Contents
PHYSIOLOGICAL EFFECT OF CAPSAICIN
Intro
|
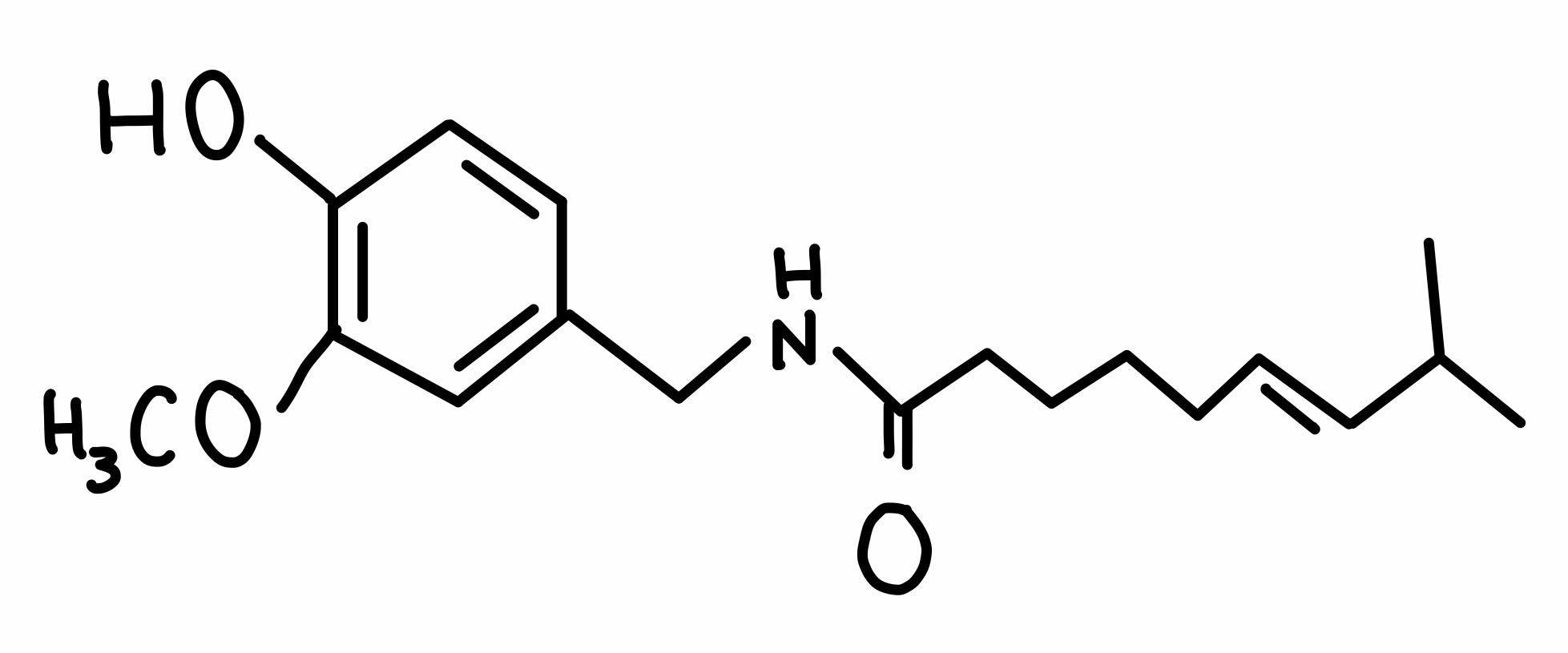
Figure 1 'Chemical structure of capsaicin' |
Have you heard of ‘mukbang’? It is a new trend that is popular among those who create their own YouTube videos. It means broadcasting yourself eating. This is becoming an increasingly popular trend and lots of people watch this. If you search the word mukbang on YouTube, you may find lots of videos people eating many kinds of food. As you scroll down the video list, you will see a variety of foods that are eaten include ‘artificially made spicy’ ingredients. Furthermore, there were YouTube challenges called ‘fire noodle challenge’, or ‘one chip challenge’, which entail eating spicy food and showing people how they react. Why are the people obsessed with these spicy foods? What kinds of chemicals are used to make the food artificially spicy?
Why are people more and more obsessed with spicy food?
'Spicy’ is not a taste. It is the tongue feeling pain when a compound that makes you feel spicy sensation attaches to the surface of the tongue. To lessen the pain, the brain secretes endorphins, which has analgesic effect. Endorphins not only have painkiller effects, but also make people feel happy. This, in effect, lessens stress so people are obsessed with the so-called 'spicy' feeling!
What is capsaicin?
(Cordell et al., 1993)
- C18H27NO3 (Figure 1)
- 8-methyl-N-vanillyl-6-nonemnamide
- Alkaloid family
- Derived from the genus Capsicum
- Hydrophobic
- Colourless and odourless
- It is a compound found in chilli pepper.
- Found both in the seed and skin, but contained a lot in the seed. o To protect itself from other animals or plants o to propagate by spreading the seed
- For those animals that capsaicin is not toxic for themselves : chilli pepper seed is one of the good food for them
- For those animals that capsaicin is toxic for themselves : they avoid eating chilli pepper
- Found both in the seed and skin, but contained a lot in the seed. o To protect itself from other animals or plants o to propagate by spreading the seed
- Is used for medicine and spices
- It makes mucous membranes in the mouth feel burning sensations. ( affecting nociceptor )
- Compounds that are related to capsaicin are called ‘capsaicinoid’.
