|
Size: 43540
Comment:
|
← Revision 104 as of 2016-05-05 19:33:12 ⇥
Size: 43581
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 15: | Line 15: |
| . {{attachment:1.jpg|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''Limbic System Schematic Diagram'' |
. . {{attachment:1.jpg|pop-up text}} <<BR>>'''Fig 1.'''<<BR>>''Limbic System Schematic Diagram'' |
| Line 45: | Line 22: |
| . {{attachment:2.jpg|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''Sheep Brain: Image of sheep brain was taken in 2008 at St. Mary’s College of Maryland under the guidance of Dr. Anne Marie Brady in a Biological Psychology course'' | . {{attachment:2.jpg|pop-up text}} <<BR>>'''Fig 2.'''<<BR>>''Sheep Brain: Image of sheep brain was taken in 2008 at St. Mary’s College of Maryland under the guidance of Dr. Anne Marie Brady in a Biological Psychology course'' |
| Line 53: | Line 58: |
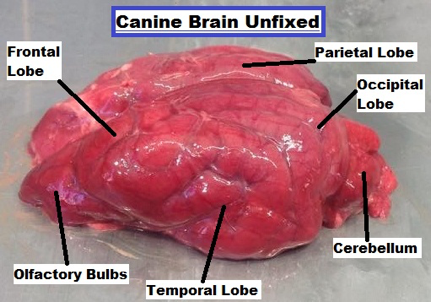
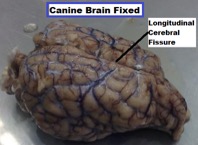
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:3.png|pop-up text}} <<BR>>'''Fig 3.'''<<BR>>''Canine Brain Unfixed Dorsal lateral view'' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:4.jpg|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''Canine Brain Fixed Dorsal View'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:3.png|pop-up text}} <<BR>>'''Fig 3.'''<<BR>>''Canine Brain Unfixed Dorsal lateral view'' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:4.jpg|pop-up text}} <<BR>>'''Fig 4.'''<<BR>>''Canine Brain Fixed Dorsal View'' || |
| Line 59: | Line 64: |
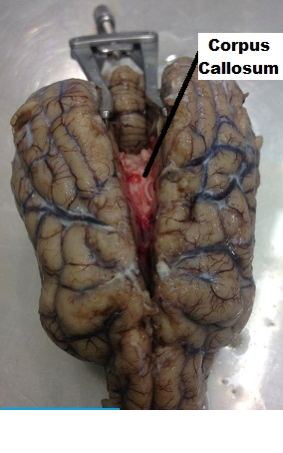
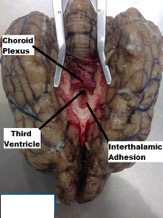
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:5.png|pop-up text}} <<BR>>'''Fig 5.'''<<BR>>''Canine Brain Ventral View Corpus Callosum'' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:6.png|pop-up text}} <<BR>>'''Fig 6.'''<<BR>>''Canine Brain Choroid Plexus Ventral View '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:7.png|pop-up text}} <<BR>>'''Fig 7.'''<<BR>>''Canine Brain Dorsal View Hippocampus '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:8.png|pop-up text}} <<BR>>'''Fig 8.'''<<BR>>''Canine Brain Dorsal View Hippocampus, Fornix and Fimbria. Remainding part of Cortex Removed '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:10a.png|pop-up text}} <<BR>>'''Fig 9.'''<<BR>>''Canine Brain Dorsal View Left Hemisphere Removed '' || |
||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:5.png|pop-up text}} <<BR>>'''Fig 5.'''<<BR>>''Canine Brain Ventral View Corpus Callosum'' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:6.png|pop-up text}} <<BR>>'''Fig 6.'''<<BR>>''Canine Brain Choroid Plexus Ventral View '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:7.png|pop-up text}} <<BR>>'''Fig 7.'''<<BR>>''Canine Brain Dorsal View Hippocampus '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:8.png|pop-up text}} <<BR>>'''Fig 8.'''<<BR>>''Canine Brain Dorsal View Hippocampus, Fornix and Fimbria. Remainding part of Cortex Removed '' || ||<style="padding:0.5em; ;text-align:center"> {{attachment:10a.png|pop-up text}} <<BR>>'''Fig 9.'''<<BR>>''Canine Brain Dorsal View Left Hemisphere Removed '' || |
| Line 68: | Line 73: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:11a.png|pop-up text}} <<BR>>'''Fig 10.'''<<BR>>''Canine Brain Transection Dorsal to Ventral Position Limbic System '' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:11a.png|pop-up text}} <<BR>>'''Fig 10.'''<<BR>>''Canine Brain Transection Dorsal to Ventral Position Limbic System '' || |
| Line 137: | Line 142: |
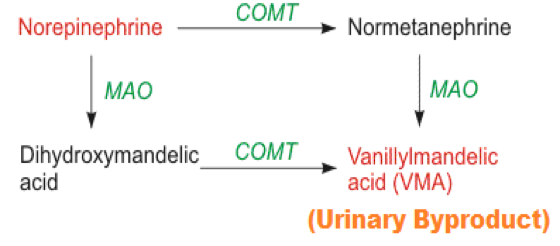
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:18.png|pop-up text}} <<BR>>'''Fig 15.'''<<BR>>''Schematic Diagram of Norepinephrine Metabolism '' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:18.png|pop-up text}} <<BR>>'''Fig 15.'''<<BR>>''Schematic Diagram of Norepinephrine Metabolism '' || |
| Line 162: | Line 167: |
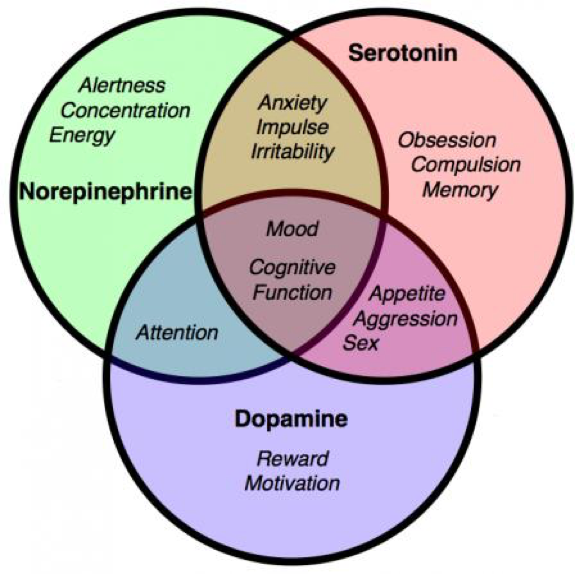
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:20.png|pop-up text}} <<BR>>'''Fig 17.'''<<BR>>''Schematic Graph of Monoamine Roles '' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:20.png|pop-up text}} <<BR>>'''Fig 17.'''<<BR>>''Schematic Graph of Monoamine Roles '' || |
| Line 184: | Line 189: |
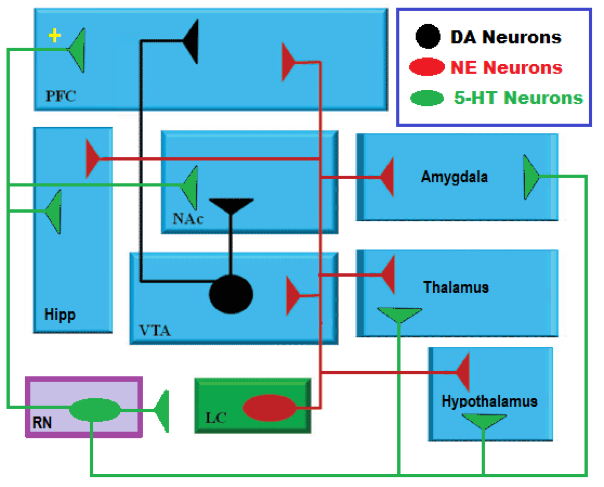
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:22.png|pop-up text}} <<BR>>'''Fig 18.'''<<BR>>''Schematic Diagram of Neural Pathways Within Monoamines '' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:22.png|pop-up text}} <<BR>>'''Fig 18.'''<<BR>>''Schematic Diagram of Neural Pathways Within Monoamines '' || |
