|
Size: 27733
Comment:
|
← Revision 23 as of 2016-04-25 19:51:03 ⇥
Size: 27727
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 26: | Line 26: |
| * __Location__: The CB1 receptors are thought to be one of the most widely expressed G-protein coupled receptors in the brain. These receptors are found predominantly in the central nervous system but are also found in adipose tissue, myocardium, vascular endothelium and sympathetic nerve terminals (Mackie et al., 2008). CB1 receptors are widely distributed throughout the brain with high concentrations found in the basal ganglia, hippocampus, cerebellum, cerebral cortex and moderate levels in the hypothalamus (Jansen et al., 1992) (Fig.1). These receptors are responsible for mediating the effects of cannabinoid binding in the brain and the effects produced. CB1 receptors have also been found in some non-neuronal cells and tissues, such as leukocytes and in the testes (Pertwee et al., 2002). | * '''Location:''' The CB1 receptors are thought to be one of the most widely expressed G-protein coupled receptors in the brain. These receptors are found predominantly in the central nervous system but are also found in adipose tissue, myocardium, vascular endothelium and sympathetic nerve terminals (Mackie et al., 2008). CB1 receptors are widely distributed throughout the brain with high concentrations found in the basal ganglia, hippocampus, cerebellum, cerebral cortex and moderate levels in the hypothalamus (Jansen et al., 1992) (Fig.1). These receptors are responsible for mediating the effects of cannabinoid binding in the brain and the effects produced. CB1 receptors have also been found in some non-neuronal cells and tissues, such as leukocytes and in the testes (Pertwee et al., 2002). |
| Line 28: | Line 28: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:1.jpg|pop-up text}} Fig 1. ''Nervous System'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:1.jpg|pop-up text}} Fig 1. ''Nervous System'' || |
| Line 31: | Line 31: |
| * __Function__: The CB1 receptors are located pre-synaptically, and upon post-synaptic depolarization, endocannabinoids are capable of a depolarization-induced suppression of inhibition on the presynatptic neuron (Graham et al., 2009). Endocanabinoids are released from the depolarized post-synaptic neuron bind to CB1 receptors in the pre synaptic neuron and cause a reduction in GABA release (Walker et al., 2002). This very short term plasticity in which the depolarization of a single neuron induces a reduction in GABA-mediated neurotransmission by stimulating potassium ion release and inhibiting both calcium ion channels and adenylate cyclase (Graham et al., 2009). Stimulation of these receptors produce several responses to THC and the other cannabinoid receptor agonists in the nervous system such as therapeutically beneficial effects of analgesia (Walker et al., 2002), attenuation of the nausea and vomiting in cancer chemotherapy (Mechoulam et al., 2001), appetite stimulation and neuroprotection (Pertwee et al., 2012). Unfortunately, there are some side effects that occur through stimulation of these receptors that include alterations in cognition and memory (Regio et al., 2003), dysphoria/euphoria and sedation (Walker et al., 2002). It is generally accepted that the CB1 receptor functions to mediate inhibition of on-going release of certain excitatory and inhibitory neurotransmitters (Szabo et al., 2005). Inside of the hippocampus, activation of the CB1 receptor disrupts long term potentiation and memory formation ( The CB1 receptor is often localised in axon terminals, and its activation leads to inhibition of transmitter release through a multi-step retrograde signaling pathway (Graham et al., 2009). The consequence is the inhibition of neurotransmission via a presynaptic mechanism demonstrated in the figure. Through the inhibition of voltage-dependent calcium channels, activation of potassium channels and direct interference with the synaptic vesicle release, these mechanisms are all implicated in the cannabinoid-evoked inhibition of transmitter release (Szabo et al., 2005). Inhibition of neurotransmission is not limited to GABA and Glutamate, but also Acetylcholone, Serotonin, and Norepinephrine and has been observed in many regions of the central nervous system (Pertwee et al., 2012). In the peripheral nervous system, CB1 receptor-mediated inhibition of adrenergic, cholinergic and sensory neuroeffector transmission has been frequently observed (Munro et al., 1993). Peripherally speaking, endocannabinoids affect adipose tissue by increase lipogenesis and adipocyte size while decreasing adipocentin (Wenzel et al., 2014). In liver cells, CB1 stimulation increases insulin resistance and dyslipidermia; and in the gastrointestinal tract decreases intestinal motility, gastric emptying and satiety, producing the symptoms of hunger (Reggio et al., 2002). | * '''Function:''' The CB1 receptors are located pre-synaptically, and upon post-synaptic depolarization, endocannabinoids are capable of a depolarization-induced suppression of inhibition on the presynatptic neuron (Graham et al., 2009). Endocanabinoids are released from the depolarized post-synaptic neuron bind to CB1 receptors in the pre synaptic neuron and cause a reduction in GABA release (Walker et al., 2002). This very short term plasticity in which the depolarization of a single neuron induces a reduction in GABA-mediated neurotransmission by stimulating potassium ion release and inhibiting both calcium ion channels and adenylate cyclase (Graham et al., 2009). Stimulation of these receptors produce several responses to THC and the other cannabinoid receptor agonists in the nervous system such as therapeutically beneficial effects of analgesia (Walker et al., 2002), attenuation of the nausea and vomiting in cancer chemotherapy (Mechoulam et al., 2001), appetite stimulation and neuroprotection (Pertwee et al., 2012). Unfortunately, there are some side effects that occur through stimulation of these receptors that