- This is 2nd metabolites produced by chilli pepper.
Effect of capsaicin
Promotes appetite (Yoon HeeJun et al., 2003)
- Accelerates metabolism à burns fat à prevents body from storing fat (Kang Ji-Hye et al., 2010)
- Analgesic à is used in painkillers (Yaksh et al., 1979)
- Recently, it has been revealed that capsaicin has an anti-cancer effect (Munkyung Hwang, 2012)
- Makes adrenaline secretion more active from adrenal medulla (Emily Robinson, 2020)
- Sterilizing effect
Raises body temperature (Yoon HeeJun et al., 2003)
- Decreases blood cholesterol level à can be adequate for remedies of arteriosclerosis or diabetes mellitus (Emily Robinson, 2020)
- Recovering fatigue (Satyanarayana, 2004)
- Because chilli pepper contains vitamin A, B, and C, it is good for recovering fatigue.
- Specifically, it has 9 times more vitamin C than a tangerine (mandarin), 18 times more than an apple.
- Increases immunity (Deng, Yaxiong, et al., 2016)
- Chilli pepper contains B-carotene, which is converted into vitamin A.
- Vitamin A increases immunity and prevents from nyctalopia.
Disadvantages of capsaicin
- Having too much spicy food can harm your health
- Potential for damage of the mucous layer of stomach (Satyanarayana,2006)
- Wall of stomach becomes thinner (Satyanarayana,2006)
- Stomach ulcer or chronic gastritis can occur (Satyanarayana,2006)
- Can cause skin irritation
- Bad for people who have acne or face redness (flashes)
- This is because
- Capsaicin activates sympathetic n. à heart beats faster
- Capsaicin causes people to sweat à dilates blood vessel
- Disturbs deep sleep (REM cycles) (Obál Jr, Ferenc et al., 1983)
- Stomach-ache (Hutchinson et al., 1990)
- Feeling burning sensation in the stomach
- More general to people who have irritable bowel syndrome.
- Capsaicin burn (Hutchinson et al., 1990)
- When you drop liquid form capsaicin on your skin, it feels burning sensation.
- Can feel it when cutting chilli peppers
Additional
- As you eat spicy food more often, you become accustomed to the stimulus because TPRV 1, which causes pain, decreases (Ludy et al., 2012).
- Appropriate consumption of capsaicin depends on the person’s weight and age, but it is dangerous when having more than 50um of capsaicin (Jieun Park,2018).
- It is about having 20 chilli peppers
- Medically, it is used as medical cream for treating shingles and psoriasis
- It contains only about 0.025% - 0.1%, but still!!!
- Oleoresin capsicum, a compound extracted from chilli pepper, is an important ingredient of peppers (Haas, J. S., et al., 1997).
- Foods high in capsaicin (Yoonjung Kim, 2019)
- Chilli peppers, habanero peppers, pepperoni, jalapeno, piri-piri
- NO capsaicin in plain red pepper
- Capsaicin itself can be bought as liquid / powder form to add spicy feeling in your food
- Fluid capsaicin ; even putting one drop of capsaicin makes you feel burning sensation
As stated, capsaicin is a chemical compound that can be extracted from many kinds of chilli peppers. Capsaicin has many beneficial effects. When a person consumes adequate amount of capsaicin by chilli pepper, he or she can expect weight loss effect, increased metabolism, fatigue recovery, and painkilling effect. But when a person overeat capsaicin, it causes because stomach problems like simple stomach ache, to stomach ulcer or chronic gastritis. It is also bad for skin irritation, so be aware not to eat too much spicy food.
TRP (transient receptor potential) channel family
TRP channels are integrated membrane proteins and they work as cationic ion channels, which increase the intracellular cation levels by receiving the chemical or physical stimuli. This changes the membrane potentials. TRP channels have a characteristic structure, which is 6 transmembrane spanning helices, and both N- and C-terminals are located inside the cell. It is understood that the complete channels are tetramers with a pore formed by them. These channels are present in all types of cells in the body (Tominaga, 2013).