| Line 198: | Line 203: |
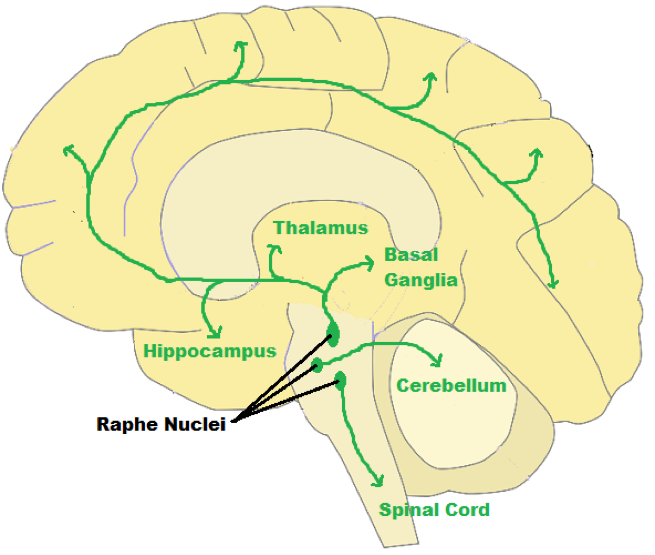
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:23.png|pop-up text}} <<BR>>'''Fig 19.'''<<BR>>''Schematic Diagram of Raphe Nuclei Pathway'' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:23.png|pop-up text}} <<BR>>'''Fig 19.'''<<BR>>''Schematic Diagram of Raphe Nuclei Pathway'' || |
| Line 234: | Line 239: |
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:25.png|pop-up text}} <<BR>>'''Fig 20.'''<<BR>>''Schematic Diagram of Acute Amphetamine Exposure'' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:25.png|pop-up text}} <<BR>>'''Fig 20.'''<<BR>>''Schematic Diagram of Acute Amphetamine Exposure'' || |
| Line 240: | Line 245: |
| ||<style="padding:0.5em; ;text-align:center"> {{attachment:26.png|pop-up text}} <<BR>>'''Fig 21.'''<<BR>>''Schematic Diagram of Chronic Amphetamine Exposure'' || | ||<style="padding:0.5em; ;text-align:center"> {{attachment:26.png|pop-up text}} <<BR>>'''Fig 21.'''<<BR>>''Schematic Diagram of Chronic Amphetamine Exposure'' || |
Amphetamines and The Limbic System
Contents
The term limbic system is applied to a collection of brain structures found in all mammals which is involved with a variety of functions including epinephrine flow, emotion, behavior, motivation, long-term memory, and olfaction. Emotional life is largely housed in the limbic system, and it has a great deal to do with the formation of memories. The limbic system has great input on thirst, hunger, sexual behavior, and reward. Many drugs of abuse such as cocaine and amphetamines directly affect the structures of the limbic system. Perhaps the simplest way to understand the functions of the limbic system is to use the mnemonic “M-O-V-E.”
M – Motivation and Memory formation
O – Olfaction or sense of smell
V – Visceral of autonomic nervous system functions
E – Emotional components of behaviour
.
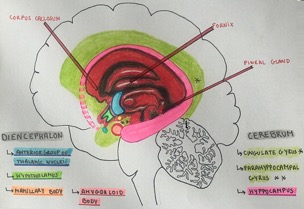
Fig 1.
Limbic System Schematic Diagram
All mammals have a limbic system, however many specie variants developed related to the magnitude olfaction plays in the animal’s evolution. In species where olfaction development and function is high (canines, rodents, etc.), the limbic system constitutes more of the brain mass and less devotion to outer cortex development (Finlay et al., 2001). Primates, insectivores, and ungulates collectively demonstrate an inverse relationship between cerebral cortex and limbic volumes, but terrestrial carnivores have high relative volumes of both; meanwhile bats have low relative volumes of both (Reep et al., 2007). Marine mammals with reduced olfactory bulbs also have a reduced limbic system overall (Clark et al., 2001).
Structures of the Limbic System
The Limbic System consists of cortical and subcortical components that have developed from the telencephalon, diencephalon and mesencephalon. The cortical part comprises interconnected telencephalic structures on the medial and basal aspect of the hemispheres, namely the cingulated gyrus, hippocampus and the piriform lobe. The subcortical part includes components of the diencephalon (habenula, hypothalamus, thalamus), midbrain (tegmental nuclei) and the amygdala. The limbic system receives olfactory input from the amygdala and piriform lobe that can trigger emotional behavior such as fear, aggression, and apparent pleasure.
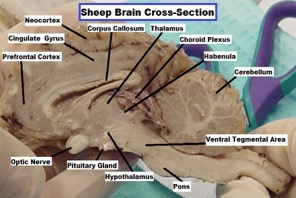
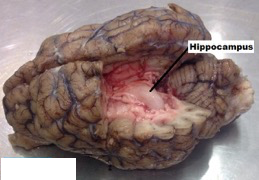
Fig 2.
Sheep Brain: Image of sheep brain was taken in 2008 at St. Mary’s College of Maryland under the guidance of Dr. Anne Marie Brady in a Biological Psychology course
Dog Brain Extraction
The extraction, fixation, and dissection of the dog brain was completed at Szent Istvan University under the guidance of Dr. Kalman Czeibert. Skin surround skull was incised, then using scalpel, the temporal and masseter muscles were transected and scrapes off skull. Using a surgical saw, a rostral cut was made into the frontal sinus. Superficial cuts were made laterally on the temporal bone starting at the occipital condyles, through the nuchal crest and along the temporal bones connecting to the rostral cut at the frontal sinus. Using a surgical chisel and hammer, the superficial cuts were widened allowing the skull to easily be removed dorsally. Carefully, the cranial nerves were removed and the brain was extracted from the cranial cavity.