include alterations in cognition and memory (Regio et al., 2003), dysphoria/euphoria and sedation (Walker et al., 2002). It is generally accepted that the CB1 receptor functions to mediate inhibition of on-going release of certain excitatory and inhibitory neurotransmitters (Szabo et al., 2005). Inside of the hippocampus, activation of the CB1 receptor disrupts long term potentiation and memory formation ( The CB1 receptor is often localised in axon terminals, and its activation leads to inhibition of transmitter release through a multi-step retrograde signaling pathway (Graham et al., 2009). The consequence is the inhibition of neurotransmission via a presynaptic mechanism demonstrated in the figure. Through the inhibition of voltage-dependent calcium channels, activation of potassium channels and direct interference with the synaptic vesicle release, these mechanisms are all implicated in the cannabinoid-evoked inhibition of transmitter release (Szabo et al., 2005). Inhibition of neurotransmission is not limited to GABA and Glutamate, but also Acetylcholone, Serotonin, and Norepinephrine and has been observed in many regions of the central nervous system (Pertwee et al., 2012). In the peripheral nervous system, CB1 receptor-mediated inhibition of adrenergic, cholinergic and sensory neuroeffector transmission has been frequently observed (Munro et al., 1993). Peripherally speaking, endocannabinoids affect adipose tissue by increase lipogenesis and adipocyte size while decreasing adipocentin (Wenzel et al., 2014). In liver cells, CB1 stimulation increases insulin resistance and dyslipidermia; and in the gastrointestinal tract decreases intestinal motility, gastric emptying and satiety, producing the symptoms of hunger (Reggio et al., 2002). |
| Line 33: | Line 33: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:2.jpg|pop-up text}} Fig 2.''' '''CB1 Receptor Activation || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:2.jpg|pop-up text}} Fig 2.''' '''CB1 Receptor Activation || |
| Line 38: | Line 38: |
| * __Location__: The CB2 receptor is found primarily in immune/ lymphoid tissue, specifically T cells of the immune system, on macrophages and B cells, and in hematopoietic cells mi(Gallegue et al., 1998; Pacher et al., 2011). They also have a function in kertatinocytes and are expressed on peripheral nerve terminals where receptors play a role in nociception and the relief of pain (Gallegue et al., 1998). In the brain, they are mainly expressed by the immune cells of brain such as on microglia or dendritic cells (Guidon et al., 2011). The number of other potential cellular targets is expanding, and now includes smooth muscle cells, fibroblasts of various origins, and cardiomyocytes (Pacher, 2011). | * '''Location:''' The CB2 receptor is found primarily in immune/ lymphoid tissue, specifically T cells of the immune system, on macrophages and B cells, and in hematopoietic cells mi(Gallegue et al., 1998; Pacher et al., 2011). They also have a function in kertatinocytes and are expressed on peripheral nerve terminals where receptors play a role in nociception and the relief of pain (Gallegue et al., 1998). In the brain, they are mainly expressed by the immune cells of brain such as on microglia or dendritic cells (Guidon et al., 2011). The number of other potential cellular targets is expanding, and now includes smooth muscle cells, fibroblasts of various origins, and cardiomyocytes (Pacher, 2011). |
| Line 40: | Line 40: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:3.jpg|pop-up text}} Fig 3.'' CB2 Receptor Activation'' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:3.jpg|pop-up text}} Fig 3.'' CB2 Receptor Activation'' || |
| Line 43: | Line 43: |
| * __Function__: Changes in endocannabinoid levels and/or CB₂ receptor expressions have been reported in almost all diseases affecting humans, ranging from cardiovascular, gastrointestinal, liver, kidney, neurodegenerative, psychiatric, bone, skin, autoimmune, lung disorders to pain and cancer, and modulating CB₂ receptor activity holds tremendous therapeutic potential in these pathologies (Mackie et al., 2008). Inflammation/tissue injury triggers rapid elevations in local endocannabinoid levels, which in turn regulate signaling responses in immune and other cells modulating their critical functions (Pacher et al., 2011). While CB₂ receptor activation in general mediates immunosuppressive effects, which limit inflammation and associated tissue injury in large number of pathological conditions, in some disease states like cancer activation of the CB₂ receptor may enhance or even trigger tissue damage plus anti-proliferative and pro-apoptotic properties (Guidon et al., 2011; Nikan et al., 2016). The CB2 receptor has been shown to signal a response through the inhibition of adenylate cyclase, and the primary effect of the CB2 receptor is a variety of modulatory functions, including immune suppression, induction of apoptosis, and induction of cell migration (Zendulka et al., 2016) (Fig.3). | * '''Function:''' Changes in endocannabinoid levels and/or CB₂ receptor expressions have been reported in almost all diseases affecting humans, ranging from cardiovascular, gastrointestinal, liver, kidney, neurodegenerative, psychiatric, bone, skin, autoimmune, lung disorders to pain and cancer, and modulating CB₂ receptor activity holds tremendous therapeutic potential in these pathologies (Mackie et al., 2008). Inflammation/tissue injury triggers rapid elevations in local endocannabinoid levels, which in turn regulate signaling responses in immune and other cells modulating their critical functions (Pacher et al., 2011). While CB₂ receptor activation in general mediates immunosuppressive effects, which limit inflammation and associated tissue injury in large number of pathological conditions, in some disease states like cancer activation of the CB₂ receptor may enhance or even trigger tissue damage plus anti-proliferative and pro-apoptotic properties (Guidon et al., 2011; Nikan et al., 2016). The CB2 receptor has been shown to signal a response through the inhibition of adenylate cyclase, and the primary effect of the CB2 receptor is a variety of modulatory functions, including immune suppression, induction of apoptosis, and induction of cell migration (Zendulka et al., 2016) (Fig.3). |