|
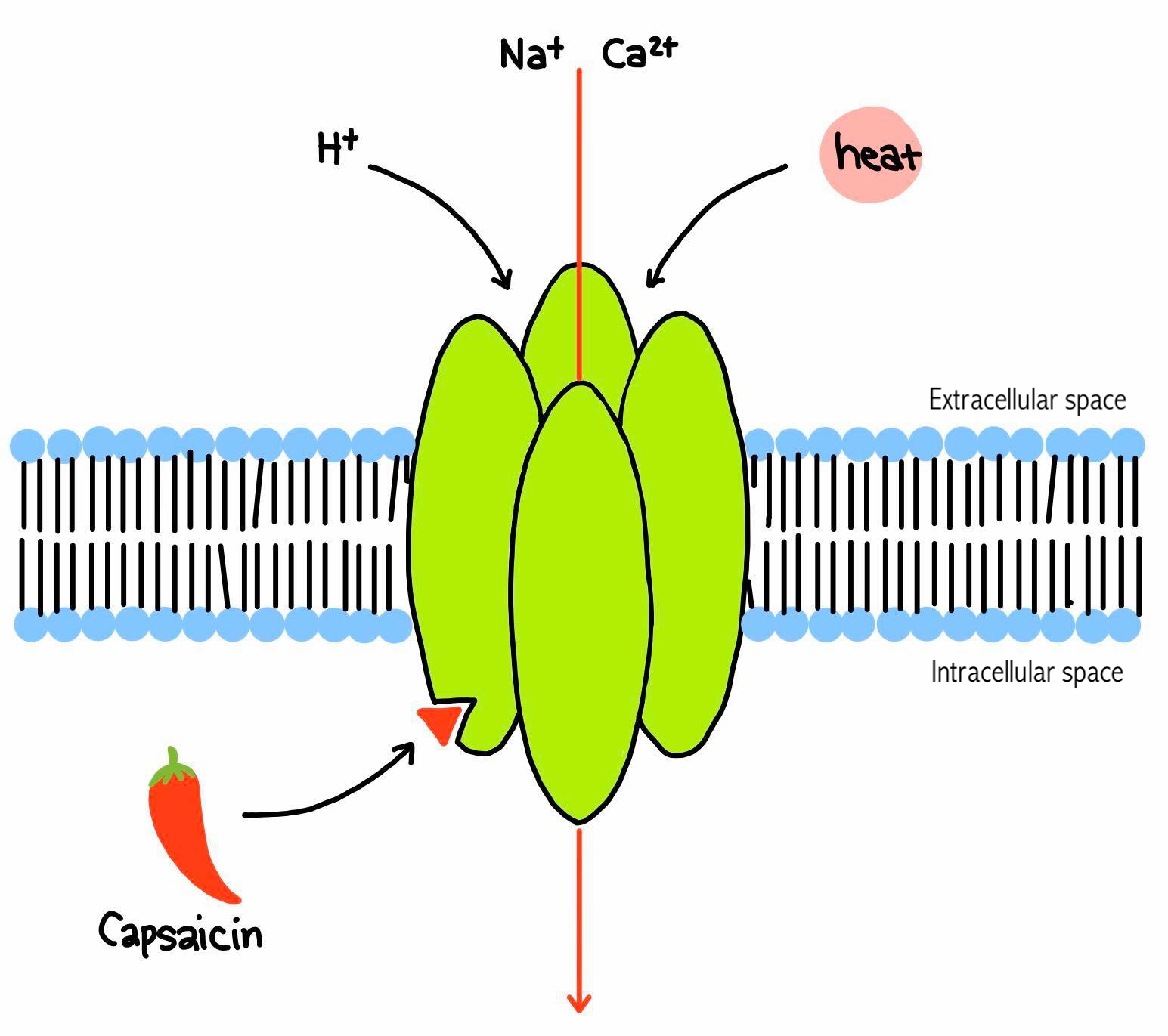
Figure 2 'TRPV1 receptor' |
Mechanisms of TRPV1
TRPV1 as a heat sensitive ion channel
TRPV1 (Transient receptor potential vanilloid member 1 channel) is one of TRP ion channel family, and it is also well known as “Capsaicin receptor”. This receptor is aligand-gated (nonselective, cation) ion channel receptor, but it also has the property of the voltage-gated ion channel as well (Rosenbau & Simon, 2007). However, in this essay we only focus on ligand-gated ion channel property since its voltage-gated property is minor and not clearly identified. It detects the heat (43℃: the threshold that the organisms feel pain) and opens the ion channel, so Ca2+ and Na+ flow into the cell (Rosenbau & Simon, 2007). The mechanism of the heat sensation has not been clarified yet, but some of the TRP ion channel family are considered to be the heat sensors (Tominaga, 2013). However, other TRPV receptors such as TRPV2-6 are not heat sensitive (Samanta et al., 2018). The influx of cations causes the depolarization of the cell and then emission of the action potential. This signal is recognized as pain. Those TRPV1 receptors are mainly located in the nociceptive neurons of peripheral nervous systems but it is ubiquitous (Mucosa of GI tract etc.) (Tominaga, 2013; Rosenbau & Simon, 2007).
TRPV1 as a capsaicin receptor
TRPV1 can also be sensitized by vanilloid groups such as capsaicin etc. Capsaicin is a lipophilic substance, so it can pass through the membrane and attach to the receptor intracellularly in the peripheral terminals of afferent sensory neurons. When capsaicin is attached to the receptor, the receptor can be activated and it allows Ca2+ and Na+ to flow into the cell. (Figure 2) At the same time, in the presence of capsaicin, the threshold of the heat sensitivity (43℃) is lowered to around 30-35℃, which is lower than the body temperature in the most of domestic animals, and therefore it causes the painful and burning sensation. This means that the hotter the food is, the more pain the individual feels when they eat capsaicin-containing food. Other than capsaicin, other factors such as proton (acidic conditions) can also activate the receptor, and cause the pain (Tominaga, 2013; Rosenbau & Simon, 2007).
Desensitization of TRPV1
In the presence of capsaicin, it causes the burning or painful sensation as described above, but the prolonged exposure to capsaicin actually leads to TRPV1 activity to be declined. This is so-called desensitization of the receptors, and it is caused by increased concentration of intracellular calcium ions. Enzymes such as calcineurin play role in this case to dephosephorylate the receptor proteins. This dephosphorylation makes TRPV1 less sensitive and decrease in response to agonists like capsaicin (Smutzer & Devassy, 2016).