Fixation
- The structure and consistency of the dog brain upon removal was not ideal for dissecting in order to expose the limbic system. Upon removal of the canine brain, it was placed in a 7% formaldehyde solution for 24 hours than transferred into a 3% formaldehyde solution for another 24 hours.
|
|
|
Dissection
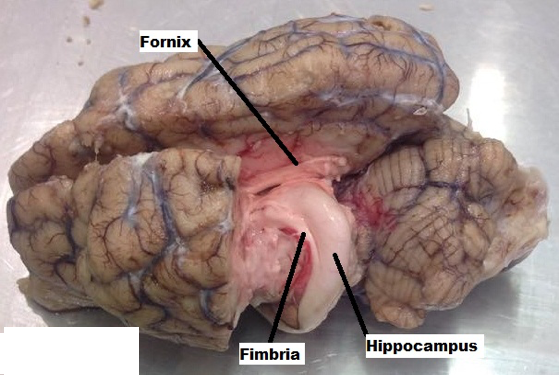
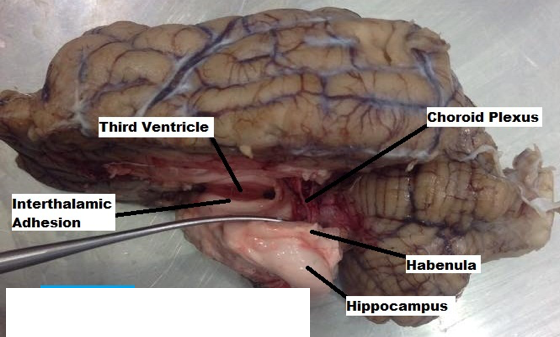
- The two hemispheres were gently widened, exposing the white matter of the corpus callosum. A light incision was made on the corpus callosum (Figure 5), removing the dense axon fibers connecting the two hemispheres, and making visible the third ventricle and choroid plexus (Figure 6). Using the borders of the lateral ventricles, gently a transverse incision was made through the parietal lobe as well as a lateral cut that would continue all the way through the occipital lobe (Figure 7). The horn of the hippocampus was made visible. Using finite and precise cuts, the remainder of the cortex was removed around the hippocampus (Figure 8). Through a similar process, the remaining part of the left hemisphere was removed, making the thalamus and other components of the limbic system visible (Figure 9).
|
|
|
|
|
|
|
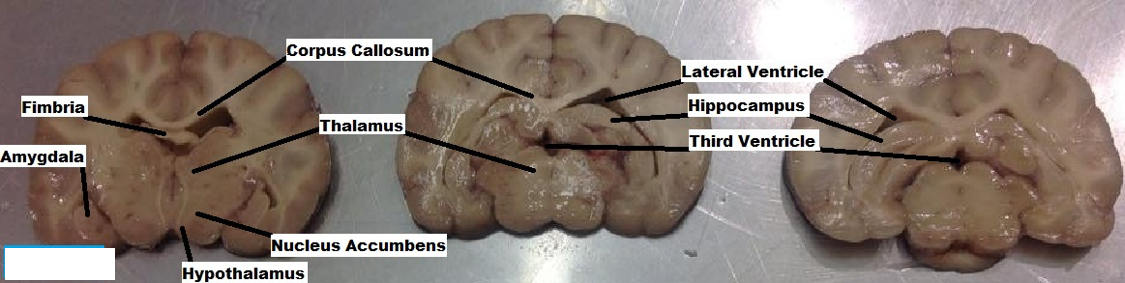
- A second brain was similarly fixed and used for cross sectional dissections. Approximately 5-9 mm thick cuts were made transecting through the canine brain from a dorsal to ventral position. Brain slices allowed the exposure of the internal components of the limbic system (Figure 10).
|
The various components of the limbic system are each associated with different functions. The Amygdala is involved with several emotional responses (Sato et al., 2016); the Hippocampus is involved with learning and memory formation (Mao et al., 2015); the Thalamus relays sensory and motor signals plus regulates sleep and alertness (Li S et al., 2014); the Nucleus Accumbens with reward, pleasure and addiction (West et al., 2016); Hypothalamus is involved with regulation of the pituitary gland and hormonal function (Perez et al., 2016); Olfactory bulbs involved in the perception of smell and connect to piriform lobe & amygdala (De La Rosa et al., 2015); the Habenula receives input from the limbic system and basal ganglia, influencing the brains response to pain, stress, anxiety, sleep and reward (Velasquez et al., 2014).
Neurotransmission
As an action potential travels down the axon, the depolarized cell membrane opens voltage gated calcium channels at the terminal button and stimulates vesicular release. These synaptic vesicles are filled with a particular neurotransmitter (neuro-chemical). There are over 100 different neurotransmitters that have identified in the mammalian nervous system. The location, post-synaptic effect, metabolism, and removal of these neurotransmitters significantly differ from one another. Neurotransmitter transport systems are integral to the release, re-uptake and recycling of neurotransmitters at synapses. High affinity transport proteins found in the plasma membrane of presynaptic nerve terminals and glial cells are responsible for the removal from the extracellular space of released-transmitters, thereby terminating their action. In addition to these removal systems, several neurotransmitter-specific enzymes are present to inactivate or regulate these chemicals released into the synapse. Upon bonding to a post-synaptic receptor, these neurochemicals will typically have an excitatory or inhibitory response on the sequential neuron through ionotropic or metabotropic functions. Through electrophysiological studies, immunohistochemistry, and genetic knock-out models, the understanding of these brain chemicals has vastly improved in the past couple decades. In regards to the topic of this essay and its relation to the Limbic System, we are only going to focus on the neurotransmitters specifically relevant.
Neurotransmitters And Corresponding Receptors
Catecholamines
Classification of neurotransmitters based on their organic structure. Dopamine (DA), Norepinephrine (NE), and Epinephrine (E) belong to this class based on the fact that they are all variants of the organic Diphenol, Catechol, as well as containing an amine group. These biogenic amines are derived from the amino acid tyrosine. Tyrosine is created from phenylalanine via hydroxylation by the enzyme phenylalanine hydroxylase or is ingested directly from dietary protein. It is then sent to catecholamine-secreting neurons where many kinds of reactions convert it to Dopamine, to Norepinephrine, and eventually to Epinephrine depending on the neuron type. Dopamine and Norepinephrine have strong implications in the Limbic System and will be described in detail.
Dopamine
- This neurotransmitter has many effects systemically, but in relation to its function in the central nervous system, Dopamine has been connected to the involvement in the reward circuitry of the brain as well as control of locomotion, cognition, endocrine function and feelings of engagement or excitement (Vokow et al, 1998; Shen, 2008).
Dopamine Receptors
- There are five sub-classes of Dopamine receptors scattered throughout the body of all mammalian species and they are all members of the Rhodopsin like G-Protein Receptor family (7-helix trans-membrane proteins). All of these receptors are metabotropic and involve a second messenger G-Protein (Kienast et al., 2006). Dopamine receptors are typically classified as either a D1 or D2 class, depending on their ability to either inhibit or activate adenylate cyclase and a cascade of events in the post-synaptic neuron (Le Crom et al., 2003).
Fig 11.
Schematic Diagram Distribution of Dopamine Receptors Throughout the Brain
Dopamine Metabolism
- As dopamine is released into the synapse, there are many mechanisms designed to remove or inactivate this chemical following neurotransmission.
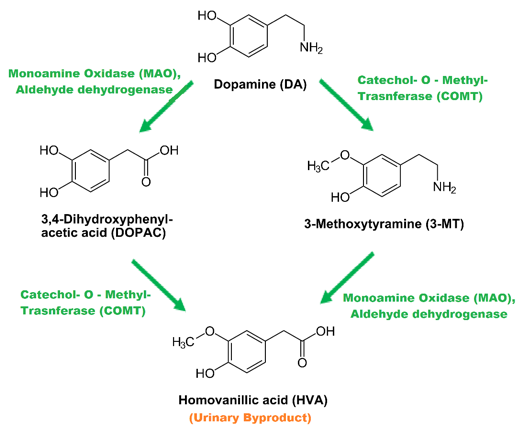
Fig 12.