| Line 49: | Line 49: |
| * __CB1–OX1__: The localization of the CB1 receptor in the endocannabinoid system has a very large degree of overlap with the orexinergic projection system, which mediates many of the same functions, both physical and cognitive. Moreover, CB1 is co localized on orexin projection neurons in the lateral hypothalamus and many output structures of the orexin system, where the CB1 and orexin receptor 1 (OX1) receptors physically and functionally join together to form the CB1–OX1 receptor heterodimer (Graham et al., 2009). | * '''CB1–OX1:''' The localization of the CB1 receptor in the endocannabinoid system has a very large degree of overlap with the orexinergic projection system, which mediates many of the same functions, both physical and cognitive. Moreover, CB1 is co localized on orexin projection neurons in the lateral hypothalamus and many output structures of the orexin system, where the CB1 and orexin receptor 1 (OX1) receptors physically and functionally join together to form the CB1–OX1 receptor heterodimer (Graham et al., 2009). |
| Line 51: | Line 51: |
| * __TRPV1__: Endocannabinoids have also been shown to act on TRPV1 receptors which are transient receptor potential cation channels subfamily V member 1, also known as the “capsaicin receptor” and “vanilloid receptor” 1 (Ross 2003). These receptors are co-localized to some extent in sensory neurons of the spinal cord and dorsal root ganglia (Hermann et al., 2003) | * '''TRPV1:''' Endocannabinoids have also been shown to act on TRPV1 receptors which are transient receptor potential cation channels subfamily V member 1, also known as the “capsaicin receptor” and “vanilloid receptor” 1 (Ross 2003). These receptors are co-localized to some extent in sensory neurons of the spinal cord and dorsal root ganglia (Hermann et al., 2003) |
| Line 53: | Line 53: |
| * __GPR__: In addition, cannabinoid compounds are able to activate other “non-CB” receptors, such as GPR18 & GPR55 which are regulators of neuroimmune function (Pertwee et al. 2010). Proposed receptors (also called putative or nonclassical cannabinoid receptors) include GPR18, GPR55 and GPR119 that have structural similarity to CB1 and CB2 (Zubrzycki et al. 2014). | * '''GPR:''' In addition, cannabinoid compounds are able to activate other “non-CB” receptors, such as GPR18 & GPR55 which are regulators of neuroimmune function (Pertwee et al. 2010). Proposed receptors (also called putative or nonclassical cannabinoid receptors) include GPR18, GPR55 and GPR119 that have structural similarity to CB1 and CB2 (Zubrzycki et al. 2014). |
| Line 59: | Line 59: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:4.jpg|pop-up text}} Fig 4. || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:4.jpg|pop-up text}} Fig 4. || |
| Line 65: | Line 65: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:5.jpg|pop-up text}} Fig 5.'' Synthesis of Endocannabinoids'' || | |
| Line 66: | Line 67: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:5.jpg|pop-up text}} Fig 5.'' Synthesis of Endocannabinoids'' || | |
| Line 70: | Line 71: |
| ||<tablebgcolor="#eeeeee" tablestyle="font-weight:bold;font-size:0.85em;margin:0px; "style="padding:0.5em; ;text-align:center"> {{attachment:6.jpg|pop-up text}} Fig 6. ''Hydrolysis of Endocannabinoids'' || | ||<tablebgcolor="#eeeeee" tablestyle="margin: 0px; font-size: 0.85em;"style="padding:0.5em; ;text-align:center">''' {{attachment:6.jpg|pop-up text}} '''Fig 6. ''Hydrolysis of Endocannabinoids'' || |
| Line 90: | Line 91: |
| • __∆ -9 Tetrahydrocannabinol (THC) (Fig.7)__ | • '''∆ -9 Tetrahydrocannabinol (THC) '''(Fig.7) |
| Line 93: | Line 94: |
| ||<tablebgcolor="#eeeeee" tablestyle="font-weight:bold;font-size:0.85em;margin:0px; "style="padding:0.5em; ;text-align:center"> {{attachment:7.jpg|pop-up text}} Fig 7. ''∆ -9 Tetrahydrocannabinol (THC) '' || | ||<tablebgcolor="#eeeeee" tablestyle="margin: 0px; font-size: 0.85em;"style="padding:0.5em; ;text-align:center">''' {{attachment:7.jpg|pop-up text}} '''Fig 7. ''∆ -9 Tetrahydrocannabinol (THC) '' || |
| Line 98: | Line 99: |
| • __Cannabidiol (CBD) (Fig.8)__ | • '''Cannabidiol (CBD) '''(Fig.8) |
| Line 101: | Line 102: |
| ||<tablebgcolor="#eeeeee" tablestyle="font-weight:bold;font-size:0.85em;margin:0px; "style="padding:0.5em; ;text-align:center"> {{attachment:8.jpg|pop-up text}} Fig 8. ''Cannabidiol (CBD) '' || | ||<tablebgcolor="#eeeeee" tablestyle="margin: 0px; font-size: 0.85em;"style="padding:0.5em; ;text-align:center">''' {{attachment:8.jpg|pop-up text}} '''Fig 8. ''Cannabidiol (CBD)''' ''''' || |
| Line 106: | Line 107: |
| • __Cannabinol (CBN) (Fig.9)__ | • '''Cannabinol (CBN)''' (Fig.9) |
| Line 109: | Line 110: |
| ||<tablebgcolor="#eeeeee" tablestyle="font-weight:bold;font-size:0.85em;margin:0px; "style="padding:0.5em; ;text-align:center"> {{attachment:9.jpg|pop-up text}} Fig 9. ''Cannabinol (CBN) '' || | ||<tablebgcolor="#eeeeee" tablestyle="margin: 0px; font-size: 0.85em;"style="padding:0.5em; ;text-align:center">''' {{attachment:9.jpg|pop-up text}} '''Fig 9. ''Cannabinol (CBN) '' || |
| Line 114: | Line 115: |
| • __Cannabigerol (CBG) (Fig.10)__ | • '''Cannabigerol (CBG) '''(Fig.10) |
| Line 119: | Line 120: |
| ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:10.jpg|pop-up text}} Fig 10. ''Cannabigerol (CBG) '' || | ||<tablebgcolor="#eeeeee" tablestyle="float:center;font-size:0.85em;margin:0 0 0 0; "style="padding:0.5em; ;text-align:center"> {{attachment:10.jpg|pop-up text}} Fig 10. ''Cannabigerol (CBG) '' || |
Cannabinoid Receptors
Table of Contents
- Introduction
- Classes of Receptors
- Endocannabinoids
- Exogenous Cannabinoids
- Potential Therapeutic/Medicinal Uses
Introduction:
