Cancer therapy by inhibition of negative immune regulation
Contents
- Cancer therapy by inhibition of negative immune regulation
- 1. Introduction
- 2. Immune checkpoints in T-cells
- 3. The different immune checkpoints and their mechanisms
- 4. Immune checkpoint inhibitors in cancer therapy
- 5. The drawbacks of cancer therapy via negative immune response
- References
- Figures
1. Introduction
The most fundamental property of the immune system is to distinguish between "self" to "non-self" in order to recognize and eliminate invaders. Hence, for proper growth and development, tumor cells need to avoid detection. One of the mechanisms tumor cells use is negative immune regulation, for which James P. Allison and Tasuku Honjo won Nobel Prize in Physiology or Medicine in 2018.
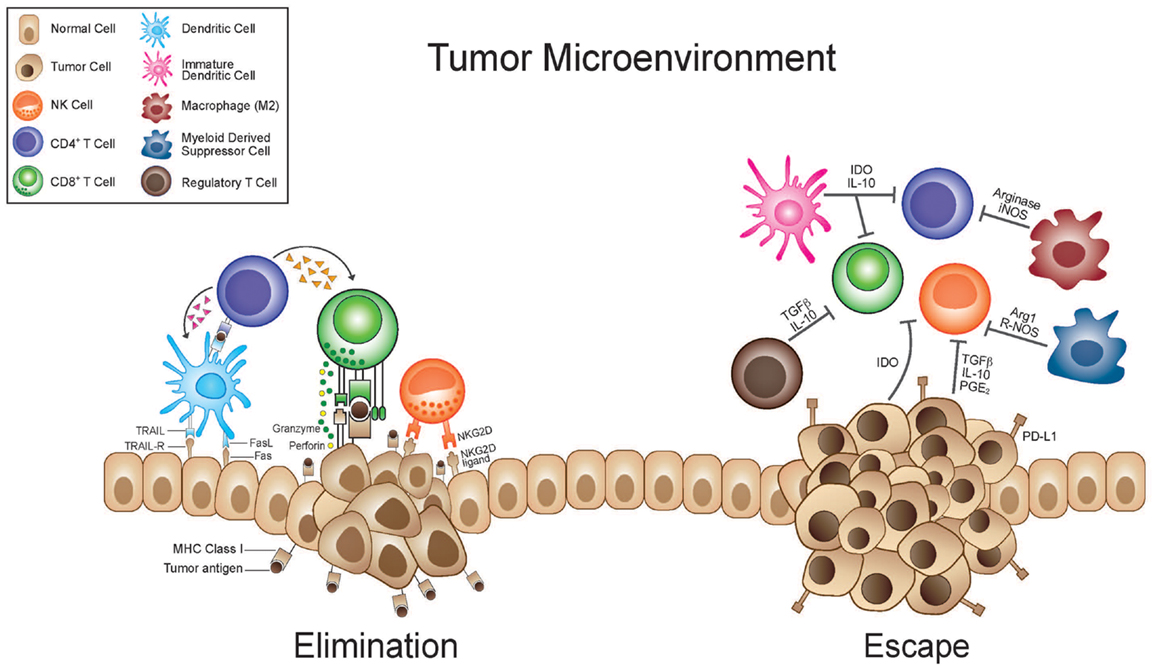
Negative immune regulation suppresses T-cell activation and by that, diminishes the immune system response to the tumor. By inhibiting this process, we promote stronger immune response and thus, causing the tumor to regress as seen in figure 1.
|
Figure 1: Growing tumour cells release inflammatory cytokines that activate several effector cells such as natural killer cells and T-cells. During the elimination phase, the recruited tumour-infiltrating NK cells and macrophages produce interleukin 12 which kills tumour cells by cytotoxic mechanisms. During escape phase of cancer, the immune system fails to prevent tumor outgrowth so tumor cells emerge, causing clinically apparent disease. CTLA-4 is the one of most studied immune check-point and its inhibitor was the first to be accepted as a treatment of cancer patients. Nowadays, increasing number of immune checkpoints inhibitors are tested and approved by the European Union (EU) and the Food Drug Administration (FDA) (Seidel et al, 2018).
2. Immune checkpoints in T-cells
The main function of T- cells is to recognize and react to external and internal antigens. External antigen, such as bacteria, will be recognized by the antigen-presenting cell's MHCII (cell membrane protein that helps to distinguish between self and non self). In the case of internal antigen, such as tumor cell, recognition occurs by the APC's MHCI. In order to prevent an autoimmune response, a mechanism called Negative immune regulation, or in its other name, immune checkpoints will be activated. Under physiological conditions, immune checkpoint maintain self-tolerance and limit collateral tissue damage when responding to microbial invaders. This mechanism can also be exploited by tumor cells to evade from the immune system (Topalian et el, 2015).