Other activating factors of TRPV1
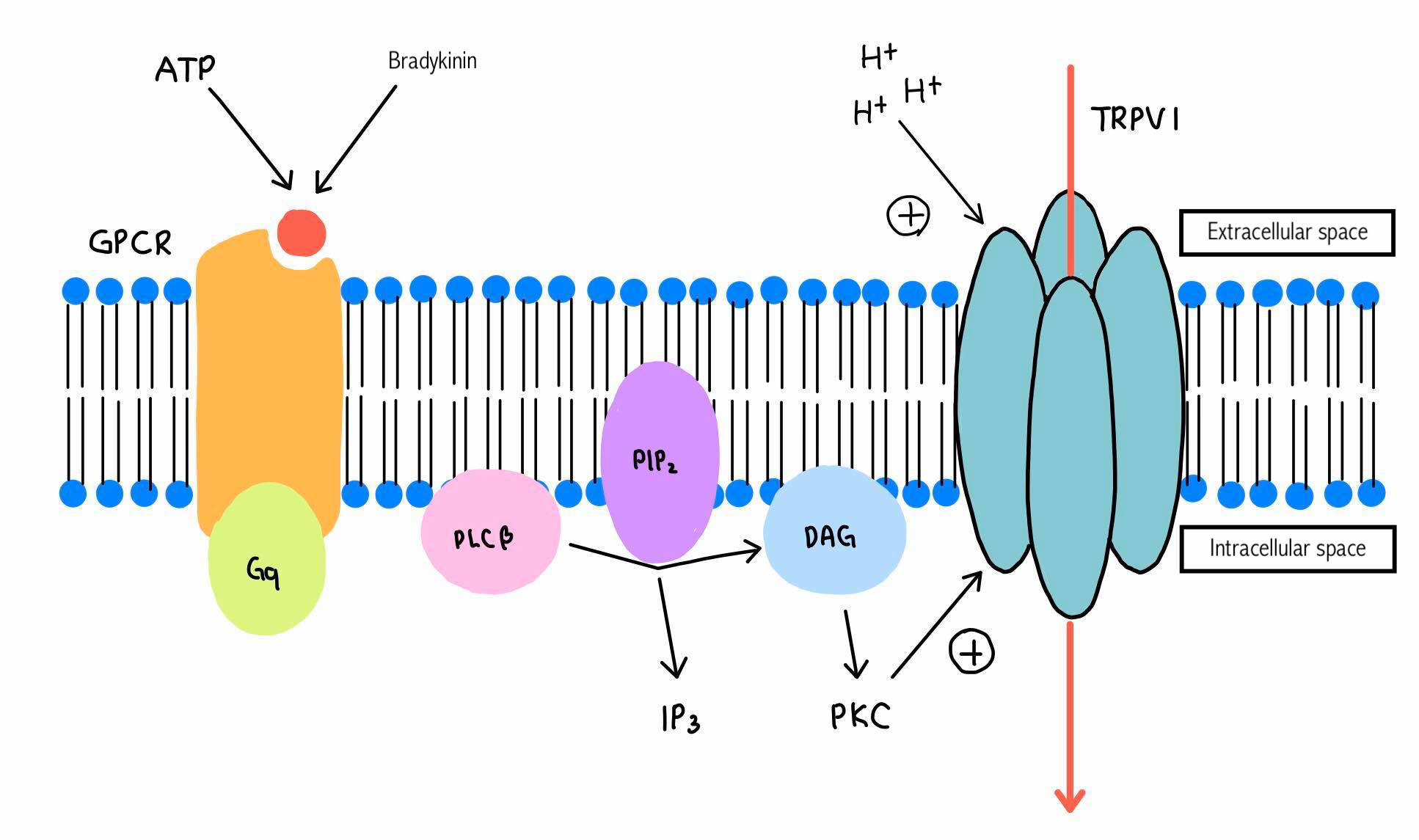
The sensation of pain through this receptor can be enhanced by phosphorylation of the receptor too. GPCR (G-protein-coupled receptor) has a role on this phosphorylation of the receptor. The inflammatory mediators, such as bradykinin, ATP, protease etc. bind to GPCR and this G-protein activation stimulates PLC (phospholipase C) on the intracellular membrane. The activated PLC breaks down PIP2 (phosphatidylinositol-4, 5-bisphosphate) into IP3 (inositol-1,4,5-triphosphate) and DAG (diacylglycerol). This DAG activates PKC (protein kinase C), which phosphorylate TRPV1. It can also be phosphorylated by PKA, which is activated by cAMP. Stimulatory G-protein plays a role when prostaglandin or serotonin binds to it, and AC (adenylate cyclase) synthesizes cAMP (Figure 3) (Tominaga, 2013).