Cannabinoids have been used in traditional medicine for thousands of years. There are reports going back to ancient China ( Zuardi 2006), medieval Persia (Gorji et al., 2002) and even in 19th century Europe (Kalant 2001). The cannabinoid receptors and their ligands have been researched heavily in the past decade for their potential therapies in many medical ailments. There have been many physiological functions of cannabinoids outlined including the regulation of neurotransmitter release, appetite, mood and memory, pain and analgesia, energy homeostasis, and control of immune cell function (Graham et al., 2009). With new receptors still being discovered and studied to this day, the possibilities of cannabinoids for use in medicine is growing every day. The goal of this physiology research project is to inform the reader of the following:
1. The various classes of cannabinoid receptors location and function
2. Classification and identification of the endogenous cannabinoids, focusing on their structure, function, synthesis, degradation, and pharmacodynamics
3. Classification and identification of exogenous cannabinoids sources, focusing on their structure, function, metabolism and pharmacodynamics
4. Potential medical therapies and uses of exogenous cannabinoids in medicine
Classes of Receptors:
The endocannabinoid system consists of several subtypes of cannabinoid receptors. In the recent years, there have been thirteen different cannabinoid receptors identified in the mammalian body; however only two of these receptors (CB1 and CB2) have properly been researched, cloned and had medical therapies outlined (Mackie et al., 2008). Cannabinoid receptors are a class of cell membrane receptors that belong to the rhodopsin-like G-protein coupled receptor (GPCR) family that contain seven transmembrane spanning domains and are phylogenetically restricted to the chordate branch of the animal kingdom (Graham et al., 2009). Cannabinoid receptors are activated by three major groups of ligands: firstly, the endocannabinoids which are produced naturally by the mammalian body, such as N-arachidonoylethanolamine and 2-arachidonoyl glycerol (Pertwee et al., 2010); secondly, exogenous cannabinoids produced by the plant Cannabis sativa , such as tetrahydrocannabinol (Munro et al., 1993); and finally synthetic cannabinoids, such as HU-210 (Felder et al., 1999).
CB1 Receptor:
Location: The CB1 receptors are thought to be one of the most widely expressed G-protein coupled receptors in the brain. These receptors are found predominantly in the central nervous system but are also found in adipose tissue, myocardium, vascular endothelium and sympathetic nerve terminals (Mackie et al., 2008). CB1 receptors are widely distributed throughout the brain with high concentrations found in the basal ganglia, hippocampus, cerebellum, cerebral cortex and moderate levels in the hypothalamus (Jansen et al., 1992) (Fig.1). These receptors are responsible for mediating the effects of cannabinoid binding in the brain and the effects produced. CB1 receptors have also been found in some non-neuronal cells and tissues, such as leukocytes and in the testes (Pertwee et al., 2002).
|
Function: The CB1 receptors are located pre-synaptically, and upon post-synaptic depolarization, endocannabinoids are capable of a depolarization-induced suppression of inhibition on the presynatptic neuron (Graham et al., 2009). Endocanabinoids are released from the depolarized post-synaptic neuron bind to CB1 receptors in the pre synaptic neuron and cause a reduction in GABA release (Walker et al., 2002). This very short term plasticity in which the depolarization of a single neuron induces a reduction in GABA-mediated neurotransmission by stimulating potassium ion release and inhibiting both calcium ion channels and adenylate cyclase (Graham et al., 2009). Stimulation of these receptors produce several responses to THC and the other cannabinoid receptor agonists in the nervous system such as therapeutically beneficial effects of analgesia (Walker et al., 2002), attenuation of the nausea and vomiting in cancer chemotherapy (Mechoulam et al., 2001), appetite stimulation and neuroprotection (Pertwee et al., 2012). Unfortunately, there are some side effects that occur through stimulation of these receptors that include alterations in cognition and memory (Regio et al., 2003), dysphoria/euphoria and sedation (Walker et al., 2002). It is generally accepted that the CB1 receptor functions to mediate inhibition of on-going release of certain excitatory and inhibitory neurotransmitters (Szabo et al., 2005). Inside of the hippocampus, activation of the CB1 receptor disrupts long term potentiation and memory formation ( The CB1 receptor is often localised in axon terminals, and its activation leads to inhibition of transmitter release through a multi-step retrograde signaling pathway (Graham et al., 2009). The consequence is the inhibition of neurotransmission via a presynaptic mechanism demonstrated in the figure. Through the inhibition of voltage-dependent calcium channels, activation of potassium channels and direct interference with the synaptic vesicle release, these mechanisms are all implicated in the cannabinoid-evoked inhibition of transmitter release (Szabo et al., 2005). Inhibition of neurotransmission is not limited to GABA and Glutamate, but also Acetylcholone, Serotonin, and Norepinephrine and has been observed in many regions of the central nervous system (Pertwee et al., 2012). In the peripheral nervous system, CB1 receptor-mediated inhibition of adrenergic, cholinergic and sensory neuroeffector transmission has been frequently observed (Munro et al., 1993). Peripherally speaking, endocannabinoids affect adipose tissue by increase lipogenesis and adipocyte size while decreasing adipocentin (Wenzel et al., 2014). In liver cells, CB1 stimulation increases insulin resistance and dyslipidermia; and in the gastrointestinal tract decreases intestinal motility, gastric emptying and satiety, producing the symptoms of hunger (Reggio et al., 2002).
