Mechanism of the Inflammatory and Neuropathic pain (Hyperalgesia) - The Role of the Pro-Inflammatory Cytokines
When researching both inflammatory pain and neuropathic pain, it can be stated that they both differ but also are analogous. They contrast as inflammatory pain is the damage of the bodies’ tissues whereas neuropathic pain is the damage of the somatosensory system of the body, the PNS and CNS. Both however are strongly related with regards to cytokines. Pro-inflammatory cytokine production is stimulated, either induced or increased with the onset of either of these chronic pains.
Contents
Inflammatory Pain
Inflammation is a vital immune response in the nervous system to many pathological pain states. This response is induced by harmful stimuli such as pathogens, xenobiotics or mechanical damage (injury). The aim of this complex defense mechanism is to retain a homeostatic balance of the organism. Inflammatory pain is a non-neuropathic pain, which is therefore known as nociceptive, in other words the pain is caused by damage to the tissue.
Inflammatory responses can be either beneficial or harmful to the body depending on the types of cells involved and how badly the tissue is damaged. The initiation, maintenance and resolution of the inflammation greatly impact the effect the inflammation has on the organism. Inflammatory responses have been vital throughout evolution as an important mechanism of survival and self-defence.
Inflammatory responses lead to pain due to the excitation of nociceptive pathways. Inflammation and damage to tissues stimulate the nociceptors. Inflammation is an endogenous stress meaning that it originates from within the organism instead of being an external factor. Nociceptors are specialized sensory neurons. These peripheral neurons have an important role in the detection of stimuli to the skin that may cause harm. For example, they detect both extreme pressure and temperature in order to avoid tissue damage. Chemicals that may result in injury are also detected. These harmful stimuli are picked up by the nociceptors are brought to the brain by electrical signals. The sensation of these neurons picking up the stimuli is pain and it differs in intensity depending on both the strength of the stimuli and also the area of the body where the nociceptors are located.
Increased excitability of the nociceptors alters the ion channel activity in the affected fibers. This then leads to molecular change in the nerves involved.
Phases of Inflammatory Responses
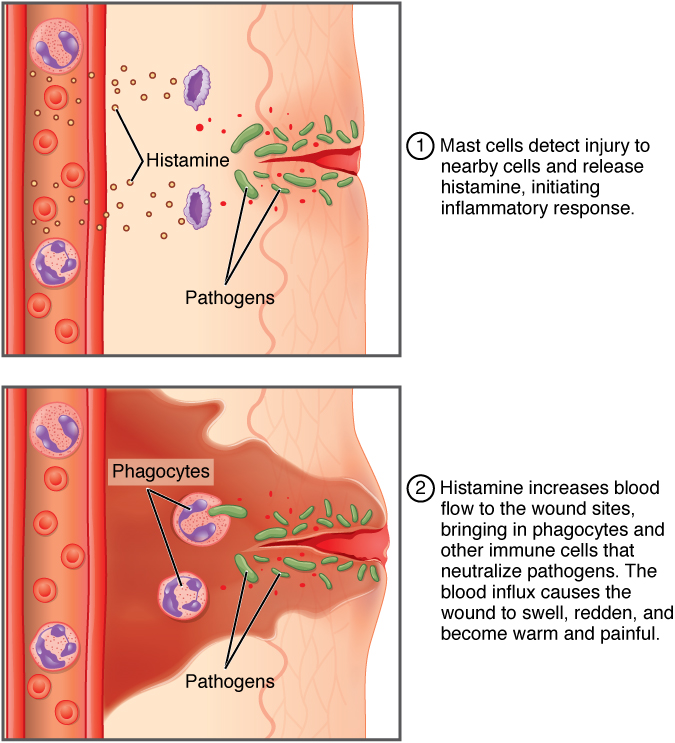
The first stage is known as the acute phase. These initial reactions occur just seconds to minutes after the injury to the tissue occurs. During this phase, there is a change in the plasma concentration of a type of proteins called Acute Phase Proteins (APP). If there is an increase in concentration of these proteins they are positive APP while a decrease in the concentration means that they are negative APP. The response of APP differs in different species. Different types APP genes are regulated by specific cytokines. Pro-inflammatory cytokines are secreted which cause a series of molecular changes in endothelial cells, fibroblasts and neutrophils. Examples of these cytokines are IL-8 and TNF-α. Macrophages, mast cells and lymphocytes are important immune cells in the secretion of these cytokines. Blood flow will increase around the affected area leading to an external red appearance. The transport of macrophages to the damaged area will activate the complement system. Edema will occur during the acute phase due to the discharging of the excess blood plasma, which contain immunoglobulins or fibrins, to the site of inflammation. This is what causes the tissue to swell. T and B cells of the lymphatic system recognize the antigens and release the swelling of the tissue.
|
This acute stage of inflammatory pain could become chronic if the inflammatory response is not rapid enough. This chronic stage causes significantly more damage to the tissue. Chronic inflammation is associated with diseases such as cardiovascular diseases, neurodegenerative disorders and cancer, in comparison to mechanical damage due to injury. To avoid the occurrence of chronic inflammation and serious tissue damage, the quick regulation of the homeostasis is important. Inflammatory mediators and both the innate and adaptive immune systems coordinate acute and chronic inflammations that underlie many diseases of the organism.