Schematic Summary of Dopamine Metabolism
Dopmanine Transporter
- The dopamine transporter is critical for the removal of dopamine from the extracellular space, following its release, and is the principal site of action for psycho-stimulant drugs, such as cocaine and amphetamines, which inhibit the transporter's activity (Giros et al., 1992). A single form of dopamine transporter has been isolated from humans and other mammals. Targeted gene disruption of the dopamine transporter has confirmed its importance in maintaining low extracellular dopamine levels (Gainetdinov et al., 1999).
Monoamine Oxidase
- Attached to the mitochondria, this family of enzymes catalyzes the oxidation of monoamines through deamination including serotonin, dopamine, norepinephrine and epinephrine. They are found bound to the outer membrane of mitochondria in most cell types in the body (Kumagae, 1991).
Catachol-O-Methyl-Transferase
- Found membrane bound inside of neurons in nearby glia, this enzyme catalyzes the transfer of a methyl group from S-adenosylmethionine to catecholamines, including the neurotransmitters dopamine, epinephrine, and norepinephrine. (Lachman et al., 1996). This O-methylation results in one of the major degradative pathways of the catecholamine transmitters. It is involved in the inactivation of dopamine in brain regions in which the dopamine transporter (DAT1) is sparsely expressed (Jugurnauth et al., 2012).
Trace Amine Associated Receptor
- A recently discovered endogenous receptor that stores amine metabolites and monoamines that is also a member of the rhodopsin-like G-protein coupled receptor (GPCR) family. (Miller, 2011; Maguire et al., 2009) Since trace amine associated receptors are putative endogenous receptors for trace amines. believed to be a key regulator of common and trace brain monoamines (Lindemann et al., 2008)
Veiscular Monoamine Transporter (VMAT)
- The VMAT is a transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons.(Yulung et al., 2015). It acts to transport monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons (Wang et al., 2016; Freyberg, 2016)
Fig 13.
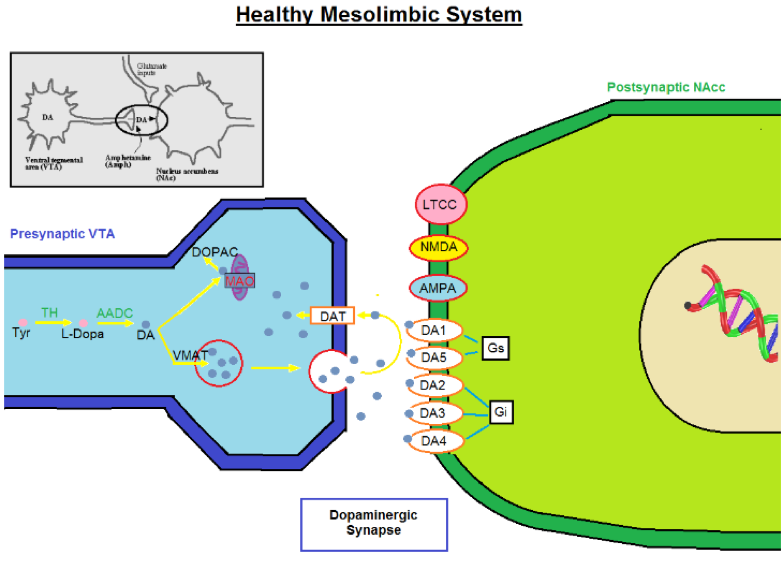
Schematic Diagram of Healthy Mesolimbic system
Neural Pathways
- Brain regions such as the substantia nigra (SN) and ventral tegmental area (VTA) are known to contain dopaminergic cell bodies (Giros et al., 1992). These regions have projects to other locations in the brain where dopamine is released at the axon terminal
.
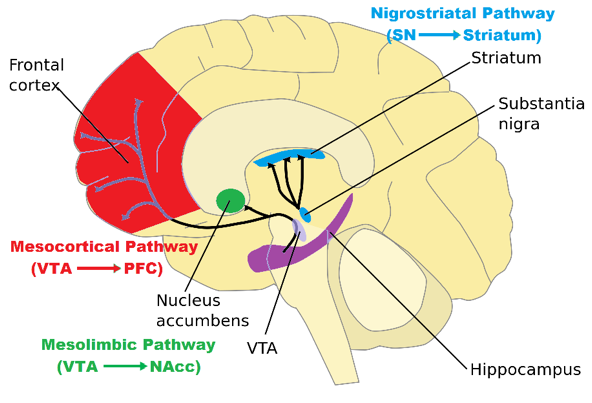
Fig 14.
Schematic Summary of Dopamine Pathways
Mesolimbic Pathway
- Dopaminergic tracts originate from the ventral tegmental area and synapse with the nucleus accumbens. This pathway is known as the reward circuit and is activated during pleasurable stimuli such as food, success, sex, and psychoactive drugs (Joseph et al., 2016). Drug addiction has been associated to this pathway.
Mesocortical Pathway
- Dopamine releasing pathway that connects the ventral tegmental area (VTA) with the prefrontal frontal cortex (PFC). It is essential for normal cognitive functioning and is involved in cognition, motivation and emotional response. The D1 class of receptors are more common found in the cerebral cortex (Puig et al., 2014).
Nigrostriatal Pathway
- Although irrelevant to the functioning of the limbic system, some of the side effects of drugs that effect the physiology of dopamine will also impair this pathway. The nigrostriatal pathway in locomotion and motor activity. D1A and D2 Receptors are in high concentration inside this system (Song et al., 2016).
Tuberoinfundibular Pathway
- A recently discovered pathway of the nervous system. Dopamine producing neurons inside of hypothalamic arcuate nucleus have projections to the pituitary gland. Acting as a feeback control system, the hormone prolactin is tonically inhibited by dopamine secretion. Implications for this system may involve the control of lactation, sexual libido, fertility, and body weight (Ramirez et al., 2015).
Norepinephrine
Norepinephrine has multiple roles within the central nervous system and the periphery. First, it relays messages in the sympathetic nervous system, as part of the autonomic nervous system's fight-or-flight response. Secondly, norepinephrine prepares the brain to encounter and respond to stimuli from the environment, thereby facilitating vigilance. So in both roles, norepinephrine mediates arousal (Zaniewska et al., 2015).
Receptors
There are three-classes of norepinephrine adrenoreceptors scattered throughout the body of all mammalian species and they are all members of the Rhodopsin like G-Protein Receptor family (7-helix trans-membrane proteins). All of these receptors are metabotropic and involve a second messenger G-Protein (Shen et al.; 2008). The three classes are alpha 1- α1 (a Gq coupled receptor- postsynaptically excitatory), alpha 2- α2 (a Gi coupled receptor- presynaptically inhibitory of noradrenaline and beta ᵝ (a Gs coupled receptor), and each can be further divided into subtypes (Ma et al; 2004).
Metabolism
- Since Norepinephrine is also a monoamine and catecholamine, similar metabolism occurs that were previously reviewed with dopamine.
|
Plasma Membrane Monoamine Transporter
- General transporter located on the presynaptic neuron for all monoamines (Serotonin, Norepinephrine, Epinephrine, and Dopamine). It is critical for the removal of epinephrine from the extracellular space (Naganuma, 2014).
Monoamine OxidaseCatechol-O-Methyl-Transferase
- Attached to the mitochondria, this family of enzymes catalyzes the oxidation of monoamines through deamination including serotonin, dopamine, norepinephrine and epinephrine. They are found bound to the outer membrane of mitochondria in most cell types in the body (Kumagae et al., 1991).
