Itt írjon a(z) cAMP_Muscle-ról/ről
The Role of cAMP in Skeletal Muscle Cell Adaptation
Helena Pope
Melanie Lean
Josefin Jansson
Contents
INTRODUCTION
Cyclic adenosine monophosphate (cAMP) (see Figure 1 for structure) is a secondary messenger molecule, which helps to regulate numerous cell functions by way of signal transduction (Sjaastad et al, 2010). G protein (guanylate nucleotide binding protein) activates the enzyme adenlyl cyclase, which stimulates the formation of cAMP from ATP (adenosine triphosphate). cAMP subsequently binds to and activates protein kinase A, which is a key enzyme in the pathway of signal transduction (see Figure 2) (Sjaastad et al, 2010). Fluctuations in intracellular concentration of cAMP result in changes in cAMP dependent protein kinase activity (Korenman and Krall, 1977). The role of cAMP is extensive, much of which lies beyond the remit of discussion here (Rybalkin, et al, 2003). The review will instead focus on the role of cAMP in skeletal muscle adaptation.
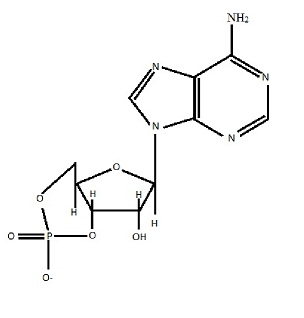
Figure 1 Structure of cAMP
(Adapted from Sjaastad et al, 2010)
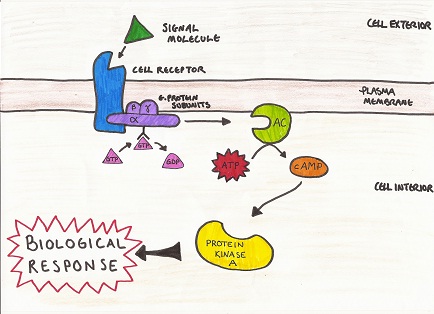
Figure 2 G protein pathway
(Adapted from Sjaastad et al, 2010)
CHARACTERISTICS OF SKELETAL MUSCLE
Skeletal muscle is composed of large multinucleated cells and heterogeneous muscle fibres, which differ in their physiological and metabolic capacity (Rasmussen et al, 1987; Bassel-Duby and Olson, 2006). A direct correlation can be found between muscle strength and contractile ability, a key component of which is cAMP and its role in adaptation of skeletal muscle to exercise training (Rasmussen et al, 1987; Jorgensen et al, 2006). Skeletal muscle, in contrast to smooth muscle, exhibits repetitive contractile responses to recurring neural stimulation. Smooth muscle displays biphasic or monotonic contraction, the difference being the type of action potential created (Rasmussen et al, 1987).
cAMP EFFECTS ON MUSCLE MASS
Skeletal muscle is made up of units known as fibres (Nunamaker et al, 1985) (as seen in Figure 3). Fibres are classified by means of their expression of MHC (myosin heavy chain) isoforms, which are proteins with special contractile properties, and the way in which they generate energy (Rockl et al, 2007). Muscle myofibrils are able to physiologically respond to environmental demands by transforming and remodeling (Bassel-Duby and Olson, 2006). There are multiple signaling pathways involved, many of which interact with one another to reprogram gene expression in order to meet the demands of muscle performance.
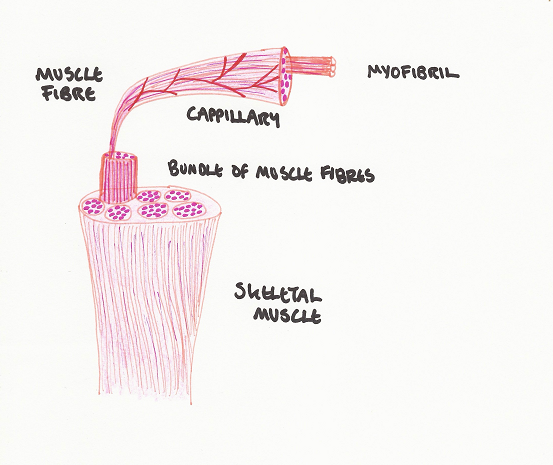
Figure 3 Muscle Fibre Components
(Adapted from Sjaastad et al, 2010)
A key mechanism in muscle hypertrophy is the cAMP cascade (Bassel-Duby and Olson, 2006). Hypertrophy can be characterised by enlargement of cell size due to synthesis of cytoplasmic components in response to training (Glass, 2003: Sjaastad et al, 2010). Depending on the type of training- strength training or anaerobic training, the muscle responds accordingly by either increased sarcoplasmic volume, or increased contractile components (Helander, 1961). In contrast, atrophy can be recognised by a reduction in cell size (organelles, cytoplasm and proteins) as experienced during periods of muscle disuse/denervation (box rest/ equine non weight bearing lameness) and or disease (Glass, 2003; Bassel-Duby and Olson, 2006; Sandri, 2008).
