|
Size: 11012
Comment:
|
Size: 11023
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 83: | Line 83: |
| {{attachment:food intake.jpg|alt text}} | {{attachment:food intake.jpg|alt text|height=400}} |
Obesity and the brain
Hadar Zutta
Annelise Svindland
Verity Walsh
Contents
Introduction
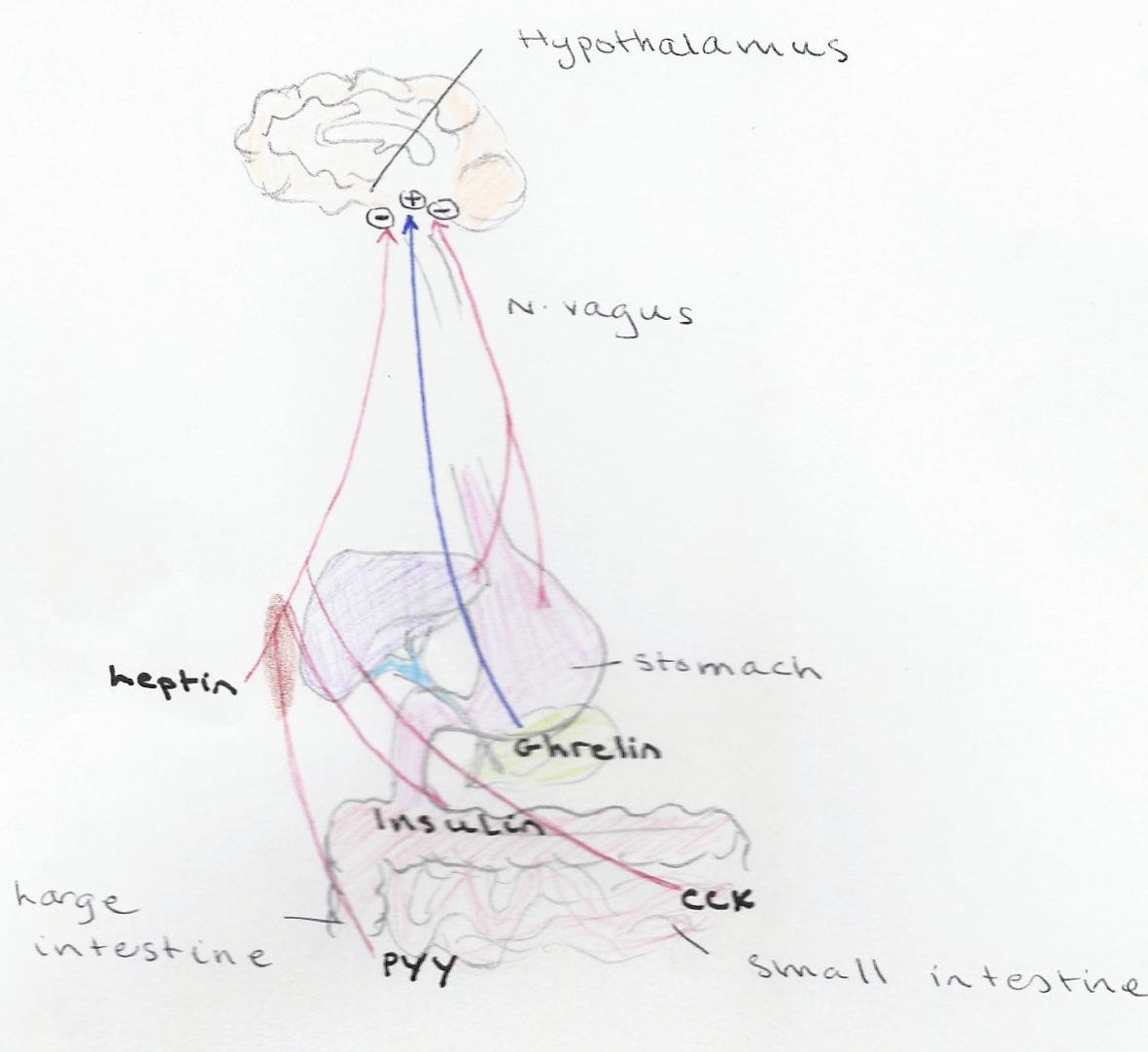
Obesity is defined as an excess of body fat. A good indication of body fat content is body mass index (BMI) BMI= Kg/height m2 .BMI over 30 kg is defined as obese. The problem with this method is that it doesn't take into account large muscle mass and doesn't measure direct adiposity so the best way to define obesity is percentage of total body fat 25% or greater in men and 35% greater in women is classified as obese. (med. Physi: guyton)when appetite evokes a caloric intake above the metabolic use of the calories obesity results (page 530 phathophysio Barbra h. bulloch). Food intake is regulated by neural centers, craving for food is a hunger sensation and the desire for food is appetite, normally a certain type. A satisfaction after the search and consumption of food is called satiety both the hunger and satiety center are contained in the hypothalamus of the brain. Control of food intake is participated in by several neuronal centers of hypothalamus. Feeding centers are the lateral nuclei of the hypothalamus and stimulating them cause's animal to eat greedily (hyperphagia). The lateral nuclei operates by exciting motor drive to look for food. The satiety centers are located on the ventromedial nuclei of the hypothalamus and gives nutritional satisfaction the inhibits feeding center. Complete satiety (aphagia) can be caused by electrical stimulation of the region. (pg 868 Med physio).