Catechol-O-Methyl-Transferase
- Attached to the mitochondria, this family of enzymes catalyzes the oxidation of monoamines through deamination including serotonin, dopamine, norepinephrine and epinephrine. They are found bound to the outer membrane of mitochondria in most cell types in the body (Kumagae et al., 1991).
Trace Amine Associated Receptor
- A recently discovered endogenous receptor that stores amine metabolites and monoamines that is also a member of the rhodopsin-like G-protein coupled receptor (GPCR) family. (Miller, 2011) Since trace amine associated receptors are putative endogenous receptors for trace amines. believed to be a key regulator of common and trace brain monoamines (Lindemann et al., 2008)
Veiscular Monoamine Transporter
- A transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons.(Yulung et al., 2015). It acts to transport monoamine neurotransmitters – such as dopamine, serotonin, norepinephrine, epinephrine, and histamine – into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons (Wang et al., 2016; Freyberg et al., 2016)
Neural Pathways
- The axons of neurons in the loci coerulei project to both sides of the brain where they innervate and release norepinephrine in wide ranging areas. Branching axons of norepinephrine-producing neurons in the loci coerulei innervate the brain stem, spinal cord, and cerebellum, as well as the hypothalami, thalamic relay nuclei, amygdalae, and neocortex (Aston-Jones et al., 2016). Norepinephrine which is most associated with learning and attention problems in our modern-day life (Zaniewska et al., 2015). Selective depletion of NE in the forebrain makes animals more distractible. It is the neurotransmitters norepinephrine and dopamine that accomplish attentiveness in the prefrontal cortex (Golirzaei, 2016).
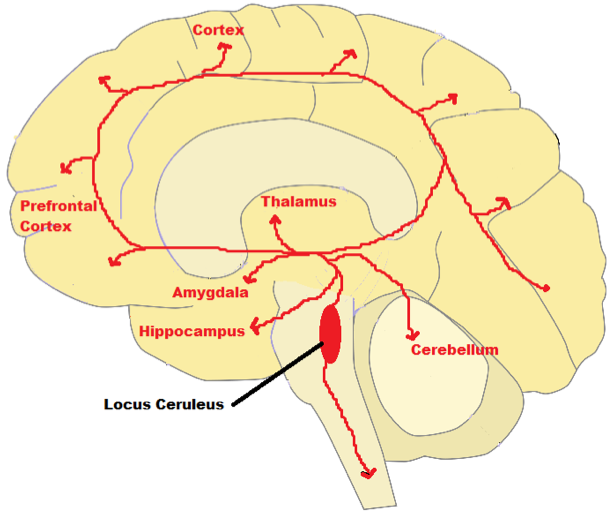
Fig 16.
Schematic Diagram of Norepinephrine Neural Pathway
Monoamines
Monoamines are general description of neurotransmitters that are all derived from the amino acids phenylalanine, tryptophan or tyrosine. As previously described, the catecholamines (dopamine, norepinephrine and epinephrine) are specific class of monoamines. Similarly, histamine, melatonin and serotonin are classes of monoamines due to their derivation from one of these aromatic amino acids.
|
Serotonin (5-HT)
From a broad perspective, whereas it is dopamine that primarily drives the seeking and reward system, it is both norepinephrine and dopamine that drive the vigilance system, while serotonin acts as a modulator of these neurotransmitters. The transmission of serotonin has a strong influence on the transmission of these and other neurotransmitters. Serotonergic neurons play a fundamental role in the integration of behavior in the central nervous system, but it also holds large roles in the gastrointestinal tract and blood platelets (Nicholas et al., 2008). Our sense of well-being and our capacity to organize our lives and to relate to others depend profoundly on the functional integrity of the serotonergic system. Many distinct emotions share generalized components such as acetylcholine, norepinephrine, and serotonin systems for the control of attention and general arousal functions. There are only a few hundred thousand serotonergic neurons in the human brain, roughly one millionth of the total population of neurons in the human central nervous system (Burnet et al., 1995).
Receptors
Fourteen types of serotonin receptors have been discovered so far in the brains of mammals, located in different places and acting in different ways Due to the magnitude of serotonin receptors located throughout the body, this project will only focus on the receptors relevant to the limbic system and amphetamines. Receptors for 5-HT mediate both excitatory and inhibitory neurotransmission, and modulate the release of many neurotransmitters including glutamate, GABA, dopamine, epinephrine/norepinephrine, and acetylcholine, as well as many hormones, including oxytocin, prolactin, vasopressin and cortisol (Nicholas et al., 2008). In the CNS, 5-HT receptors can influence various neurological processes, such as aggression, anxiety and appetite and, as a, result are the target of a variety of pharmaceutical drugs, including many antidepressants, antipsychotics and anorectics (Aznar et al., 2003). With the exception of the 5-HT3 receptor, which is a ligand-gated ion channel, all 5-HT receptors are members of the rhodopsin-like G protein-coupled receptor family and they activate an intracellular second messenger cascade to produce their responses (Le Crom, 2005).
5-HT1A
5-HT1A receptors are the most widespread of all the 5-HT receptors and are particularly high density in the limbic system (Aznar et al., 2003). They are found pre- and post-synaptically, (Burnet et al., 1995) in the raphe nuclei they are somatodendritic and act as autoreceptors to inhibit cell firing; whereas postsynaptic 5-HT1A receptors are present in a number of limbic structures, particularly the hippocampus (Aznar et al., 2003). 5-HT1A receptors are involved in many neuromodulative processes, and are potential anxiolytic and hypertensive targets. In the CNS, 5-HT1A receptors exist in the cerebral cortex, hippocampus, septum, amygdala, and in the raphe nucleus in high densities, whilst lower amounts also exist in the basal ganglia and thalamus (Burnet et al., 1995).
5-HT2A
5-HT2A receptors are one of the main excitatory serotonin receptors and are expressed throughout the central nervous system in high concentrations found on the apical dendrites of hippocampal pyramidal cells (Aznar et al., 2003). These receptors regulate dopamine release as well as modulate cognitive processes by enhancing glutamate release in the cerebral cortex (Burnet et al., 1995).
5-HT2C
5-HT2C receptor distribution is limited to the central nervous system and the choroid plexus (Nicholas et al., 2008). Activation of the receptor has been shown to exert an inhibitory influence upon frontal cortical and striatal dopamine and NE (Milan et al., 1998)
5-HT6
5-HT6 receptors decrease monoamines in the prefrontal cortex by stimulating; antagonism of these receptors facilitates dopamine and norepinephrine release in the frontal cortex (Lacroix et al., 2004).
|
Metabolism
Serotonin Transporter
The serotonin transporter is critical for the removal of serotonin from the extracellular space, following its release, and is an active site of action for antidepressants (Giros et al., 1992). Targeted gene disruption of the transporter has confirmed its importance in maintaining positive emotions (Gainetdinov et al., 1999).
Monoamine Oxidase
Attached to the mitochondria, this family of enzymes catalyzes the oxidation of monoamines through deamination including serotonin, dopamine, norepinephrine and epinephrine. They are found bound to the outer membrane of mitochondria in most cell types in the body (Kumagae et al., 1991).