1. Elimination of tumors by T-cells
There are two known pathways by which T-cells can eliminate tumor cells. The first way is performed by cytotoxic T-cell which will directly kill the tumor cell by the release of cytotoxins and induced apoptosis due to FAS and FAS-ligand interaction. The second pathway is by CD4+ T cells that can recognize a tumor-specific peptide in the MHCII of APC's or the tumor cells themselves. Those tumor reactive CD4+ T-cells will develop cytotoxic activity like those of CD8+ T-cells, but they will have higher antitumor capabilities (Quezada et al, 2010).
2. How immune checkpoints suppress T cell activity
Naive T-cell activation occurs when T-cell receptor (TCR) recognizes an antigen presented by the major histocompatibility complex (MHC) of another cell. Recognition of MHC itself is not enough to initiate activation of the T-cell; effective co-stimulation of T-cell's receptor membrane, CD28, by CD80/86 on antigen presenting cells (APC's) must occur. This results in CTLA-4 upregulation on the T-cell membrane, which acts as a competitive inhibitor for the co-stimulators CD80 and CD86 to suppress T-cells activation, proliferation and effector functions (Pianko et el, 2017).
Another immune checkpoint ligand are PDL-1 and PDL-2 which are members of B7 family and are ligands for PD-1 which result in tremendous decreased proliferation and IL‐2 production (Carter et al, 2002).
3. Immune checkpoints and their ligands can occur at different stages of T-cell activation
Naïve and activated T-cells can be regulated by negative immune checkpoints in several different modes of expression of the immune checkpoints and their ligands. Most immune checkpoints will be expressed only after activation, such as CTLA4 and PD-1, there are some immune checkpoints that are continuously expressed and found in similar level in both naïve and activated T-cells (e.g. VISTA). Furthermore, immune checkpoints can be highly expressed in naïve T-cells but are downregulated upon their activation. LAIR-1 is an example to such an expression. HVEM has a special form of expression, in which in both naïve state and several days after activation it shows a high level of expressivity, but shortly after activation this immune checkpoint is downregulated (Śledzińska et el, 2015).
3. The different immune checkpoints and their mechanisms
|
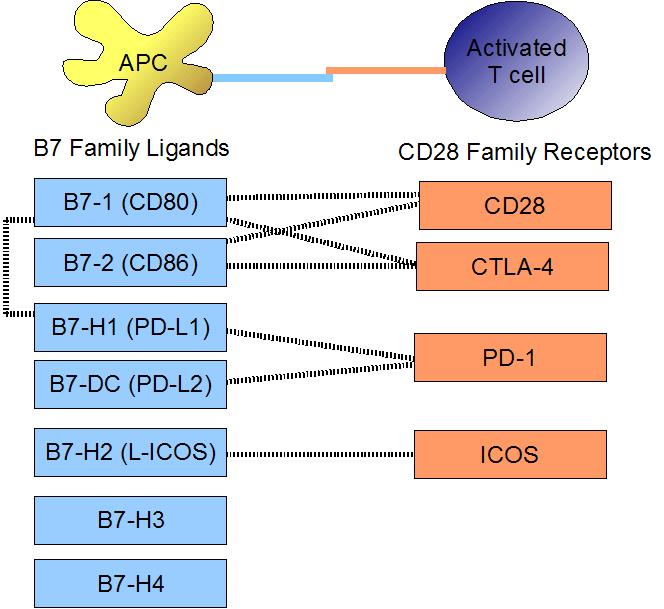
Figure 2 : Binding of costimulatory molecules on APC's and T-cells, B7 family ligands and their interactions with CD28 family receptors.
Immune checkpoints have different ligands and can prevent the T-cell from functioning through multiple ways.
1. CTLA-4
1. How CTLA-4 inhibits T-cell function
CTLA-4 is an inhibitory receptor which supresses T-cell responses as seen in figure 2(Linsley et al, 1991).
For the activation of T-cell, we need two signals: firstly the recognition of an antigen presented by MHC and secondly the binding of CD28 to CD80 or CD86 expressed by APC's.
