This is an exciting period in hepatology and advances, especially in the veterinary field (e.g. transplantation in animals) can and may change everyday clinical approaches to patients which before may have had a poor prognosis.
ABBOTT, N. J., PATABENDIGE, A. A., DOLMAN, D. E., YUSOF, S. R. & BEGLEY, D. J. 2010. Structure and function of the blood-brain barrier. Neurobiol Dis, 37, 13-25.
ALDRIDGE, D. R., TRANAH, E. J. & SHAWCROSS, D. L. 2015. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol, 5, S7-S20.
BERNAL, W., AUZINGER, G., DHAWAN, A. & WENDON, J. 2010. Acute liver failure. Lancet, 376, 190-201.
BERNAL, W., LEE, W. M., WENDON, J., LARSEN, F. S. & WILLIAMS, R. 2015. Acute liver failure: A curable disease by 2024? J Hepatol, 62, S112-20.
BLEI, A. T. & LARSEN, F. S. 1999. Pathophysiology of cerebral edema in fulminant hepatic failure. J Hepatol, 31, 771-6.
BOWER, W. A., JOHNS, M., MARGOLIS, H. S., WILLIAMS, I. T. & BELL, B. P. 2007. Population-based surveillance for acute liver failure. Am J Gastroenterol, 102, 2459-63.
BUTTERWORTH, R. F. 2015. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J Clin Exp Hepatol, 5, S96-S103.
DRAKE, R. L., DRAKE, R. L. & GRAY, H. 2008. Gray's atlas of anatomy, Philadelphia, Churchill Livingstone.
GUYTON, A. C. & HALL, J. E. 2006. Textbook of medical physiology, Philadelphia, Elsevier Saunders.
HENRY, C. J., HUANG, Y., WYNNE, A., HANKE, M., HIMLER, J., BAILEY, M. T., SHERIDAN, J. F. & GODBOUT, J. P. 2008. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation, 5, 15.
JIANG, W., DESJARDINS, P. & BUTTERWORTH, R. F. 2009. Minocycline attenuates oxidative/nitrosative stress and cerebral complications of acute liver failure in rats. Neurochem Int, 55, 601-5.
KATO, M., HUGHES, R. D., KEAYS, R. T. & WILLIAMS, R. 1992. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology, 15, 1060-6.
KOSENKO, E., KAMINSKI, Y., LOPATA, O., MURAVYOV, N. & FELIPO, V. 1999. Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic Biol Med, 26, 1369-74.
LARSEN, F. S., HANSEN, B. A., EJLERSEN, E., SECHER, N. H., CLEMMESEN, J. O., TYGSTRUP, N. & KNUDSEN, G. M. 1996. Cerebral blood flow, oxygen metabolism and transcranial Doppler sonography during high-volume plasmapheresis in fulminant hepatic failure. Eur J Gastroenterol Hepatol, 8, 261-5.
LIU, S. Q., LEI, P., CUI, X. H., LV, Y., LI, J. H., SONG, Y. L. & ZHAO, G. 2013. Sutureless anastomoses using magnetic rings in canine liver transplantation model. J Surg Res, 185, 923-33.
MARCAIDA, G., FELIPO, V., HERMENEGILDO, C., MINANA, M. D. & GRISOLIA, S. 1992. Acute ammonia toxicity is mediated by the NMDA type of glutamate receptors. FEBS Lett, 296, 67-8.
MOSBY INC. 2013. Mosby's dictionary of medicine, nursing & health professions, St. Louis, Mo., Elsevier/Mosby.
NICKEL, R., SCHUMMER, A. & SEIFERLE, E. 1954. Lehrbuch der Anatomie der Haustiere, Berlin,, Parey.
NICKEL, R., SCHUMMER, A., SEIFERLE, E. & SACK, W. O. 1979. The viscera of the domestic mammals, Berlin
New York, P. Parey ;
Springer-Verlag.
O'GRADY, J. G., SCHALM, S. W. & WILLIAMS, R. 1993. Acute liver failure: redefining the syndromes. Lancet, 342, 273-5.
SAMUELSON, D. A. 2007. Textbook of veterinary histology, St. Louis, Mo., Saunders-Elsevier.
SCOTT, T. R., KRONSTEN, V. T., HUGHES, R. D. & SHAWCROSS, D. L. 2013. Pathophysiology of cerebral oedema in acute liver failure. World J Gastroenterol, 19, 9240-55.
SHESTOPALOFF, Y. K. & SBALZARINI, I. F. 2014. A method for modeling growth of organs and transplants based on the general growth law: application to the liver in dogs and humans. PLoS One, 9, e99275.
TAPPER, E. B., JIANG, Z. G. & PATWARDHAN, V. R. 2015. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc, 90, 646-58.
ZWIRNER, K., THIEL, C., THIEL, K., MORGALLA, M. H., KONIGSRAINER, A. & SCHENK, M. 2010. Extracellular brain ammonia levels in association with arterial ammonia, intracranial pressure and the use of albumin dialysis devices in pigs with acute liver failure. Metab Brain Dis, 25, 407-12.