|
• CB2 Receptor
Location: The CB2 receptor is found primarily in immune/ lymphoid tissue, specifically T cells of the immune system, on macrophages and B cells, and in hematopoietic cells mi(Gallegue et al., 1998; Pacher et al., 2011). They also have a function in kertatinocytes and are expressed on peripheral nerve terminals where receptors play a role in nociception and the relief of pain (Gallegue et al., 1998). In the brain, they are mainly expressed by the immune cells of brain such as on microglia or dendritic cells (Guidon et al., 2011). The number of other potential cellular targets is expanding, and now includes smooth muscle cells, fibroblasts of various origins, and cardiomyocytes (Pacher, 2011).
|
Function: Changes in endocannabinoid levels and/or CB₂ receptor expressions have been reported in almost all diseases affecting humans, ranging from cardiovascular, gastrointestinal, liver, kidney, neurodegenerative, psychiatric, bone, skin, autoimmune, lung disorders to pain and cancer, and modulating CB₂ receptor activity holds tremendous therapeutic potential in these pathologies (Mackie et al., 2008). Inflammation/tissue injury triggers rapid elevations in local endocannabinoid levels, which in turn regulate signaling responses in immune and other cells modulating their critical functions (Pacher et al., 2011). While CB₂ receptor activation in general mediates immunosuppressive effects, which limit inflammation and associated tissue injury in large number of pathological conditions, in some disease states like cancer activation of the CB₂ receptor may enhance or even trigger tissue damage plus anti-proliferative and pro-apoptotic properties (Guidon et al., 2011; Nikan et al., 2016). The CB2 receptor has been shown to signal a response through the inhibition of adenylate cyclase, and the primary effect of the CB2 receptor is a variety of modulatory functions, including immune suppression, induction of apoptosis, and induction of cell migration (Zendulka et al., 2016) (Fig.3).
• Other CB Receptors
The endocannabinoid system has been studied using genetic and pharmacological methods. In addition to CB1 and CB2, certain orphan receptors are known to bind endocannabinoids as well. Other cannabinoid receptors have been speculated, but none of them have been cloned. These studies have revealed that cannabinoids act as neuromodulators for a variety of processes, including motor learning, appetite, and pain sensation, among other cognitive and physical processes.
CB1–OX1: The localization of the CB1 receptor in the endocannabinoid system has a very large degree of overlap with the orexinergic projection system, which mediates many of the same functions, both physical and cognitive. Moreover, CB1 is co localized on orexin projection neurons in the lateral hypothalamus and many output structures of the orexin system, where the CB1 and orexin receptor 1 (OX1) receptors physically and functionally join together to form the CB1–OX1 receptor heterodimer (Graham et al., 2009).
TRPV1: Endocannabinoids have also been shown to act on TRPV1 receptors which are transient receptor potential cation channels subfamily V member 1, also known as the “capsaicin receptor” and “vanilloid receptor” 1 (Ross 2003). These receptors are co-localized to some extent in sensory neurons of the spinal cord and dorsal root ganglia (Hermann et al., 2003)
GPR: In addition, cannabinoid compounds are able to activate other “non-CB” receptors, such as GPR18 & GPR55 which are regulators of neuroimmune function (Pertwee et al. 2010). Proposed receptors (also called putative or nonclassical cannabinoid receptors) include GPR18, GPR55 and GPR119 that have structural similarity to CB1 and CB2 (Zubrzycki et al. 2014).
Endocannabinoids:
Endocannabinoids are marijuana-like compounds that are produced by most animals, including all vertebrates (Lu et al., 2016). Quite surprisingly, the endocannabinoid system has an all-pervasive role in maintaining homeostasis. All living creatures suffer from a common biochemical imbalance through aging and develop illnesses including cardiovascular diseases, autoimmune diseases, neurological disorders, and cancers. Modern science indicates that stimulating the endocannabinoid system benefits all of these conditions (Pacher, 2011).
The endocannabinoids are arachidonic acid containing messengers generated by phospholipidase action that are produced on demand at the site of the location needed (Flores et al., 2013). The endocannabinoids are not stored in vesicles like classical neurotransmitters and are known to act as retrograde regulators of synaptic transmission (DiMarzo et al., 2009). The most important endocannabinoids identified are N-arachidonylethanolamine (anandamide) and 2-arachidonylglycerol (2-AG) (Fig.4). Endocannabinoids are endogenously formed from membrane phospholipids in response to increases in intracellular calcium; they are immediately released and act as ligands of cannabinoid receptors (Flores et al., 2013). Despite similarities in chemical structure, 2-arachidonoyl glycerol and anadamide are synthesized and degraded by distinct enzymatic pathways, which impart fundamentally different physiologic and pathophysiologic roles to these two endocannabinoids (Lu et al., 2016).