Neuropathic Pain (Hyperalgesia)
Hyperalgesia is the increased sensitivity to painful stimuli. Neuropathy is defined as the disturbance of function or pathological change in a nerve. Since 1994, the definition for neuropathic pain by the International Association for the Study of Pain (IASP) has been ‘pain initiated or caused by a primary lesion or dysfunction of the nervous system’. However, in 2008 IASP approved a change of definition to ‘pain arising as a direct consequence of a lesion or disease affecting the somatosensory system’. This effectively means that dysfunction without evidence of injury is no longer regarded as meeting the criteria for neuropathic pain.
Mechanism
Neuropathic pain is best thought of as an abnormal activation of pain pathways and can occur as a result of injury to peripheral nerves and posterior roots (peripheral neuropathic pain) and spinal cord and brain (central pain). Cytokines role in regards to pain and hyperalgesia and link with inflammatory pain: There is ample evidence from experimental studies that pro-inflammatory cytokines induce or increase neuropathic pain as well as inflammatory pain. Direct actions of cytokines on afferent nerve fibers have been shown as well as actions involving further mediators such as prostaglandins or nerve growth factor. Inhibition of pro-inflammatory cytokines, either by synthesis inhibitors, inhibitors of cleavage from the cell membrane, by direct antagonists, or by anti-inflammatory antibodies, reduces pain and hyperalgesia in most models studied. Cytokine inhibition may add to the panel of treatment modalities for neuropathic pain
Experimental Investigation
Bites from the brown recluse spider (BRS) can cause extreme pain. We propose cytokine release as a cause of the discomfort and a central mechanism through glial cell upregulation to explain measured pain levels and time course.
|
Observations
Twenty-three BRS bites were scored at a probable or documented level clinically, and an enzyme-linked immunosorbent assay was used to confirm the presence of BRS venom. The mean (SD) pain level in these cases 24 hours after the spider bite was severe: 6.74 (2.75) on a scale of 0 to 10. Narcotics may be needed to provide relief in some cases. The difference in pain level by anatomic region was not significant. Escalation observed in 22 of 23 cases, increasing from low/none to extreme within 24 hours, is consistent with a cytokine pain pattern, in which pain increases concomitantly with a temporal increase of inflammatory cytokines.
Conclusions and Relevance
These findings in BRS bites support the hypothesis of cytokine release in inflammatory pain. A larger series is needed to confirm the findings reported here. The extreme pain from many BRS bites motivates us to find better prevention and treatment techniques.
Cytokines
Cytokines are small regulatory, non-structural proteins produced by white blood cells. Their molecular weight ranges between 8-40,000 Daltons, and their structure is quite diverse. Some of them form long-chain 4-helix bundles, e.g. interleukin (IL)-6, others jelly rolls, e.g. tumor necrosis factor-a (TNF-a ), and others beta-trefoils, e.g. IL-1b.
Cytokines can be divided into; lymphokines (cytokines made by lymphocytes), monokines (cytokines made by monocytes), chemokines (cytokines with chemotactic activities), and interleukin (cytokines made by one leukocyte and acting on other leukocytes). (Zhang and An, 2009) There is no amino acid sequence motif or 3D structure that links cytokines; rather it’s their biological activities that allow us to group them into different classes. Cytokines are primarily involved in host responses to disease or infection. They act at hormonal concentrations through high affinity receptors whose binding constants range between10-12 and 10-10 M. In some cases only a few dozen receptors need to be activated per cell to elicit an effect. They are produced on demand and travel only over short distances. (Sommer and Kress, 2004)
Cytokines may act on the cells that secrete them (autocrine action), on nearby cells (paracrine action), or on distant cells (endocrine action). It is common for different cell types to secrete the same cytokine or for a single cytokine to act on several different cell types (pleiotropy). Cytokines are redundant in their activity, meaning similar functions can be stimulated by different cytokines. In addition to that they are often produced in a cascade, in other words one cytokine stimulates its target cells to create additional cytokines.