The structure of CTLA-4 is similar to the structure of CD28, hence CTLA-4 binds to CD80 or CD86. This binding occurs at an even higher affinity than for CD28 (Der Merwe et al, 1997), most likely due to the fact that CTLA-4 reduces CD28 binding affinity to CD80 or CD86 (Alegre et al, 2001 and Mastellar et al, 2000). The binding of CTLA-4 to APCs supresses T-cell responses.
Other ways to supress T-cell activation are by the inhibition of TCR and CD28-induced genes, by the reduction in CD25 expression or by an increase in T-cell motility (Schneider et al, 2006 and Krummel et al, 1996).
2. The dual function of CTLA-4
CTLA-4 is constitutively expressed on Treg cells but can also be expressed on effector T-cells after their activation (Alegre et al, 2001 and Takahashi et al, 2000). However, Treg cells express CTLA-4 on their cell surface whereas activated T-cells express CTLA-4 intracellularly. This difference of expression leads to a dual function of CTLA-4. CTLA-4 expressed on Treg cells lead to the suppression of excessive T-cell responses, while CTLA-4 expressed intracellularly on activated T-cells prevent tissue damage by self-reactive pathogenic T-cells (Jago et al, 2004).
3. CTLA-4 targeted in treatment of cancer
It has been shown that high CTLA-4 expression on T-cells is crucical for the suppressive function of Treg cells (Wing et al, 2008). Therefore, blocking CTLA-4 could prevent Treg cells from being inactivated thus promoting antitumor immunity. This has been demonstrated in vivo in a mouse model for melanoma, where it resulted in enhanced effector T-cell function (Peggs et al, 2009).
2. PD-1
1. PD-1 and its ligands PD-L1 and PD-L2
PD-1 is another inhibitory receptor. When PD-1 of a T-cell binds to a ligand found on tumor cells, it leads to the suppression of the T-cell’s function.
PD-1 has two ligands named PD-L1 and PD-L2 (Latchman et al, 2001 and Freeman et al, 2000). Those ligands are expressed by many different cell types, including tumor cells, myeloid cells and T-cells. PDL-1 and PDL-2 are both ligands of PD-1, however they have two different patterns of expression. PDL-1 is constitutively expressed at low levels on antigen-presenting cells (APCs) whereas PD-L2 expressed on lung dendritic cells and has an important role in pulmonary tolerance (Boussiotis, 2017).
PD-1 is expressed on T-cells but also on B cells and myeloid cells even though its role is best seen in T-cells. Its expression on T-cell is induced by antigen stimulation (Pauken et al, 2015).
While CTLA-4 limits early T-cell activation, PD-1 on the other hand has inhibitory effect on T-cells in the periphery, where T-cells encounter PD-1 ligands (Toplian et al, 2012). However, not all the cell types expressing PD-L1 are capable of inhibiting T-cells through PD-1 ligation (Iraolagoitia et al, 2016).
2. How PD-1 inhibits T-cell function
PD-1 ligation inhibits T-cell activation, but only upon TCR engagement. PD-1 has two motifs: an intracellular immunoreceptor tyrosine-based inhibition motif (ITIM) and an immunoreceptor tyrosine-based switch motif. PD-1 ligation leads to the recruitment of the phosphatases src domain, containing tyrosine phosphatase SHP-1 and SHP-2, to the immunoreceptor tyrosine-based switch motif (Chemnitz et al, 2004).
SHP-2 affects T-cell function in various ways. SHP-2 interferes with TCR downstream signaling by preventing protein kinase 70 and CD3 phosphorylation (Sheppard et al, 2004).
Moreover, PD-1 ligation interferes with signaling molecules which are important for T-cell proliferation, cytokine secretion and metabolism (Pauken et al 2015 and Patsoukis et al, 2012). Triggering of PD-1–mediated signals inhibited PI3K–Akt activation, which leads to inhibition of T-cell proliferation and differentiation. Another consequence of triggering PD-1 is a suppression of interleukin-2 production. IL-2 is a cytokine which acts as a co-stimulator for T-cells activation (Boussiotis, 2017).
Ligation of PD-1 can also induce metabolic alterations in T-cells. Enhanced expression of inhibitory receptors results in mitochondrial dysfunction of T-cells (Scharping et al, 2012). Moreover, PD-1 leads to induced defects in mitochondrial respiration and glycolysis, leading to impaired T-cell effector function. In addition to inhibiting effector T-cell responses, PD-1 – PD-L1 connection can promote the induction of Treg cells (Bengsh et al, 2016).