|
Figure 3 'Phosphorylation of the receptor' |
Other factors released after activation of TRPV1
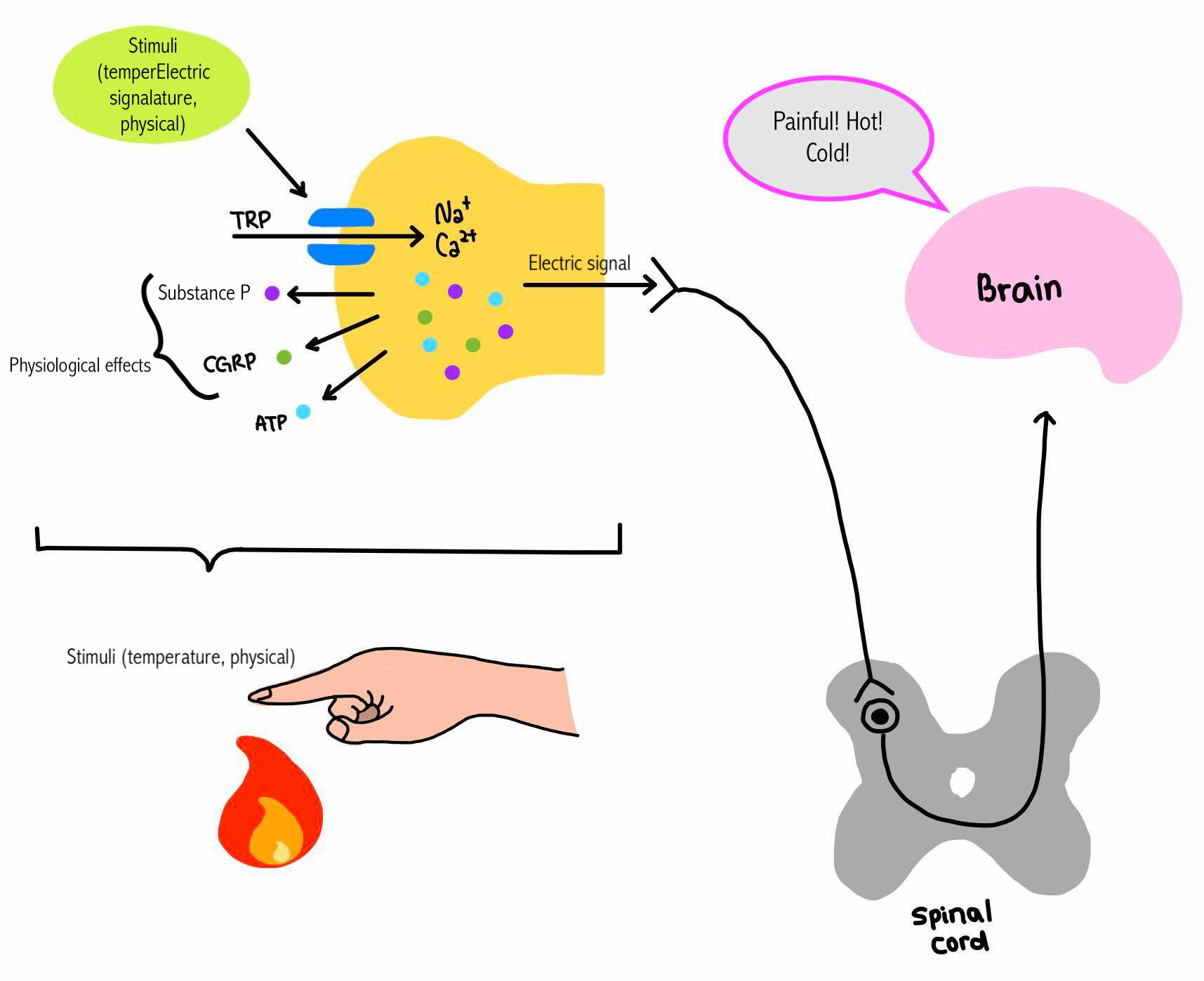
Activation of TRPV1 induces the secretion of CGRP (Calcitonin gene-related peptide) and substance P (and ATP, which is not well clarified) (Figure 4)(Tominaga, 2013). CGRP is known for its vasodilator effect in the cerebral arteries and therefore induces migraine. However, they also have advantageous physiological effects on the GI tract, such as increasing blood circulation, inducing GI tract activity, and suppressing secretion of gastric juice. Its vasodilator effect can be beneficial in neurovascular protection from vasoconstriction due to cerebral or cardiac ischemia (Grell et al., 2019).
|
Figure 4 'Signal of the sensation' |
Capsaicin on the sensory neurons
Capsaicin is a general stimulator of nociceptive sensory neurons which translates signals to the brain and the spinal cord in case of an injury. Capsaicin stimulates afferent sensory neurons primarily by opening a cation channel. This process is mediated directly by a specific membrane receptor. Antagonists of capcasain give rise to the activation of the nociceptive sensory neurons. Subsequently capsaicin exhibits anti inflammatory property and is wildly used in clinical practice (Szolcsányi, 2004).