Vesicular Monoamine Transporter
A transport protein integrated into the membrane of synaptic vesicles of presynaptic neurons.(Yulung et al., 2015). It acts to transport monoamine neurotransmitters (such as dopamine, serotonin, norepinephrine, epinephrine, and histamine) into the vesicles, which release the neurotransmitters into synapses as chemical messages to postsynaptic neurons (Wang, 2016; Freyberg et al., 2016)
|
NeuralPathways
The axons of the serotonergic neurons project in rich profusion to every part of the central nervous system (the brain and spinal cord), where they influence the activity of virtually every neuron. This widespread influence implies that the serotonergic neurons play a fundamental role in the integration of behavior (Nicholas et al., 2008). One exception where serotonin directly excites neurons is in the cerebral cortex, where it excites pyramidal neurons (Aznar et al., 2003). Our sense of well-being and our capacity to organize our lives and to relate to others depend profoundly on the functional integrity of the serotonergic system. Axons of neurons in the lower raphe nuclei terminate in the spinal cord as well as the cerebellum's deep nuclei and cortex. Axons of neurons in the higher raphe nuclei terminate in multiple regions; including the centrally located thalamus; the nucleus accumbens; the hypothalamus, hippocampus, and amygdala (Aznar et al., 2003).
Other Relevent Neurotransmiters
Glutamate
There are three classes of ionotropic glutamate receptors (iGluRs), namely NMDA (N-methyl-D-aspartate), AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-4-propionic acid) and kainate receptors (Paladini et al., 2001). They are believed to play critical roles in synaptic plasticity. At many synapses in the brain, transient activation of NMDA receptors leads to a persistent modification in the strength of synaptic transmission mediated by AMPA receptors and kainate receptors can act as the induction trigger for long-term changes in synaptic transmission by resulting in an influx of calcium ions into the cell (Bortolotto et al., 1999). At many synapses in the brain, transient activation of NMDA receptors leads to a persistent modification in the strength of synaptic transmission mediated by AMPA receptors and kainate receptors can act as the induction trigger for long-term changes in synaptic transmission.
Behavioural Effects of Amphetamines
- Amphetamines are stimulants of the central nervous system that specifically target many regions of the limbic system by altering the normal neurochemistry. Short term effects of amphetamine use include reduced appetite, dilation of pupils, insomnia, euphoria, and an increase in heart rate, blood pressure, alertness, concentration, energy and motor activity. With higher doses, or chronic use, these effects are intensified, leading to exhilaration and euphoria, rapid flow of ideas, feelings of increased mental and physical ability, excitation, agitation, fever, weight loss, tremors and sweating. Paranoid thinking, confusion and hallucinations have been observed. Severe overdose may lead to high fever, convulsions, coma, cerebral haemorrhage and death.
Amphetamine Effect on Neurotransmitters
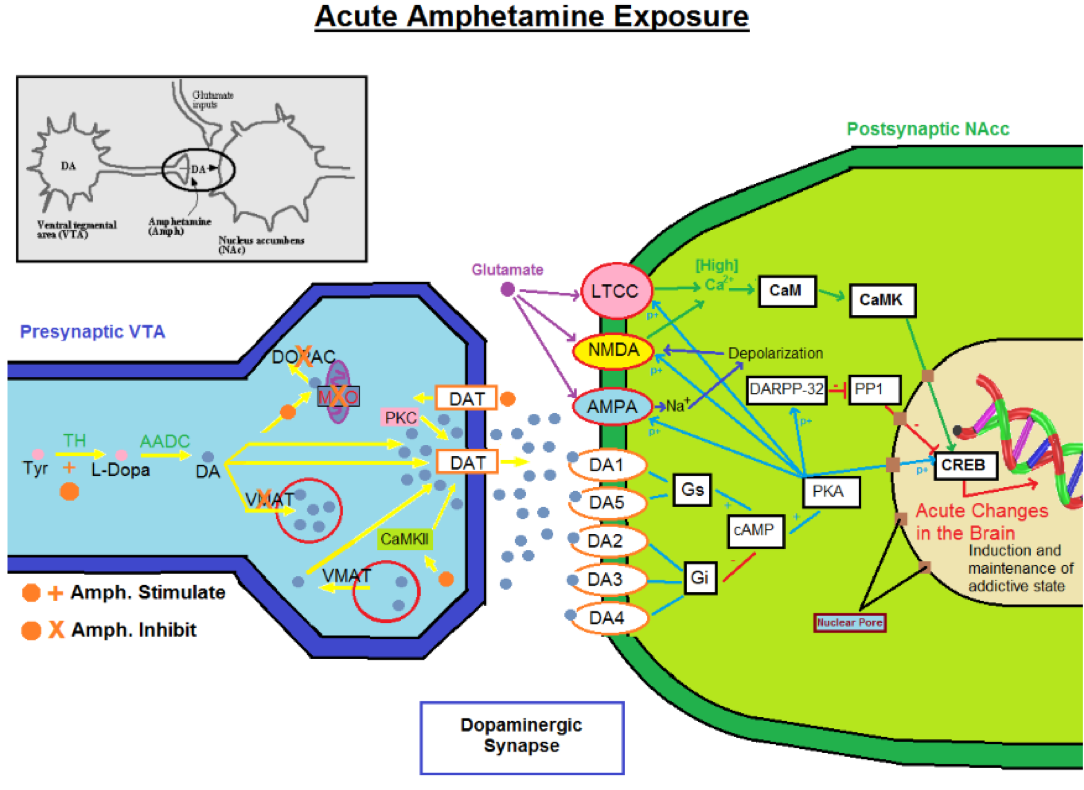
Amphetamines are stimulants of the central nervous system and sympathetic division of the peripheral nervous system. Similar to cocaine, amphetamines are used to combat fatigue by amplifying our body’s neurochemistry and increasing the concentration of dopamine in the synaptic gap, but through a more successful mechanism. Amphetamines are similar in structure to dopamine and norepinephrine, and due to this organic structure similarity, can mimic the effect of these neurotransmitters inside of the neuron. These compounds enter the brain very easily and cause significant changes in monoaminergic neurotransmission.
The main action of amphetamines is to increase the synaptic activity of the dopamine and norepinephrine neurotransmitter systems. These psychostimulants are capable of entering the terminal button of the pre-synaptic neuron via monoamine transporters, as well as by simply diffusing through the neural membrane directly. Once inside the presynaptic neuron, amphetamines are capable of increasing the concentration of the dopamine system in 5 ways:
Dopmanine Transporter
Amphetamines bind to the dopamine re-uptake transporter preventing for removal of dopamine from the synapse. In higher concentrations, amphetamines cause the dopamine transporter to act in reverse and transport free dopamine out of the nerve terminal. Amphetamines act as competitive substrates at the membrane transporters of noradrenaline (NAT), dopamine (DAT), and serotonin (5-HTT; SERT) (Zaniewska et al., 2015)
Monoamine Oxidase Inhibitor
Amphetamines bind to monoamine oxidase in dopaminergic neurons and prevent the degradation of dopamine, leaving free dopamine in the nerve terminal. Similarly inside of serotonergic and androgenic receptors, deactivation of the mitochondrial enzyme significantly elevates serotonin and norepinephrine levels inside the central nervous and limbic system (Miller, 2011).
VMAT Malfunction
Amphetamines work on the vesicle packing system by acting as a competitive antagonist of the VMAT protein and blocking the presynaptic cell's ability to use VMAT for vesicular packaging, affecting the distribution of dopamine in the cell. By interacting with the dopamine containing synaptic vesicles, a greater amount of dopamine is released into the nerve terminal (Freyberg Z et al.,2016).