3. Preclinical studies with PD-1 and PD-L1
There is increasing evidence from animal models and clinical data that PD-1 plays a crucial role in limiting T-cell reponses against tumor cells. An analysis of twelve different epithelial cancers revealed significantly shorter survival for patients with lymphocytes expressing PD-1 (Zhang et al, 2015).
Aberrant PD-L1 expression on tumor cells has been associated with immune escape in multiple cancers. This means that PD-L1 structural differences contribute to tumor development (Kataoka et al, 2016).
In some diseases, like melanoma, RCC and hepatocellular carcinoma, the expression of PD-L1 correlates with worse prognosis (Massi et al, 2014; Leite et al, 2015 and Jung et al, 2015).
In other diseases, no correlation exists. In some cases, such as for colorectal cancer patients and breast cancers, PD-L1 expression has even been correlated with better survival (Dunne et al, 2016). In those latter cases, the expression of PD-L1 might be due to enhanced antitumor immune responses, associated with improved patient prognosis (Dunne et al, 2016).
There is little evidence of a correlation between PD-L2 expression and cancer prognosis, mainy because PD-L1 expression on tumors is more prevalent than PD-L2 expression. No correlation was found, for example in breast cancer patients (Baptista et al, 2016).
3. Other immune checkpoints
1. LAG-3 and TIM-3 immune checkpoints
LAG-3, TIM-3 and TIGIT are other immune checkpoints that are being targeted in cancer immunotherapy. CD4+ binding to MHC class II activates T-cells. However, LAG-3 binds to MHC class II with higher affinity than CD4+ therefore reducing T-cell activation (Huard et al, 1995). As LAG-3 expression is also associated with deterioration of CD8+ and NK cell function, this suggests that there may exist additional LAG-3 ligands (Anderson et al, 2016).
TIM-3 can be expressed by T-cells, NK cells and myeloid cells. Binding of galectin-9 to TIM-3 on T-cells induces cell death (Kang et al, 2015).
High motility group box 1 is a cell marker triggering innate cell responses by binding to certain receptors. The binding of TIM-3 to this cell marker prevents the activation of innate cells (Chiba et al, 2012).
2. PD-1 of NK cells and TIGIT immune checkpoints
NK cells express immune checkpoints as well especially PD-1. Because of this, their protective role in early immune responses against cancer may be compromised. For example in patients with multiple myeloma (Benson et al, 2010) or infected with HIV (Norris et al, 2012) or tuberculosis (Alvarez et al, 2010), the expression of PD-1 is enhanced on NK cells. NK-cells express the transmembrane glycoprotein CD226. This glycoprotein promotes NK-cell function and cytotoxicity when binding to its ligand CD155 (Bottino et al, 2003). On activated NK-cells, the immune checkpoint TIGIT is upregulated and binds to CD155 at a higher affinity than the glycoprotein CD226. This binding therefore inhibits the CD226-mediated activation (Stanietsky et al, 2013).
TIM-3 is an exhaustion marker on NK cells (Da Silva et al, 2014). Patients with melanoma have NK cells that overexpress TIM-3 and are functionally impaired. This can be reversed by treatment with anti-TIM-3 antibody (Da Silva et al, 2014).
4. Immune checkpoint inhibitors in cancer therapy
1. Aims of immune checkpoint inhibitors
Immune checkpoint inhibitors have been licensed for use in humans with cancers, especially melanoma. These inhibitors are mostly monoclonal antibodies against immune checkpoints or immune cells and block the interaction with the resepctive checkpoint ligands. The primary aim of immune checkpoint blockade is to reduce suppression of effector T-cells and thereby improve tumor-specific immune responses. In addition, immune checkpoint blockade can increase the effectiveness of other immunotherapeutic approaches such as cancer vaccines, by ending T-cell suppression and thus enhancing T-cell function. For most of these inhibitory receptors, antibodies have been developed and tested in clinical models. Some have even been licensed for use in humans (Ai and Curran, 2015).
2. CTLA-4 inhibitor: ipilimumab
CTLA-4 was the first immune checkpoint targeted in treatment of cancer. Blocking of CTLA-4 reduced tumor growth in mouse models of melanoma, colon carcinoma and many others (Elsas et al, 1999 and ). The efficacity of CTLA-4 therapy in models is associated with increased T-cell infiltration into tumors (Duraiswamy et al, 2013).
Antibodies targeting CTLA-4 include ipilimumab. It is the first checkpoint inhibitor to be approved by the US food and drug administration (FDA) in 2011. It is approved for treatment of metastatic melanoma and has shown great success in patients, increasing the survival by ten months (Hodi et al, 2010) and survival up to ten years in some melanoma patients (Ribas et al, 2015).