Administrating capsaicin physiological effects
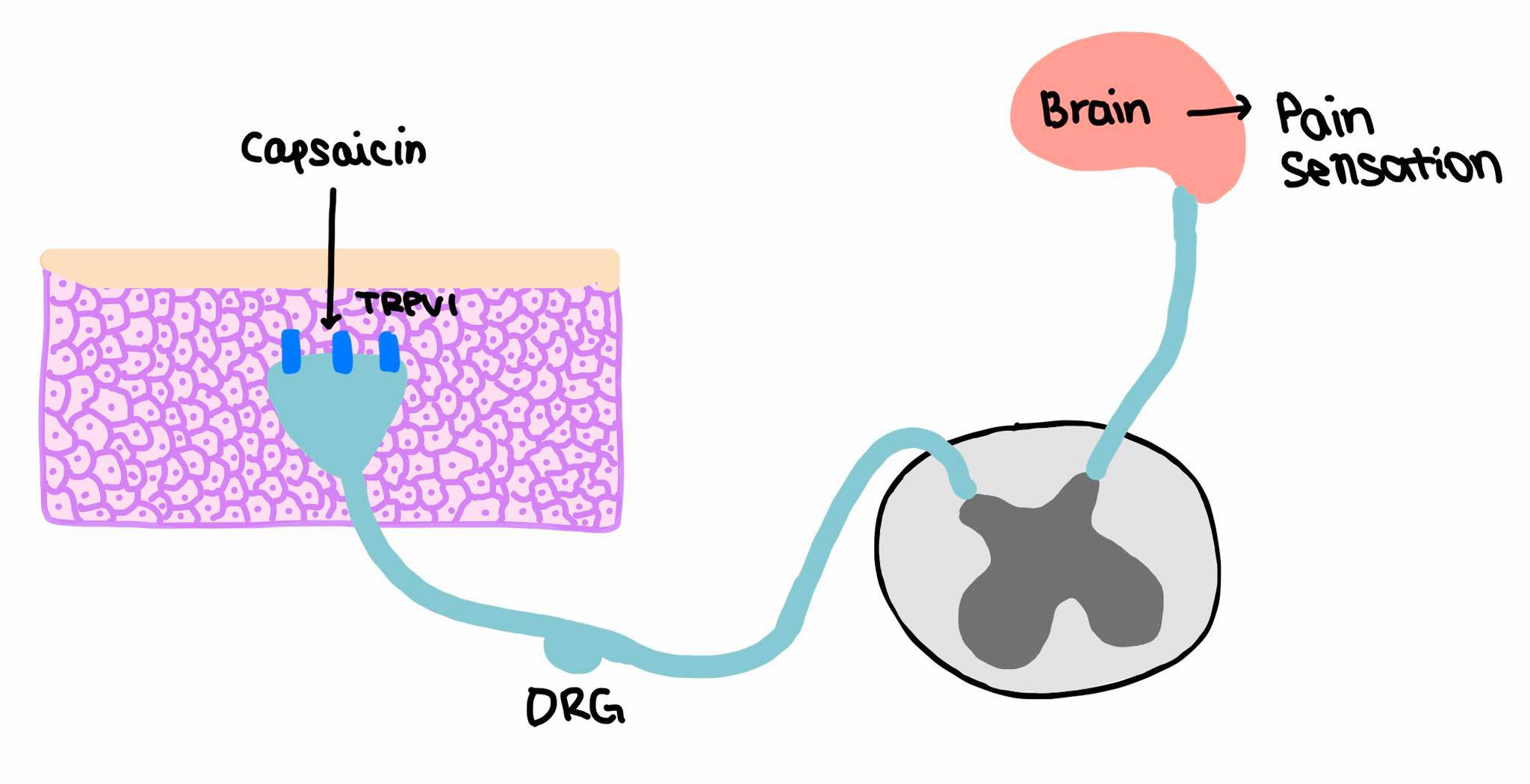
Effects of capsaicin vary according to the route of administration. The Dorsal Root Ganglion (DRG) neurons (Figure 5) are destroyed however the C fibres’ (slow conducting fibres C fibres in the somatic nervous system mediate pain sensation) axons and terminal ends of the neurons undergo morphological changes in the central nervous system. There has been no evidence of the effect of capsaicin on the motor system.
|
Figure 5 'Pain sensation' |
Neurotensin, regulator of Luteinising Hormone (LH) and prolactin found in the central nervous system neurons show no changes or effects. When admistered to newborn rats, capsaicin causes the reduction of substance P, a tachykinin of paracrine effect, in the sensory neurons (Arnold et al, 1980).