MUSCLE FIBRE TRANSITION
Type I fibres exhibit slow twitch characteristics, meaning that they are able to cope with static or sustained forms of work load such as endurance running (Nunamaker et al, 1985). The source of energy in the form of ATP is mainly provided by means of oxidative (aerobic) pathways (Rockl et al, 2007). These fibres are often referred to as red muscle fibres.
Type II fibres are able to work faster but are quickly prone to fatigue, i.e. they are fast twitching and can also be referred to as white muscle fibre (Nunamaker et al, 1985). There are three subgroups assigned: IIa, IIx and IIb. The IIx and IIb fibres generate ATP mostly by means of the glycolytic (anaerobic) pathway. Type IIa, just like the slow twitching type I, works mainly aerobically (Rockl et al, 2007). Out of the three subgroups type IIb is considered to be the fastest muscle fibre, followed by IIx, IIa and finally type I (Pette et al, 2000).
The ratio of different muscle fibres in a muscle, or in an individual, defines what kind of work it will be able to cope with. Skeletal muscle tissue is very dynamic and easily adapts to change. By changing the kind of exercise or by shifting the workload up or down, muscle tissue has the ability to adjust the ratio of fibres according to its needs in order to be able to cope better (Pette et al, 2000).
Enduring exercise is proven to induce a transition of muscle fibres from fast to slower type; on the contrary absence of muscular work can convert muscle fibres from slow to fast muscle fibres, i.e. the more work a muscle has to do the more work it will eventually be able to cope with (Pette et al, 2000). Extreme workload on the muscle can make the fibres switch from slow to the more powerful fast type (Rockl et al, 2007). Thyroid hormones and age are other factors, which influence fibre type transition (Pette et al, 2000).
AMP-ACTIVATED PROTEIN KINASE
In order to be able to discuss the relevance of cAMP in fibre transition, one has to consider mechanisms at a cellular level. Transcription, translation and proteolysis alternation can be initiated by the steps which convert ATP to cAMP, outlined in earlier chapters (Pette et al, 2000). The process of which is mediated by adenylyl cyclase as a response to G-protein binding, and the resulting protein kinase (AMPK) as the subsequent effect (Sjaastad et al, 2010). The biological responses of which create an impact on the physiological functions of the muscle. Muscle contractions serve as external stimuli and initiate the entire cascade (Sakamoto et al, 2005).
The role of the cAMP cascade as a mediator in skeletal muscle fibre type transition by stimulus of muscle training was investigated by (Rockl et al, 2007). Authors used two groups of muscle specific transgenic mice (DNA from a certain species has been incorporated into DNA of a different individual’s species): one group of mice, which showed expression of inactivation of AMPK and the other group, which expressed activation of AMPK. Both groups underwent moderate exercise training, and the triceps muscle, a typical glycolytic muscle, was used as the model. Training in normal non-mutated mice should increase the transition of IIb to IIa fibres.
Results showed a lower degree, but not a complete absence; of transition of the fast fiber type IIb to the slower fiber type IIa in the set of mice with the inactivating mutation. This indicates that AMPK plays a key role in this kind of adaption but is not solely responsible, as the result in mice with inactive AMPK would show zero fiber transition of this kind. In the mice with the activating mutation exercise did not further increase the type IIa fibres; however it should be noted that these mice prior to starting the exercise scheme showed a 2.6 times higher ratio of IIa compared to non-mutated mice of the same status. Authors concluded that even though AMPK was promoting IIa fibres, an increased intensity of the mediator would not under exercise increase the fibre transition more than normal.
MUSCLE FIBRE TYPE & DIABETES
Slow twitching skeletal muscle fibres are proven to be more insulin sensitive and responsive compared to fast twitching skeletal muscle fibres (Gaster et al, 2001). Individuals suffering from insulin resistance or diabetes mellitus type 2 tend to have a higher ratio of the faster twitching skeletal muscle fibres types compared to healthy individuals (Rockl et al, 2007). Glucose uptake of the cell is determined by the phosphorylation of the protein hexokinase II and the glucose transporter GLUT 4 (of the cell membrane) (Jørgensen et al, 2006). Both of which are increased in activity during exercise (Rockl et al, 2007).