The combination of ipilimumab and dacarbazine even lead to between one- and three-years survival compared with the use of dacarbazine alone (Robert et al, 2011).
Ipilimumab has also been tested in other diseases including NSCLC, renal cancer and prostate cancer.
3. PD-1 and PD-L1 inhibitors
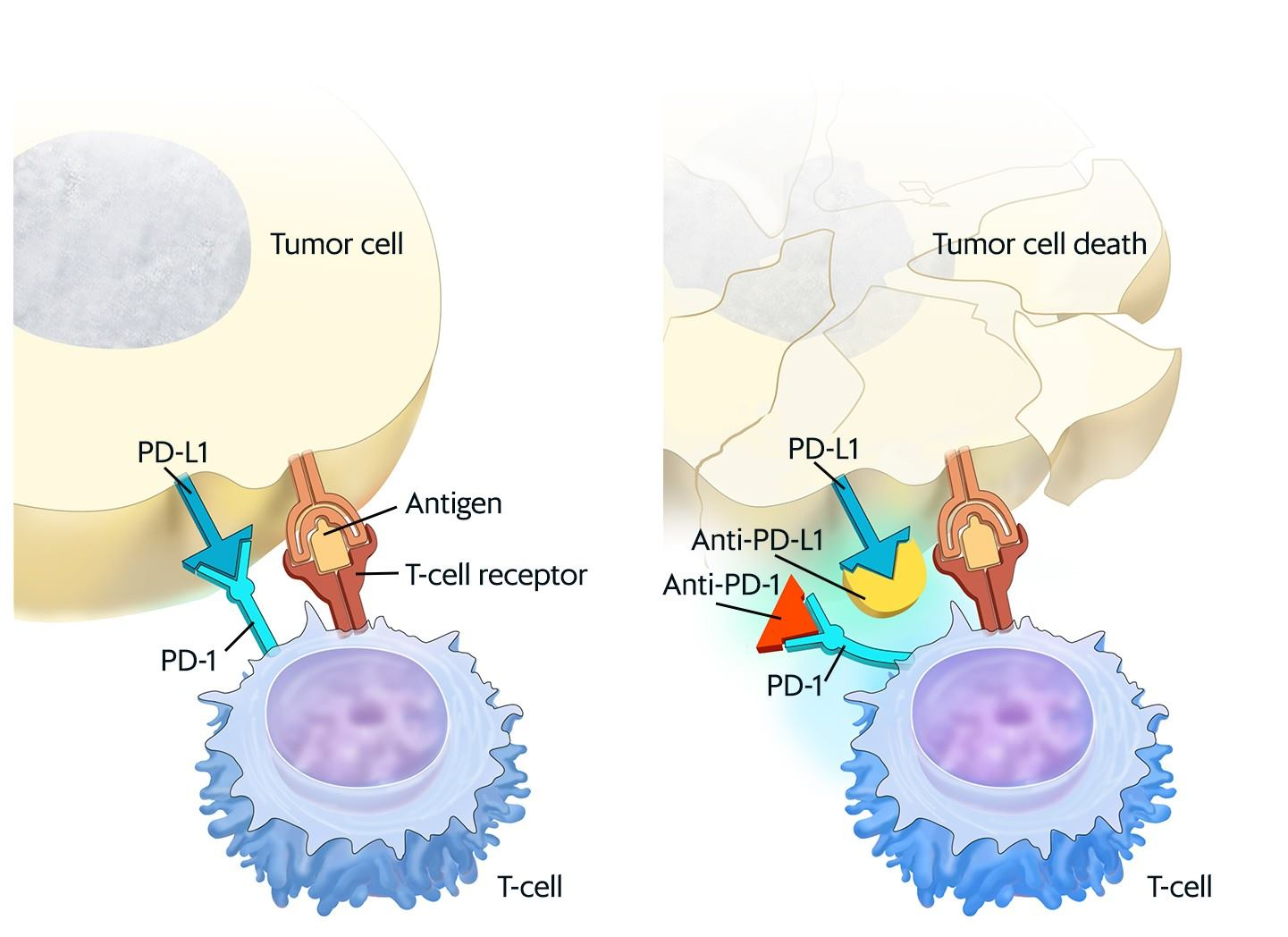
Studies with antibodies blocking PD-1 or PD-L1 demonstrated a reduction in tumor growth in several murine tumor models as seen in figure 3. Those studies showed that protection was associated with increased CD8 T-cell infiltration and function (Duraiswamy et al, 2013).