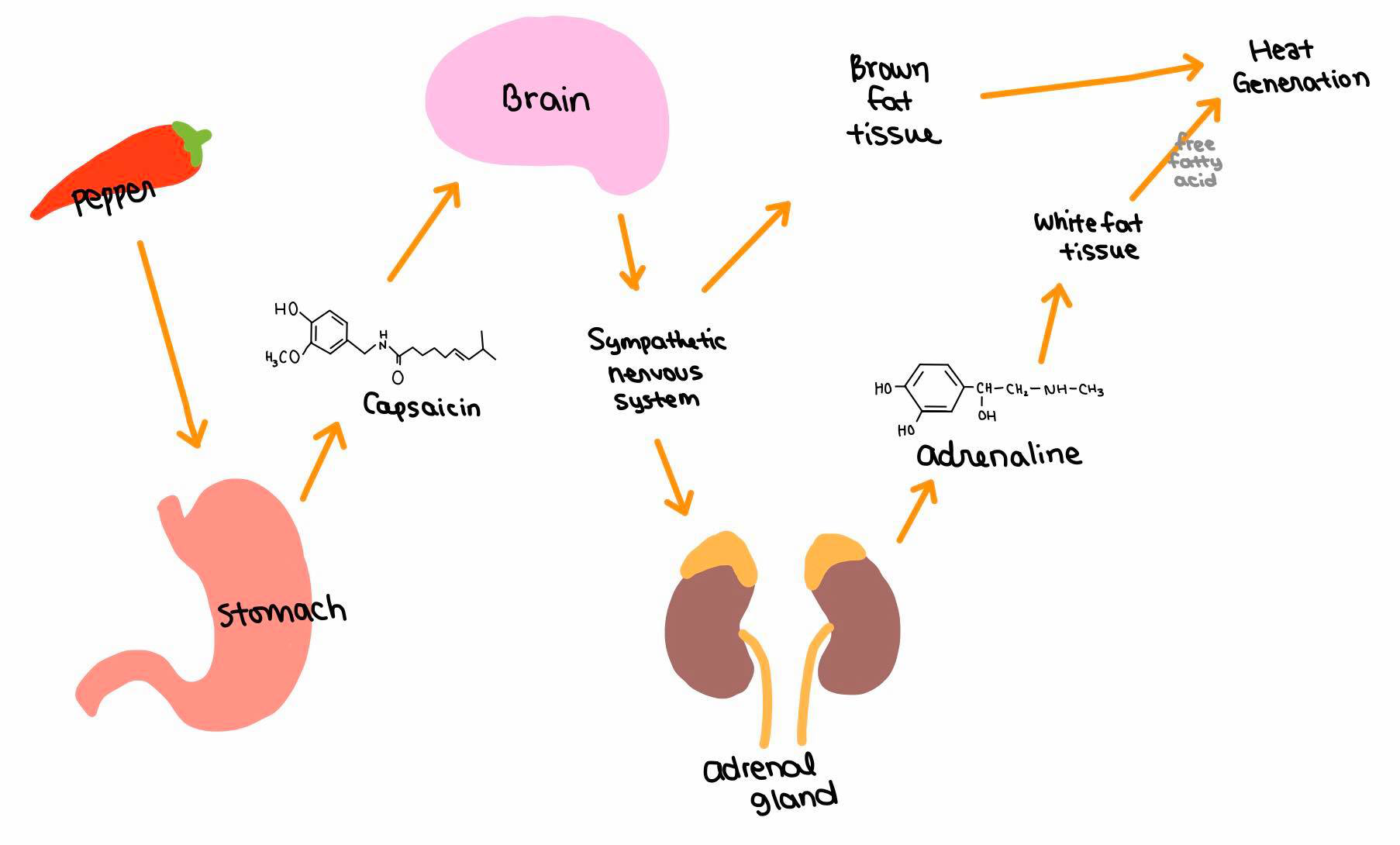
The effect of Capsaicin was investigated to determine catecholamine secretion of the adrenal medulla collective hormones. Intravenous administration of capsaicin stimulated the sympathetic activity of the adrenal gland. The higher the dosage of administration was, the higher the stimulus was in the adrenal gland sympathetic activity. It was concluded that capsaicin act mainly through the stimulation of the central nervous system. (Figure 6)
|
Figure 6 'Effects of capsaicin' |
Effect of capsaicin on sensory neurons
Capsaicin inhibits the axonal/axoplasmatic transport of sensory neurons. Evidence for this phenomenon, capsaicin was injected to the sciatic nerve blocks. An accumulation of somatostatin and substance P were measured at the site of injection within 24 hours. The long term depletion of substance P is unlikely and shall return back to normal levels within 1 or 2 weeks. The possibility of having capsaicin receptor in the axon rather than the nerve endings may exist. Capsaicin acts on the terminal ends of C fibres in both the central and peripheral nervous system without affecting the sensory neurons. Substances transported to the terminals will influence their recovery accordingly. Capsaicin stimulates or inhibits nociceptive sensory neurons called vanilloid receptor 1. These neurons exhibit inflammatory pain, regulate homeostasis as well as metamorphosis. Capsaicin sensitive neurons are modified by stress leading to a change in the metabolic development (Schultz and Ustinova, 1998).
Radio Immuno Assay (RIA) studies have shown that corticotropin releasing hormone (CRF) was found in the same location with substance P, enkephalin (involved in the nociceptice regulation in the body) and somatostatin in the dorsal horn of the spinal cord. Capsaicin administration resulted in a decline in the concentration of substance P, Vasoactive Intestinal Peptide (VIP), Cholecystokinin (CCK) and CRF whereas somatostatin and enkephalin concentration remained unchanged. It was concluded that primary afferent neurons located specifically in certain regions of the spinal cord were sensitive to the effects of capsaicin injected while topical capsaicin caused a decline of the above mentioned substances only at the terminal end of the sensory neurons (Skofitsch et al, 2003).
Effect of capsaicin on the calcium ion release
Capsaicin increases the intracellular calcium ions levels via ryanodine sensitive calcium ion channel, activated in the sarcoplasmatic reticulum. Phosphotidyl inositol triphosphate, which opens the ligand dependent intracellular calcium ion channels, however does not play a role in this phenomenon (Cholewinski et al, 2003).
Effect of capsaicin on Smooth Muscle
Capsaicin is used in identifying neurotransmitters dealing with afferent neurons through the transient receptor potential vanilloid (TRPV1) at the spinal level. The most important transmitters to stimulate a local efferent response of capsaicin are tachykinins and calcitonin. Effects of these interactions might differ in humans and animals by the mediation of Vasoactive Intestinal Peptide (VIP) and Nitric Oxide (NO) (Barthó et al, 2004).