|
Synthesis and degradation of endocannabinoids are regulated by ligand-metabolizing enzymes such as fatty acid amide hydrolase (FAAH), monoglyceride lipase (MAGL), N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD) and diacyglycerol lipases (DAGLα and DAGLß) (Flores et al., 2013). Endocannabinoids are derived from membrane phospholipids containing arachidonic acid moiety, such as phosphatidylethanolamine (PE), undergoing a transacylase reaction to form N-arachidonoyl-phosphatidylethanolamine (NAPE), which is cleaved by NAPE-specific phospholipase D-type enzyme to yield N-arachidonoyl ethanolamine (anandamide, AEA) (Fig.5). Cannabinoid receptors have affinity for anandamide and 2-aracidonoylglycerol (2-AG), the endogenous ligands (DiMarzo et al., 2009).
|
After synthesis and release, endocannabinoid signaling is terminated by reuptake into both neurons and glia followed by intracellular hydrolysis of anandamide and 2-AG, carried out by fatty acid amide hydrolase (FAAH) and Diacylglycerol lipase (DAGL), respectively (Muccioli, 2010). Post-synaptically by FAAH (fatty acid amide hydrolase) and pre-synaptically by monoacylglycerol lipase ( Pertwee et al., 2005) (Fig.6).
|
Other Endogenous Cannabinoids
1. N-palmitoylethanolamide: proposed as a selective endogenous CB2 receptor agonist
2. Docosatetraenylethanolamide: CB1 agonist
3. N-arachidonoyldopamine : a CB1 receptor selective ligand
4. Virodhamine: O-arachidonoylethanolamine; described as an endogenous CB1 receptor partial agonist and CB2 receptor agonist
5. N-arachidonoylglycine: An arachidonic acid derivative acts to suppress pain, which is often associated with agonist activity at CB1 receptors, but has no affinity for this cannabinoid receptor.
Exogenous Cannabinoids:
Phyto–cannabinoids, also known as cannabinoids or exo-cannabinoids, are differentiated from endo-cannabinoids due to their production from enzymes in a plant opposed to being manufactured in a human, or better yet, a mammal. Cannabinoids come in many shapes and functions. Today, there are many known unique phytocannnabinoids and synthetic (Kalant et al., 2001). There are 85 known cannabinoids in cannabis, but THC and CBD are the most prevalent natural cannabinoids and reputed to be the most medicinal (Fijal et al., 2011). Cannabinoids are a subset of terpenoids. Terpenoids are a very large class of naturally occurring organic chemicals that come in thousands of varieties. They contribute to scents and flavors and colors of plants, such as cinnamon or ginger. Many are valued for herbal or medicinal properties, such as menthol, camphor, or eucalyptol. Thus, all cannabinoids are terpenoids, but not all terpenoids are cannabinoids. Cannabis does produce many terpenoids that are not cannabinoids, as well, but the cannabinoids are currently the compounds in which most people are interested.
• ∆ -9 Tetrahydrocannabinol (THC) (Fig.7)
It is the most common psychoactive cannabinoid. It is best known for causing the high you get from smoking marijuana. However, it also seems to have a number of medical applications, such as pain relief and the ability to improve appetite (Kalat et al., 2001).THC is the most psychologically active compound in cannabis, and it is also one of the most therapeutic, psychotropic, analgesic (pain relieving), appetite stimulant, bronchial dilator, lowers IOP/glaucoma (Nikan et al., 2016).THC has analgesic, anti-spasmodic, anti-tremor, anti-inflammatory, appetite stimulant and anti-emetic properties that are used for a variety of ailments such as: eating disorders, side effects of chemotherapy, multiple sclerosis, spasticity, seizures and more (Fijal et al., 2011). Additionally, THC has been found to reduce tumor growth and reduce the progression of atherosclerosis in mice (Nikan et al., 2016). Patients should note THC can induce psychoactive, or cerebral, effects as well. Too much THC can cause unease, anxiety and overall discomfort.
|
• Cannabidiol (CBD) (Fig.8)
One other main endocannabinoid is 2-Arachidonoylglycerol (2-AG) which is active at both cannabinoid receptors, along with its own mimetic phytocannabinoid. 2-AG and CBD are involved in the regulation of appetite, immune system functions and pain management (Pertwee et al., 2010). CBD is the second most common cannabinoid that is non-psychoactive, reduces muscle spasms, relaxes muscles and is an analgesic for pain (Walker et al., 2002). Although it has no psychoactive effects, it appears to improve mood and alleviate pain. CBD has received a lot of attention lately because of its antipsychotic effect that calms the nervous system (Javid et al., 2015). CBD has been shown to provide anti-convulsant, anti-arthritic and neuroprotective properties while not inducing any psychoactivity (Pertwee et al., 2010).
|
• Cannabinol (CBN) (Fig.9)
CBN is a mildly psychoactive, non-narcotic analgesic found inside of cannabis (Kalant et al., 2001). Created from THC when cannabis is exposed to air, through a process called oxidization. CBN on its own provides a mild psychoactive effect, but when combined with THC can make you feel drowsy and induce sleep (Pertwee et al., 2010). CBN is a physiologically inactive crystalline cannabinoid that turns into non- tetrahydrocanabinol (THC), the most psychoactive element in cannabis. It’s also produced as THC ages and breaks down through oxidization (Pertwee et al., 2010).