Studies have shown that increases in the cAMP pathway causing AMPK amplification are parallel with an increase in the velocity of glucose uptake by the muscle cell (Kurth-Kraczek et al, 1999). Subsequently Hardie et al (1997) described AMPK as the ‘fuel gauge’ of the cell. As previously outlined, the pathway of cAMP can be initiated by muscle contraction, and together with its relation to glucose uptake it is considered to have an insulin-like effect in skeletal muscle tissue (Kurth-Kraczek et al, 1999). These combined factors make the role of cAMP in muscle adaptation an attractive subject to anti-diabetes drug research and further medical investigation (Rockl et al, 2007).
RELATIONSHIP BETWEEN cAMP AND GENE EXPRESSION
Chahine et al (1993) explain how cAMP not only plays a crucial role as a secondary messenger, but also regulates a number of gene expression pathways in relation to muscle activity. Gene expression is the transcription/translation of the DNA code, to produce proteins, which have a specific function in the body. In response to mechanical stimulus, muscle tissue is influenced by gene expression in its ability to determine muscle fibre phenotype (Goldspink et al, 1992). Muscle has an inherent ability to express variable isoforms of the same highly specific proteins, which enables different contractile characteristics (Goldspink et al, 1992). There are a number of gene expression pathways for which cAMP acts as a secondary messenger, however many lie beyond the remit of this paper. We will discuss one such pathway regarding gene expression.
One of the many proteins synthesised from DNA (deoxy ribonucleic acid) involved in gene expression is calcitonin gene related peptide (CGRP) (Laufert and Changeux, 1989). The peptide is made up of 37 amino acids linked by peptide bonds (see Figure 4), formed by the hydrolysis of an amino group and a carboxylic acid group (Laufert and Changeux, 1987).
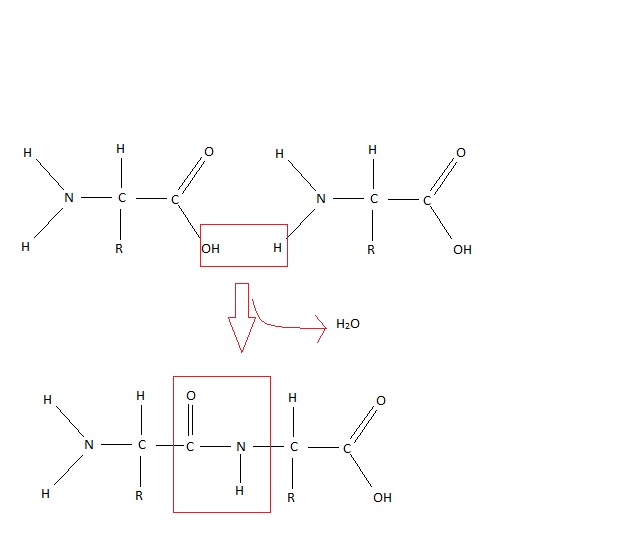
Figure 4 Peptide bond
(Adapted from Sjaad et al, 2010)
As a neuropeptide CGRP is found distributed throughout the nervous system and in some peripheral organs where it is involved in the process of sensory transmission (Laufert and Changeux, 1987). CGRP is found to stimulate adenylate cyclase in skeletal muscle cells, an enzyme previously highlighted for its stimulatory factor in the production of cAMP from ATP (Laufert and Changeux, 1989).
Laufert and Changeux in 1987 carried out experiments using skeletal muscle myofibres from chicks as a model. One element of the study involved investigation into the relationship between CGRP and maintenance of cAMP levels, and the subsequent interaction of nerve and muscle signals. Results indicate that cAMP levels in cultured chick myotubes can be enhanced by CGRP by stimulating sarcolemmal adenylate cyclase (Laufert and Changeux, 1987). Implications of the study outline the importance of cAMP and its role in neuromuscular function. Despite fluctuating levels of the molecule, the work serves to illustrate the important regulatory role of cAMP during periods of muscle differentiation such as disuse, denervation and postnatal muscle development (Laufert and Changeux, 1987).