Cytokine Responses To Infection
Pain is associated with inflammation; nerve endings sense the swelling of local tissues, which guarantees inflammation. Inflammatory pain is characterized by an increased sensitivity to mechanical or heat stimuli of the affected tissue. Following tissue injury, an inflammatory response is generated by local macrophages and this is further amplified by migrating blood cells. The various inflammatory mediators as well as the tissue acidification act synergistically to induce and maintain the development of pain and hyperalgesia. (Sommer and Kress, 2009)
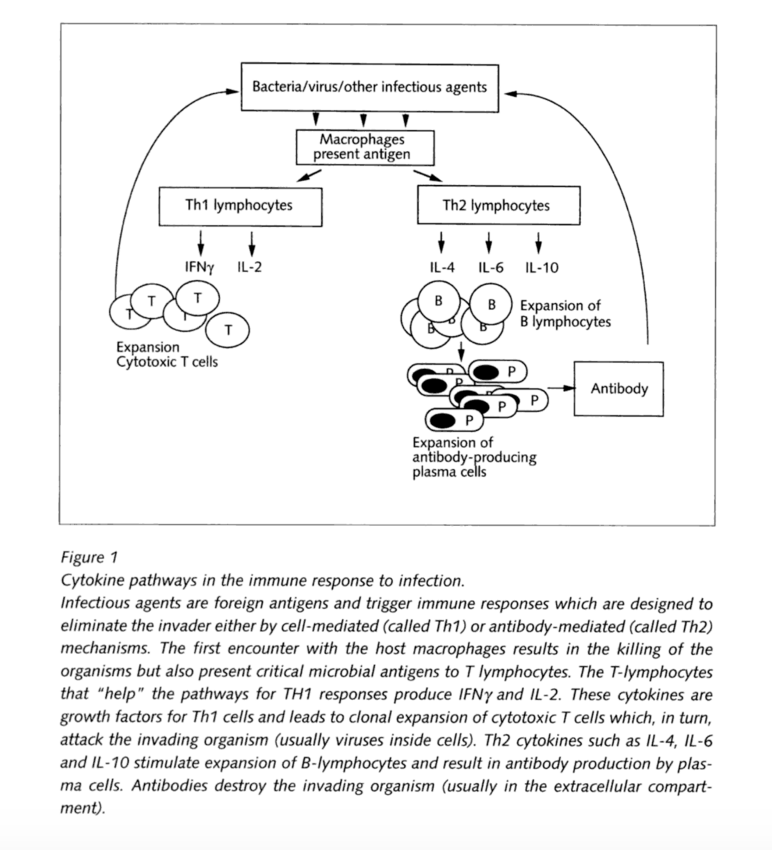
(Watkins and Maier)
The primarily used cytokines are the interleukins (IL), which indicate a product of a leukocyte and a target leukocyte cell. These types are usually associated with DNA sequences. There are 18 known cytokines of that kind (IL-1 till IL-18). They play a major role in inflammation, therefore an important role in relation to pain. Therefore some cytokines promote inflammation and are called pro-inflammatory cytokines, whereas others suppress the activity of pro-inflammatory cytokines and are called anti-inflammatory. Each type works along different kinds of cells. So IL-4 and IL-10 are potent activators of B-lymphocytes, which are responsible for antibody formation to a foreign antigen.
Cytokines that “help” promote B cells antibody formation are classified as T lymphocyte helper cell of the type 2 class (Th2); their counterpart are the cytokines that help the type 1 class (Th1). Th1 cytokines help in the process of cellular immunity where T lymphocytes attack and kill virus-infected cells. These include IL-2, IL-12, IL-18 and interferon–gamma (IFNgamma). It’s also important to mention that although IL-4, and IL-10 were both initially classified as pro-inflammatory cytokines, they are also considered anti-inflammatory for their ability to suppress genes of IL-1 and TNF, which are pro-inflammatory.