Catechol-O-Methyl-Transferase
In high concentrations of amphetamine exposure, the enzyme COMT is inactivated similarly to MAO. The inactivation of this enzyme produces an elevated concentration of both epinephrine and dopamine through out the system (Bunzow et al., 2001).
All of these actions result in more dopamine, norepinephrine and serotonin in the synaptic cleft where it can act on receptors. High-dose amphetamine can modify the action of dopamine and noradrenaline in the brain. At high doses, amphetamine has a similar effect on noradrenergic neurones; it can induce the release of noradrenaline into the synaptic cleft and inhibit the noradrenaline re-uptake transporter (Miller, 2011).
Glutamate Effect
Amphetamines also excite dopaminergic neurons via glutamate neurons. Amphetamines remove an inhibiting effect on dopamine neurons due to the metabotropic glutamate receptors on post-synaptic neurons. By releasing this natural break, amphetamines would make dopaminergic neuron more readily excitable (Underhill et al., 2014). Amphetamines achieve this by inhibiting metabotropic glutamate receptors, selectively blocking inhibitory glutamate neurotransmission, and stopping mediated IPSPs in dopamine neurons (Paladini et al., 2001). In acute exposure, these psychostimulants increase extracellular glutamate in various bran regions including the striatum, VTA and Nucleus accumbens (Danbolt et al., 2001).
|
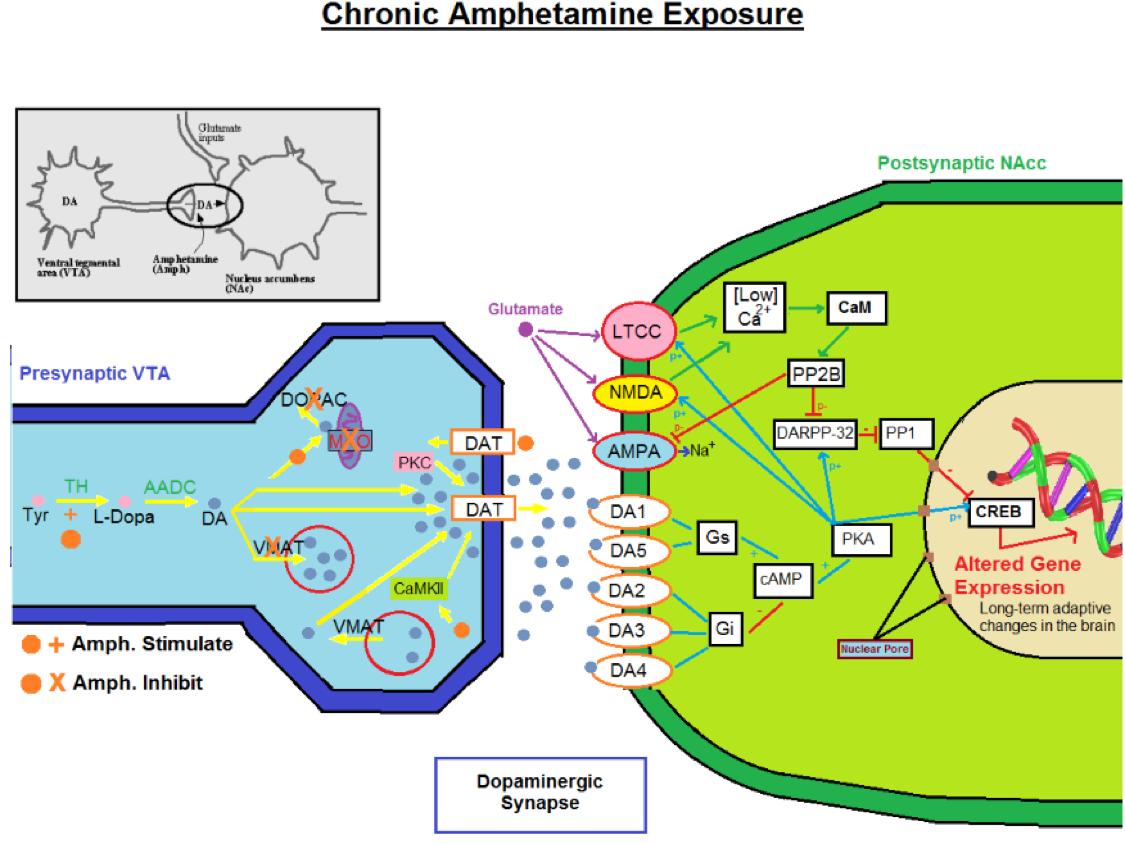
It is visible that through acute amphetamine exposure, the glutamate transmission is affecting post-synaptic neuron. The increased release of dopamine and glutamate neurotransmission will produce high levels of calcium post-synaptically and a cascade of biochemical events that will lead to acute changes in the brain. These changes are the beginning steps of tolerance and dependence (Miller, 2011). During chronic exposure of amphetamines or through very high dose exposure, the post-synaptic neuron will begin to change and truly show signs of tolerance. Due to the lower level of calcium that entered, a different cascade of events will take place that will produce long term changes on the neural system (Zaniewska et al., 2015). Receptors on the post-synaptic neuron will experience long term adaptive changes and begin to down regulate dopamine receptors due to overstimulation which led to altered gene expression (Paladini et al., 2001).
|
Dependance and Withdrawl
Following the dopamine neurons developing tolerance to the overstimulation of dopamine release caused by the amphetamines, regular rewarding behaviors like food, positive emotions and sex, produce the same amount of neurotransmitter release, but now there are far fewer receptors to stimulate due to the down regulation (Bortolotto et al., 1999). This can lead to depression and sadness following abuse of amphetamines. In addition, addicts that want to find the same “high” that was produced by the amphetamine they abused will find themselves needing to use more of the drug to produce the same effect because of the down regulation. This is known as tolerance to a psychotropic drug. Withdrawal will take place when the abuser stops using the drug, but the mesolimbic pathway desires to have dopamine stimulation (Danbolt et al., 2001). This drug induced plasticity may be the basis of the behavioral deficits in humans who abuse psychostimulants. Human patients addicted to amphetamines exhibit several cognitive abnormalities following a withdrawal period (Ornstein et al., 2000, Kalechstein et al., 2003). Humans that previously abused amphetamines have shown deficits within working memory, decision making, visual pattern recognition, and completion of a set shifting task in comparison to controls (Ornstein et al., 2000, Kalechstein et al., 2003). Chronic drug use in humans can also diminish sensitivity to non-drug sources of reinforcement (Volkow et al., 1998).
References
Aston-Jones G, Waterhouse B (2016): Locus Coeruleus: From global projection system to adaptive regulation of behavior. Brain Research 23(1):132-154.
Aznar S, Qian Z, Shah R, Knudsen G (2003): The 5-HT1A Serotonin receptor is located on calbindin and parvalbumin-containing neurons in the rat brain. Brain Research 959(1):3727-3734.
Blaylock BL, Nader MA (2012): Dopamine D3 receptor function and cocaine exposure. Neuropsychopharmacology Reviews 37(1):297-298.
Bortolotto Z, Clarke V, Parry M, Gates M, Lodge D (1999): Kainate receptors are involved in synaptic plasticity. Nature: 402(6759):297-301.