|
• Cannabigerol (CBG) (Fig.10)
CBG is a non-psychoactive cannabinoid, is the building block for THC and CBD. It has been shown to reduce intraocular pressure, making it ideal for glaucoma patients (Kalant et al., 2001) CBG is also sleep inducing and a has anti-microbial properties
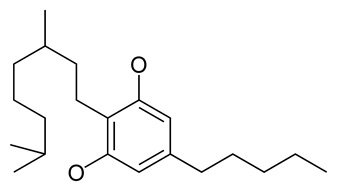 Fig 10. Cannabigerol (CBG)
Fig 10. Cannabigerol (CBG)
Potential Therapeutic/ Medicinal Uses:
There have been many therapeutic targets for cannabinoid receptor agonists which include pain epilepsy anxiety, depression, Parkinson's and Huntington’s, amyotrophic lateral sclerosis, stroke, cancer, drug dependence, glaucoma, autoimmune uveitis, osteoporosis, sepsis, and hepatic, renal, intestinal and cardiovascular disorders (Pertwee 2012). The main medicinal properties of cannabinoids include analgesic, anti-inflammatory, antitumor, appetite stimulation, antiemesis, and muscle relaxation effects (Fijal et al., 2016). Other medicinal uses cannabinoids can improve are the following:
Nausea: Several neurotransmitters mediate nausea and vomiting, including serotonin, dopamine, GABA, glutamate, cannabinoids, and others (Lynch et al., 2005). Antagonism of serotonin, dopamine, and substance P receptors (5-HT3 , D2, and NK1, respectively) produces clinically meaningful antiemetic effects. In contrast, it is the agonism of CB1 receptors, such as that produced by cannabinoid compounds, which results in antiemetic effects (Croxford et al., 2003)
Cancer: Cannabinoid compounds can reduce tumor growth in animal models of cancer by activating an ER-stress related pathway that leads to the stimulation of autophagy-mediated cancer cell death (Velasco et al., 2016). In addition, cannabinoids inhibit tumor angiogenesis, decrease cancer cell migration, reduced tumor size through decrease of cell proliferation or induction of cell cycle arrest and apoptosis along with desirable effect on decrease of tumor-evoked pain (Nikan et al 2016). Therefore, modulation of endocannabinoid system by inhibition of fatty acid amide hydrolase(FAAH), the enzyme, which metabolized endocannabinoids, or application of multiple cannabinoid or cannabis-derived compounds, may be appropriate for the treatment of several cancer subtypes (Javid et al., 2016) Cannabinoids induced autophagy mechanism in cancer and non-cancer cells (Costa et al., 2016).
Spinal cord injury (SCI): Spinal cord injuries are a devastating condition for which there is no standard treatment beyond rehabilitation strategies. The endocannabinoid system is expressed in the intact spinal cord, and it is dramatically upregulated after lesion (Arevalo-Martin et al., 2016). Endogenous activation of this system counteracts secondary damage following SCI, and treatments with endocannabinoids or synthetic cannabinoid receptor agonists promote a better functional outcome in experimental models (Arevalo-Martin et al., 2016).
References:
Arevalo-Martin A, Molina-Holgado E, Garcia-Ovejero D (2016): Cannabinoids to treat spinal cord injury. Progress in Neuro-Psychopharmacology and Biological Psychiatry 64: 190-199.
Costa L, Amaral C, Teixeriea N, Fonseca B (2016): Cannabinoid-induced autophagy: Protective or death role. Prostaglandins & Other Lipid Mediators 122: 54-63.
Di Marzo V, Matias I (2009): Endocannabinoid control of food intake and energy balance. Natural Neuroscience 8: 585-589.
Felder C, Joyce K, Briley E, Mansouri J, Blond O, Lai Y, Ma A , Mitchell R (1999): Comparison of the pharmacology and signal transduction of the human CB1 and CB2 receptors. Molecular Pharmacology 48(3): 443-450.
Fijal K, Filip M (2016): Clinical/ Therapeutic approaches for cannabinoid ligands in central and peripheral nervous system diseases. Clinical Neuropharmacology 39(2): 94-101.
Flores A, Maldonado R, Berrendero F (2013): Cannabinoid – hypocretin cross talk in the central nervous system. Frontier Neuroscience 23(2): 143-154.
Gallegue S, Mary S, Marchand J, Shire D, Casellas P (1998): Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry 232(1): 54-61.
Gorji A, Ghardi M (2002): History of headache in medieval Persian Medicine. Lancet Neurology 1:510-515.
Graham E, Asthon J, Glass M (2009): Cannabinoid receptors: a brief history and “what’s hot”. Frontiers in Bioscience 14(2):944-957.
Guidon J, Hohmann A (2011): The endocannabinoid system and cancer: therapeutic implication. British Journal of Pharmacology 111(10): 1476-1481.
Hermann H, Bisongno T, Lutz B, Di Marzo (2003): Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid receptor mediated response. Cellular Molecular Life Science 60(3):607-616. Jansen E, Haycock D, Ward S, Seybold V (1992): Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Research 575(1):93-102.
Javid F, Phillips R, Afshinjavid S, Verde R, Ligresti A (2016): Cannabinoid pharmacology in cancer research: A new hope for cancer patients. European Journal of Pharmacology 775:1-14.
Kalant H, Farraj A, Jan T (2001): Medicinal use of cannabis. Pain Research and Management 6: 80-91.
Hoffman A, Lycas M, Spivak C, Bauman M, Lupica C (2016): Disruption of hippocampal synaptic transmission and long term potentiation by psychoactive synthetic cannabinoid “spice” compounds: comparison with THC. Addiction Biology 10(11): 123-134.
Howlett A, Barth D, Bonner T, Cabral G, Casellas P, Felder C, Pertwee R (2002): International Union of Pharmacology. Classification of Cannabinoid receptors. Pharmacological Reviews 54(2): 161-202. Lu H, Mackie K (2016): An introduction to the endogenous cannabinoid system. Biological Psychiatry 79(7): 516-525.