Leptin
When we talk about obesity and the relationship with the brain, it is important to mention Leptin, which is one of the main factors when it comes to obesity. Leptin is secreted from the adipocytes in the adipose tissue. It is a peptide hormone, which plays a very important role in the regulation of energy intake and energy expenditure in the body. It also has an important role in food regulation. (physiology book)
Energy intake is food intake, and is negatively affected by leptin. The hormone works mainly on the hypothalamus through its receptor, OB-R (obese-receptor). Here it provides the central nervous system with information about the energy stored in the body, and in this way, helps the brain to establish energy homeostasis in the body. Energy homeostasis means that there is a balance between the energy coming in, the energy being used and the storage of energy. If there would be a leptin deficiency, the amount of energy stored would increase, and by this the body fat. (1+2) In the body, the plasma concentration of leptin is different in the obese then in the lean individuals. Obese animals have a higher concentration of leptin. This is due to the relationship between plasma leptin concentration and body fat. If body fat decreases, so will the concentration of leptin. (1: Leptin access into the brain) In these individuals, leptins effect is inhibited, leptin resistance occurs due to the high plasma leptin level. In other words, lack of leptin will lead to obesity. (2: leptin resistance and obesity)
The way leptin accesses the brain, is by passing across the blood-brain barrier (BBB). And in this way it can reach the appetite center of the hypothalamus, and affect the food intake, by inhibiting it. The passing of leptin through the BBB is an important step in the regulation, and several pathways have been studied. (1)
From this information, we can see that leptin is a key hormone when it comes to the regulation of food intake, and that the deficiency of it can lead to obesity. There are also other factors that play there role in the regulation, but when it comes to the hormones, leptin is the most important one.
Reward Centre; “wanting” and “liking”
Food Reward; is a process which contains “liking” (hedonic impact), “wanting” (incentive motivation) and learning (associations and predictions). Hedonic hotspot; a neurochemically stimulated specific brain site that amplifies pleasurable “liking” reactions. “Liking”; mainly generated by subcortical brain systems, a hedonic reaction detected in neural signals and behaviour. “Wanting”; a motivation for reward evoked by reward cues. The brain mesolimbic systems are important to “wanting”, especially ones involving dopamine.