Effect of capsaicin on life span
In central Asia and African countries, there is a belief that diets rich in spicy food, life expectancies of people are considerably prolonged just by incorporating a variety of spices. Scientific research has demonstrated that low concentration of capsaicin in everyday diet has shown little to no evidence in extending life. However, Capsaicin reduces the daytime activity in females contributing to a longer life (Shen J et al, 2019).
Effect of capsaicin on chronic pain
Changes in the environment surrounding the sensory endings causes hypersensitisation by the activation of TRPV1. During Prolonged pain or inflammatory processes, sensation of pain leads to an over expression of TRPV1 by the A delta and Type c fibres. In the presence of an axonal trauma, axonal transport of nociceptive transmitters contributes to the onset of chronic pain (Russell and Burchiel, 2003).
References
Articles
Cholewinski, A., Burgess, G. M., & Bevan, S. (2003, March 18). The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/0306452293903157#!
Cronkleton, E. S. and E. (2018, September 2). How Does Cayenne Pepper Help You Lose Weight? Retrieved from https://www.healthline.com/health/food-nutrition/cayenne-pepper-for-weight-loss
Grell, A.-S., Haanes, K. A., Johansson, S. E., Edvinsson, L., & Sams, A. (2019, October 4). Fremanezumab inhibits vasodilatory effects of CGRP and capsaicin in rat cerebral artery - Potential role in conditions of severe vasoconstriction. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0014299919306788
Rosenbaum, T., & Simon, S. A. (2007). TRPV1 Receptors and Signal Transduction. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK5260/?report=classic
Russell, L. C., & Burchiel, K. J. (2003, March 10). Neurophysiological effects of capsaicin. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/0165017384900055#!
Saljoughian, M. (2009, July 20). Capsaicin: Risks and Benefits. Retrieved from https://www.uspharmacist.com/article/capsaicin-risks-and-benefits
Schultz, D, H., Ustinova, & E, E. (1998, May 1). Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Retrieved from https://academic.oup.com/cardiovascres/article/38/2/348/299436
Shen, J., Shan, J., Zhu, X., Yang, P., Zhang, D., Liang, B., … Dai, Z. (2019, November 29). Sex specific effects of capsaicin on longevity regulation. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0531556519305169
Skofitsch, G., Zamir, N., Helke, C. J., Savitt, J. M., & Jacobowitz, D. M. (2003, January 27). Corticotropin releasing factor-like immunoreactivity in sensory ganglia and capsaicin sensitive neurons of the rat central nervous system: Colocalization with other neuropeptides. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/0196978185900579
Smutzer, G., & Devassy, R. K. (2016, January 14). Integrating TRPV1 Receptor Function with Capsaicin Psychophysics. Retrieved from https://www.hindawi.com/journals/aps/2016/1512457/
Suo, S., Ishiura, S., & Tol, H. H. M. V. (2004, August 23). Dopamine receptors in C. elegans. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0014299904007307
Szolcsányi, J. (2004, November 24). Forty years in capsaicin research for sensory pharmacology and physiology. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0143417904000757
Vlachová, V., Lyfenko, A., Orkand, R. K., & Vyklický, L. (2004, August 5). The effects of capsaicin and acidity on currents generated by noxious heat in cultured neonatal rat dorsal root ganglion neurones. Retrieved from https://physoc.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-7793.2001.t01-1-00717.x
Watanabe, T., Kawada, T., Kurosawa, M., Sato, A., Iwai, K., Ishii, H., … Department of Food Science and Technology. (1988, July 1). Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Retrieved from https://journals.physiology.org/doi/abs/10.1152/ajpendo.1988.255.1.E23
Websites
https://www.sciencedirect.com/science/article/abs/pii/0306452293903157#!
https://www.healthline.com/health/food-nutrition/cayenne-pepper-for-weight-loss
https://www.sciencedirect.com/science/article/abs/pii/S0014299919306788
https://www.ncbi.nlm.nih.gov/books/NBK5260/?report=classic
https://www.sciencedirect.com/science/article/abs/pii/0165017384900055#!
https://www.uspharmacist.com/article/capsaicin-risks-and-benefits
https://academic.oup.com/cardiovascres/article/38/2/348/299436
https://www.sciencedirect.com/science/article/abs/pii/S0531556519305169
https://www.sciencedirect.com/science/article/abs/pii/0196978185900579
https://www.sciencedirect.com/science/article/abs/pii/S0014299904007307
https://www.sciencedirect.com/science/article/abs/pii/S0143417904000757
https://physoc.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-7793.2001.t01-1-00717.x
https://journals.physiology.org/doi/abs/10.1152/ajpendo.1988.255.1.E23
Magazines
Tominaga, M. (2013). 温度感受性TRPチャネル. Science of Kampo Medicine '漢方医学, 37(3), 164–175. doi: http://tenaca-nips-2016.kenkyuukai.jp/images/sys\information\20161004145432-C9A0307CDC5BFFBE368042C37301506118242A368CDD5986EDA4A983ED7B8D92.pdf
Figures
Figures have been hand drawn by Minjeong Cha, one of the contributors of this essay.
Figure 2, 3, and 4: refered to Tominaga, M. (2013). 温度感受性TRPチャネル. Science of Kampo Medicine '漢方医学, 37(3), 164–175.