CONCLUSION
The cAMP cascade is just one mechanism of the numerous cell-signaling pathways, which converge to bring about activity in gene expression and remodeling of skeletal muscle myofibres. The brief review provided here serves to illustrate the importance of understanding and further developing our comprehension of the processes by which signal transduction of cAMP affects muscle fibre regeneration/remodeling, particularly in the context of disease diagnosis, management and therapeutic treatment.
REFERENCE LIST
Chahine, K.G., Baracchini, E. and Goldman, D. (1993) Coupling Muscle Electrical Activity to Gene Expression via a cAMP-dependent Second messenger System. The Journal of Biological Chemistry 268 (4) 2893-2898
Gaster, M., Peter Staehr, P., Beck-Nielsen, H., Schrøder, H.D. and Handberg, A. (2001) GLUT4 Is Reduced in Slow Muscle Fibers of Type 2 Diabetic Patients. Diabetes. 50 (6) 1324 - 1329
Glass, D.J. (2003) Singnalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nature Cell Biology 5 87-90
Goldspink, G., Scutt, A., Loughna, P.T., Wells, D.J., Jaenicke, T. and Gerlach, G.F. (1992) Gene expression in skeletal muscle in response to stretch and force generation. American Journal of Physiology 262 356-363
Hardie, D.G., Carling D. (1997) The AMP-activated protein kinase: fuel gauge of the mammalian cell European Journal of Biochemistry 246 259 - 273
Helander, E.A.S. (1961) Influence of Exercise and Restricted Activity on the Protein Composition of Skeletal Muscle. Journal of Biochemistry 78 pp478-482
Jorgensen, S.B., Richter, E.A. and Wojtaszewski, F.P. (2006) Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. Journal of Physiology 574 (1) pp17-31
Jørgensen, S.B., Treebak J.T., Viollet, B., Schjerling, P., Vaulont, S., Wojtaszewski, J.F.P. and Richter, E.A., (2006) Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. American Journal of Physiology, Endocrinology and Metabolism 292 331 – 339
Korenman, S.G. and Krall, J.F. (1977) The Role of Cyclic AMP in the Regulation of Smooth Muscle Cell Contraction in the Uterus. Biology of Reproduction 16 pp1-17
Kurth-Kraczek, E.J., Hirshman, M.F., Goodyear, L.J. and Winder, W.W. (1999) 5' AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48 (8) 1667 - 1671
Laufert, R. and Changeux, J.P. (1987) Calcitonin gene-related peptide elevates cyclic AMP levels in chick skeletal muscle: possible neurotrophic role for coexisting neuronal messenger. European Molecular Biology Organisation Journal 6 (4) pp901-906
Laufert, R. and Changeux, J.P. (1989) Calcitonin Gene-related Peptide and Cyclic AMP Stimulate Phosphoinositide Turnover in Skeletal Muscle Cells. The Journal of Biological Chemistry 264 (5) pp2683-2689
Nunamaker, D.M. and Newton, C.D. (1985) Tendons, Skeletal Muscles and Ligaments in Health and Disease. Textbook of Small Animal Orthopaedics Publisher J.B. Lippincott Company, 1985. Chapter 4, pages 60 to 61
Pette, D. and Staron, R. S. (2000) Myosin Isoforms, Muscle Fiber Types, and Transitions. Microscopy Research and Technique 50 500-509
Rasmussen, H., Takuwa, Y. and Park, S. (1987) Protein kinase C in the regulation of smooth muscle contraction. The Journal of the Federation of American Societies for Experimental Biology 1 177-185
Rockl, K.S.C., Hirshman, M.F., Brandauer, J., Fujii, N., Witters, L. A. and Goodyear, L. J. (2007) Skeletal Muscle Adaption to Exercise Training. Diabetes 56 2062 – 2069
Rybalkin, S.D., Yan, C., Bornfeldt, K.E. and Beavo, J.A. (2003) Cyclic GMP Phosphodiesterase and Regulation of Smooth Muscle Function. Circulation Research 93 280-291
Sandri, M. (2008) Singalling in Muscle Atrophy and Hypertrophy. Physiology 23 160-170
Sakamoto, K., McCarthy, A., Smith, D., Green, K.A., Grahame Hardie, D., Ashworth, A. and Alessi, D.R. (2005) Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. European Molecular Biology Organisation Journal 24 (10) 1810 – 1820
Sjaastad, V.O., Sand, O. and Hove, Knut (2010) Physiology of Domestic Animals. Scandinavian Veterinary Press 2010, Chapter 3, pp 79-104