Mackie K, Stevens L, Peterson M (2008): Cannabinoid receptors: where they are what the do. Journal of Neuroendocrinology 20(1): 10-14.
Mechoulam R, Hanu L (2001): The cannabinoids: Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion and in neuroprotection. Pain Research and Management 6(2): 67-73.
Munro S, Thomas K, Abu-Shaar M (1993): Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441): 61-65.
Nikan M, Nabavi S, Manayi A (2016): Ligands for cannabinoid receptors, promising anticancer agents. Life Sciences 146: 134-130.
Pacher P, Mechoulam R (2011): Is lipid signaling through cannabinoid 2 receptors part of a protective system? Progress in Lipid Research 50(2):193-211.
Pertwee R, Howlett A, Abood M, Alexander S, Greasley P, Hansen A, Ross R (2010): International Union of Basic and Clinical Pharmacology. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacological Reviews 62(4): 588-631.
Pertwee R (2012): Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Biological Sciences 367(1607):3353-3364.
Reggio P (2003): Pharmacophores for ligand recognition and activation/ inactivation of the cannabinoid receptors. Current Pharmaceutical Design 9(20): 1607-1633.
Szabo B, Schlicker E (2005): Effect of cannabinoids on neurotransmission. Experimental Pharmacology 168(2): 327-365.
Velasco G, Davila D, Lorente M (2016): The use of cannabinoids as anti-cancer agents. Progress in Neuropharmacology and Biological Psychiatry 64:259-266.
Walker J, Huang S (2002): Cannabinoid analgesia. Pharmacology and Therapeutics 95(2): 127-135.
Ware M, Daeninick P, Maida V (2008): A review of nabilone in the treatment of chemotherapy induced nausea and vomiting. Therapeutic Clinical Risk Management 24(3):254-263.
Wenzel J, Cheer J (2014): Endocannabinoid-dependent modulation of phasic dopamine signaling encodes external and internal reward predicting cues. Frontier Psychiatry 10(33): 89-95.
Zendullka O, Noskova K, Hanus L, Jurica K (2016): Cannabinoids and cytochrome P450 interactions. Current Drug Metabolism 17(3):206-226.
Zuardi A (2006): History of cannabis as a medicine: A review. The Revista Brasileira de Psiquiatria 28: 153-157.

 Fig 1. Nervous System
Fig 1. Nervous System 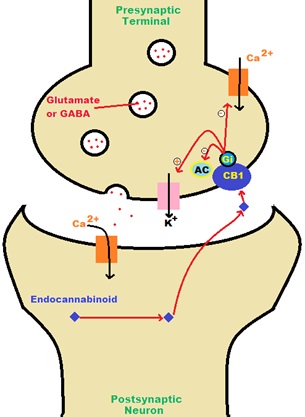 Fig 2. CB1 Receptor Activation
Fig 2. CB1 Receptor Activation 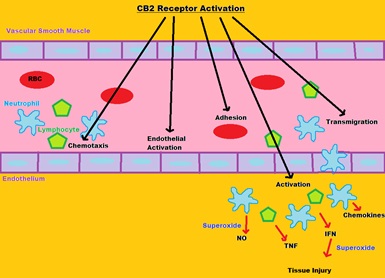 Fig 3. CB2 Receptor Activation
Fig 3. CB2 Receptor Activation 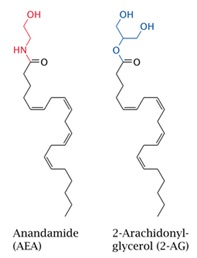 Fig 4.
Fig 4. 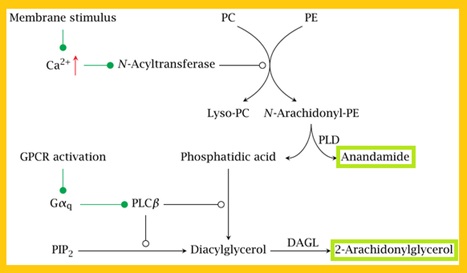 Fig 5. Synthesis of Endocannabinoids
Fig 5. Synthesis of Endocannabinoids 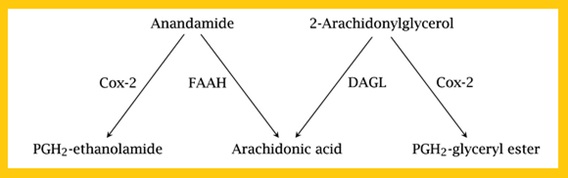 Fig 6. Hydrolysis of Endocannabinoids
Fig 6. Hydrolysis of Endocannabinoids 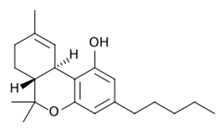 Fig 7. ∆ -9 Tetrahydrocannabinol (THC)
Fig 7. ∆ -9 Tetrahydrocannabinol (THC) 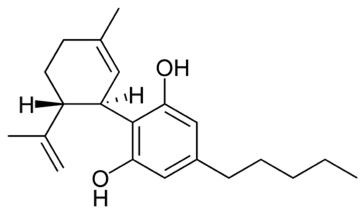 Fig 8. Cannabidiol (CBD)
Fig 8. Cannabidiol (CBD) 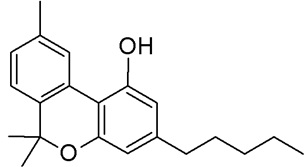 Fig 9. Cannabinol (CBN)
Fig 9. Cannabinol (CBN)