The brain reward mechanisms influence the food we eat, how much we eat and how often by generating “liking” and “wanting” sensations for food. It is thought that this maybe one of the causes for rising obesity. The “liking” mechanism contains hedonic circuits that connect together hotspots in the forebrain limbic structures e.g. nucleus accumbens and ventral pallidum. “Wanting” mechanism has larger opioid networks in nucleus accumbens, striatum and amygdala. They also have mesolimbic dopamine systems and corticolimbic glutamate signals that interact. Foods we like have motivational power, the sight or smell of the food may entice us to eat, and even a small amount of the food can trigger us to eat more. It’s not the food or cue that influences us to eat, it’s the brains response of the perceiver’s to the stimuli. If temptations themselves arise in the brain they themselves can stimulate a sudden urge to eat without food being physically in front of us (temptation or pleasure of sweet, fatty or salty foods). The temptations evoke “liking” and “wanting” reactions which are generated by neural signals that associate desire or pleasure with the sensation. The Brain Reward Centre in Growing Obesity Rates; Modern temptations to eat and to not stop are stronger than they were in the past because of the excess of sugar, fat and salt in modern food. The traditions that used to limit snacking no longer exist so more food is consumed outside of meals. Portion sizes are more likely larger than recommended which means we eat more than we should in one meal. These trends may play into normal basis of brain reward system and let us succumb to desire and eat more. Both the brains “liking” and “wanting” mechanisms are stimulates by food cues. Satiety influences can diminish the ‘go systems’, however, no strong stop signal is ever generated to stop intake, only subdue the ‘go’ signal. Potential Roles of Brain Reward Systems in Obesity; Obesity has different underlying causes which vary from individual to individual. Classification and types of overeating; • Reward Dysfunction; Food reward functions if go wrong can lead to over eating. Reward dysfunction can cause foods to be hedonically “liked” too much. Individuals can “like” and “want” food more than average person as excessive activation “liking” substrates would increase the hedonic impact of the foods. This can be a cause of excessive (binge) eating and obesity. “Wanting” to eat alone can be another possibility for overeating. Smell sight or vivid imagination of food can be a trigger for compulsion to eat. • Passively Distorted Reward Function; The initial cause of distorted to eating maybe not be the brain reward system, but its function can be abnormally passive. The mechanism for “liking” and “wanting” may try to function as normal but in studies the neuroimaging appears abnormal. • Normal Resilience in Brain Reward; The cause can lie completely outside of the brain reward system , the brain reward system continues to work normally in cases of obesity and over eating.
Stress as a promoter of eating intake
Stress promoters triggers eating palatable food in about 30% of the population. The assumption is that eating during stress acts as a hedonic self-medication for suppressing the stress. (Dallaman et. Al 2010 and Koob 2004). The suggested mechanism for this phenomenon is the CRF effect: is stress the brain releases CRF causes an aversive state which induces the will to eat comfort food as a self-medication, which results in the case of sweet comfort food in decreased HPA sensitivity and lower basal level of the CRF after stress. Blockade of CRF receptors may increase the uptake of less palatable food while decreases the intake for sucrose (Cottone et al 2009). On the other hand in the case of the central nucleus of amygldala where in the case of experimentally induced elevations of CRF level results in a decrease in the ingestive behavior and food intake. An explanation for this is certain regions of the brain the effect of stress and CRF are expressed in "wanting" food without needing aversive state to trigger the eating, seen in the nucleus accumbens where CRF microinjections triggers a "wanting" feeling for sugar by multiplying the motivational potency of sugar cues into peaks of desire of a reward, the peaks were correlated with the physical cue, though CRF was in the brain all along. It has been proven that not only distress triggers a "wanting" sensation but also incentive motivation does in the same CRF incentive mechanism.
Food addiction
Definition: the artificially intense sweet, salty or fatty sensory stimulation and the effect of technologically enhanced nature of modern processed food as a super incentive stimulus. In case of an eating behavior which is expressed as an addiction with loss of control and relapse, it has been shown that there's a high probability that those people carry both G+ alleles for the receptor gene that codes "gain of function" of the mu opoid signals, and carry A2 alleles associated with Taq1A that may increase binding to dopamine D2 receptor (Davis et al.2009). In a more general aspect it has been suggested that people with genes that promote elevated dopamine functioning are more likely to develop obesity (Campbell and Eisenberg). Compulsive level of "wanting" might be produced by sensitization-type hyper reactivity in brain mesolimbic circuits of inceptive salience.
Conclusion
References
Øystein V.Sjaastad, Knut Hove, Olav Sand. Physiology Of Domestic Animals (First edition, 2003). Page 232-233.
Barbara L. Bullock, Pearl Philbrook Rosendahl. Patho physilogy (1984). Page 530.
Arthur C.Guyton, John E.Hall. Textbook of Medical Physiology (11th edition, 2006). Page 860 and 872.
Bartolome Burguera,Marta E. Couce. Leptin access into the brain: A saturated transport mechanism in obesity. (Volume 74, issues 4-5, 12. November 2001. Page 717-720).
Kent C.Berridge, Chao-Yi Ho, Jocelyn M.Richard, Alexandra G. DiFeliceantonio. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. (Volume 1350, 2. September 2010. Neural Mechanisms of Ingestive Behaviour and Obesity. Page 46-64).
Pablo J. Enriori, Anne E. Evans, Puspha Sinnayah, Michael A. Cowley. Leptin Resistance and Obesity. Published online 6. September 2012. (Volume 14, Issue S8, Neurobiology of Obesity. Page 254S-258S. August 2006).