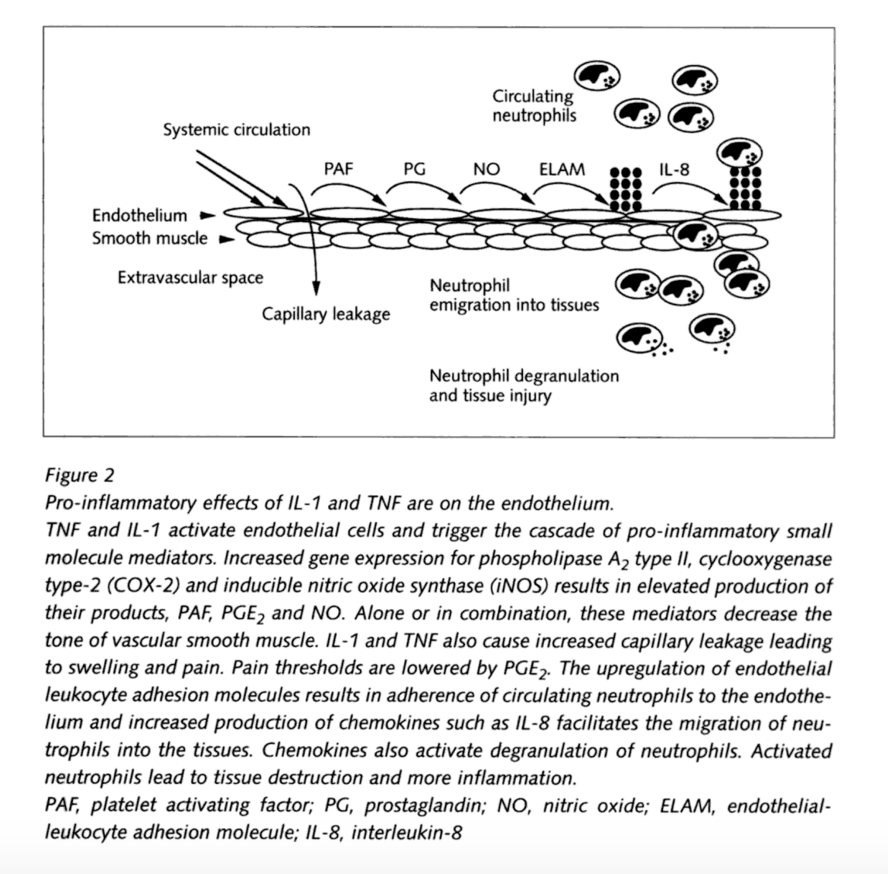
(Watkins and Maier)
Pro-inflammatory Cytokines and Pain
Neuropathic pain due to nerve injury is a prevalent condition of which syndromes include diabetic, cancer, ischemic neuropathies, and phantom limb pain. It arises when damaged or neighbouring undamaged axons are sensitized such that they induce nociceptive signaling in the central nervous system. Introduction of anti-inflammatory cytokines or the blockage of pro-inflammatory cytokines reduces hyperalgesia.
IL-1β
Interleukin-1beta is a pro-inflammatory cytokine that is produced and secreted under pathological conditions e.g. during neuropathies, tumor growth or in chronic inflammatory diseases like rheumatoid arthritis. They arise from many cell types, like mononuclear cells, fibroblasts, Schwann cells and endothelial cells. Their presence has been detected in the dorsal root ganglion neurons, which suggests possible autocrine or paracrine action on sensory processing. IL-1 also plays a central role in the generation of mechanical hyperalgesia. An experiment to prevent inflammatory hyperalgesia was performed on mice by the administration of IL-1 receptor antagonist and neutralizing antibodies to IL-1 receptors to reduce pain associated behavior. IL-1β was found to also increase the production of substance P and prostaglandin E2 (PGE2) in a number of neuronal and glial cells.
TNF-α
Tumor Nicrosis Factor - α, also known as cachectin, is another inflammatory cytokine that plays a key role in inflammatory and neuropathic hyperalgesia. TNF acts on several different signaling pathways through two cell surface receptors, TNFR1 and TNFR2 to regulate apoptotic pathways. TNF-α receptors are present in both neurons and glia. Intraplantar injection of complete Freund's adjuvant in adult rats resulted in significant elevation in the levels of TNF-α, IL-1β, and nerve growth factor (NGF) in the inflamed paw. A single injection of anti-TNF-α antiserum before the CFA significantly delayed the onset of the resultant inflammatory hyperalgesia and reduced IL-1β but not NGF levels. TNF binding protein (TNF-BP), an inhibitor of TNF, is a soluble form of a transmembrane TNF-receptor. When TNF-BP is administered systemically, the hyperalgesia normally observed is completely eliminated. (Zhang and An, 2009)
Citations
- Ann Biol Clin, “Proteins Of The Inflammatory Reaction.”
- Bennett, Michael I. (2010): OPML
- Caghan Kizil, Nikos Kyritsis, Michael Brand, “Effects Of Inflammation On Stem Cells”
- Claudia Sommer, Michaela Kress, “Recent Findings On How Pro-Inflammatory Cytokines Cause Pain: Peripheral Mechanisms In Inflammatory And Neuropathic Hyperalgesia”.
- Josef-Schneider-Strasse: Neurologische Universitätsklinik
- Jun-Ming Zhang, Jianxiong, “Cytokines, Inflammation and Pain”
- Linley JE, Rose K, Ooi L, Gamper N, “Understanding Inflammatory Pain: Ion Channels Contributing To Acute And Chronic Nociception”
- Payne KS1, Schilli K1, Meier K1, Rader RK2, Dyer JA3, Mold JW4, Green JA5, Stoecker WV6: Extreme pain from brown recluse spider bites: model for cytokine-driven pain.