Bunzow J, Sonders M, Harrison L, Zhang G, Kennedy J, Olson S, Grandy D (2001): Amphetamine, 3, 4-methylenedioxymethamphetamine, lysergic acid diethylamide and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Molecular Pharmacology 60(6):1181-1188.
Burnet P, Eastwood S, Lacey K, Harrison P (1995): The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Research 676(1):157-168.
Clark D, Mitra P, Wang S (2001): Scalable architecture in mammalian brains. Nature 411(2):189–193.
De La Rosa C, De Moya M, Arzate D, Martinez A (2015): Olfactory and cortical projections to bulbar and hippocampal adult born neurons. Frontiers in Neuroanatomy 9(2):4-13.
Finlay B, Darlington R, Nicastro N (2001): Developmental structure in brain evolution. Behavioral Brain Science 24(7):263–308.
Freyberg Z, Sonders M, Aguilar J, Hiranita T, Kara, C, Flores J, Martin C, Fei H, Lin Y, Wimalasea K, Frantz D, Sulzer D, Javitch J (2016): Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in drosophila brain. Nature Communications 7(4):106-126.
Gainetdinov R, Jones S, Caron M (1999): Functional hyperdopaminergia in dopamine transporter knock-out mice. Biological Psychiatry 46(3):303-311.
Giros B, Godinot N, Zheng K, Caron M (1992): Cloning, pharmacological characterization and chromosomal assignment of the human dopamine transporter. Molecular Pharmacology 42(3):383-390.
Golmirzaei K, Mahboobi H, Yazdanparast M, Mushtaq G, Hamzei E (2016): Psychopharmacology of attention deficit hyperactivity disorder: effects and side effects. Current Pharmaceutical Design 22(5):590-594.
Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Wess J (1999): Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. PNAS 96(18):10483-10488.
Joseph J, Zhu X, Lynam D, Kelly T (2016): Modulation of meso-limbic reward processing by motivational tendencies in youth and adults. Neuroimaging 129(7):40-54.
Jugurnauth S, Chen C, Barnes M, Li T, Lin S, Collier D, Breen G (2012): A COMT gene haplotype associated with methamphetamine abuse. Pharmacogenetics Genomics 22(7):559-564.
Kienast T, Heinz A (2006): Dopamine and the diseased brain. CNS and Neurological Disorders Drug Targers. 5(1):109-131.
Kumagae Y, Matsui Y, Iwata N (1991): Deamination of norepinephrine, dopamine and serotonin by type A monoamine oxidase in discrete regions of the brain. Journal of Pharmacology 55(1):121-128.
Lachman H, Papolos D, Saito Y, Szumlanski C, Weinshilbourm R (1996): Human Catechol-O-Methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application in neuropsychiatric disorders. Pharmacogenetics 6(3):243-250.
Lacroix L, Dawso L, Hagan J, Heidbreder C (2004): 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 51(2):158-164.
Le Crom S, Kapsimali M, Barome P, Vernier P (2003): Dopamine receptors for every species: gene duplications and functional diversification in craniates. Journal of Structural and Functional Genomics 3(1):161-176.
Lindemann L, Meyer C, Jeanneau K, Bettler B, Hoener M (2008): Trace amine –associated receptor 1 modulates dopamine activity. Journal of Pharmacology 324(4):948-956.
Li S, Shi Y, Kirouac G (2014): The hypothalamus and periaqueductal grey are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Frontiers in Neuroanatomy 8: 136-142.
Maguire J, Parker W, Foord S, Bonner T, Neubig R, Davenport A (2009): Recommendations for trace amine receptor nomenclature TAAR1. Pharmacological Reviews 61(1):1-8.
Mao L, Xue B, Jin D, Wang J (2015): Dynamic increases in AMPA receptor phosphorylation in the rat hippocampus response to amphetamine. Journal of Neurochemistry 133(6):795-805.
Milan M, Dekeyne A, Gobert A (1998): Serotonin 5-HT2C receptors tonically inhibit dopamine and norepinephrine, but not 5-HT, release in the cortex. Neuropharmacology 37(3):953-955.
Miller G (2011): The emerging role of trace amine associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. Journal of Neurochemistry 116(2):164-176.
Naganuma F, Yoshikawa T, Nakamura T, Ilda T, Miura Y, Yanai K (2014): Predominant role of plasma membrane monoamine transporter in monoamine transport. Journal of Neurochemistry 129(4):591-601.
Nicholas A, Pieribone V, Elde R, Hokfelt T (1991): Initial observations on the localization of mRNA for alpha and beta adrenergic receptors in brain and peripheral tissues of rat using in situ hybridization. Molecular and Cellular Neuroscience 2(4):344-350.
Nicholas D, Nichols C (2008): Serotonin Receptors. Chemical Reviews 108(5):1614-1641.
Perez J, Otero C, Mancebo M, Soengas J (2016): Food intake inhibition in rainbow trout induced by activation of serotonin 5-HT2C receptors is associated with increases in POMC, CART and CRG mRNA abundance in hypothalamus. Journal of Comparative Physiology 186(3):313-321.
Puig V, Rose J, Schmidt R, Freund N (2014): Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents and birds. Neural Circuits 43(2):134-144.
Ramirez M, Ornstein A, Luque G, Perez M, Garcia T, Becu-Villalobos D (2015): Pituitary and brain D2 receptors regulate liver gene sexual dimorphism. Endocrinology 156(3):1040-1051.
Reep R, Finlay B, Darlington R (2007): The Limbic System in mammalian brain evolution. Brain Behavior Evolution 70(1): 57-70.
Sato W, Kubota Y, Uono S, Sawada R, Yoshina S, Toichi M (2016): The association between perceived social support and amygdala structure. Neuropsychology 85(1):237-244.
Shen W, Flajolet M. Greengard P, Surmeier J (2008): Dichotimous Dopaminergic control of striatal synaptic plasticity. Science 321(5890):848-851.
Song J, Kim J (2016): Degeneration of dopaminergic neurons due to metabolic alterations and Parkinson’s Disease. Frontier Aging Neuroscience 8(1):65-67.
Underhill S, Wheeler D, Li M, Watts S, Ingram S, Amara S (2014): Amphetamine modulates glutamatergic transmission through endocytosis of the excitatory amino acid transporter EAAT3 in Dopamine Neurons. Neuron 83(2):404-416.
Volkow N, Gur R, Wang G, Fowler J, Moberg P, Ding Y, Hitzemann R, Smith G, Logan G (1998): Association between decline in brain dopamine activity with age and cognitive and motor impairments in healthy individuals. The American Journal of Psychiatry 155(3):344-349.
Wang Y, Li S, Liu W, Wang F, Liu C (2016): Vesicular monoamine transporter 2 (VMAT2) knockdown elicits anxiety like behavior in zebra fish. Biochemical Biopsychology 470(4):792-797.
West E, Carelli R (2016): Nucleus accumbens core and shell differentially encode reward-associated cues after reinforce devaluation.. Journal of Neuroscience 36(4):1128-1139.
Yulung B, Hanoglu L, Kilic E (2015): The neuroprotective role of vesicular monoamine transporter 2 in neurodegenerative diseases. Medical Chemistry 11(2):104-108.
Zaniewska M, Filip M, Przegalinski E (2015): The involvement of norepinephrine in behaviors related to psychostimulant addiction. Current Neuropharmacology 13(3):407-418.