In addition, the ratio of CD8+ T cells to Treg cells was elevated when PD-1 was blocked (Curran et al, 2010). Furthermore, blocking PD-1 enhanced T-cell infiltration into tumors by increasing the secretion of chemokines (Peng et al, 2012).
PD-1 and PD-L1 blocking antibodies have been tested in the clinic and have shown remarkable responses, especially in case of patients with melanoma and NSCLC. To date, three antibodies targeting the PD-1 : PD-L1 axis have been approved by the FDA. The first antibody is pembrolizumab, approved in September 2014 as first-line treatment for melanoma (Robert et al, 2014). It is now also approved for NSCLC (Garon et al, 2015).
A second PD-1- blocking antibody, called nivolumab, has been approved for the treatment of melanoma, NSCLC and even renal cancer.
Finally, atezolizumab, an anti-PD-L1, has been approved by the FDA in May 2016 for the treatment of bladder cancer (No authors listed, American Association for Cancer Research, 2016).
Anti-PD-1 therapy has also shown some efficacy against head and neck, breast, ovarian and gastric cancers (Callahan et al, 2016).
The combination of ipilimumab (anti-CTLA-1) and nivolumab (anti-PD-1) is more effective than either treatment alone (Larkin et al, 2015).
|
Figure 3 : Checkpoint proteins, such as PD-L1 on tumor cells and PD-1 on T-cells, help keep immune responses in check.For the National Cancer Institute © 2015 Terese Winslow LLC, U.S. Govt. has certain rights.
4. LAG-3, TIM-3 and TIGIT inhibitors
Immune checkpoint inhibition has progressed to other targets, with recent studies and trials using inhibitors that target the immune checkpoints LAG-3, TIM-3 and TIGIT. Studies using mouse models demonstrated significant reduction in tumor growth using blocking antibodies against LAG-3 and TIM-3 (Sakuishi et al, 2010).
Two trials with anti-TIM-3 antibodies are currently ongoing in malignancies and metastatic tumors, while anti-LAG-3 is being tested in melanoma, renal cancer and breast cancer. No clinical trials have yet evaluated TIGIT-targeted therapies. These studies are still in progress and the next years will show whether these novel immune checkpoint inhibitors are able to compete with PD-1-blocking antibodies (Woo et al, 2012).
5. Immune checkpoint inhibitor combination therapies
Combined treatment with ipilimumab and nivolumab induced better responses than either treatment alone when curing cancers. Now, several combination strategies are under investigation (Larkin et al, 2015).
Since there are nonredundant mechanisms, different checkpoint inhibitors can work synergistically. This has been shown for combinations of anti-PD-1 with anti-CTLA-4, anti-TIM-3 or anti-LAG-3 (Duraiswamy et al, 2013).
Moreover, combinations of immune checkpoint blockade with immune activators such as cancer vaccines could be particularly favourable for patients with tumors lacking T-cell infiltration. Blocking PD-1 and CTLA-4 significantly improved the efficacy of a certain vaccine (Curran et al, 2010).
Similarly, combination of a CT26 vaccine with anti-PD-L1 and anti-CTLA-4 significantly reduced tumor growth in the CT26 tumor model (Duraiswamy et al, 2013). Moreover, the addition of either anti-CTLA-4 or anti-PD-1 improved the efficacy of a vaccine comprising tumor lysates, GM-CSF and CpG in the B16 tumor model. The inclusion of anti-PD-1 or anti-CTLA-4 significantly elevated the numbers of infiltrating CD8+ T cells compared with mice that were treated with the vaccine alone (Ali et al, 2016).
Immune checkpoint blockade has also been shown to improve the efficacy of therapies that do not directly target the immune system, such as radiotherapy and targeted cancer therapies (Shi et al, 2016). Very promising results were achieved with combinations of immune checkpoint blockade and histone deacetylase inhibitors (HDACi). Consequently, the addition of anti-PD-1 significantly improved HDACi-induced reduction in tumor growth (Woods et al, 2015). Moreover, it has been suggested that HDACi enhances therapeutic effects of PD-1 blockade by stimulating the secretion of T-cell attracting chemokines into the tumor microenvironment (Zheng et al, 2016).
5. The drawbacks of cancer therapy via negative immune response
1. Biggest obstacle to antitumor immunity by immunotherapy: the tolerance to antitumor immune responses
There are various obstacles to antitumor immunity by immunotherapy which leads to the tumor avoiding recognition by the immune system, primarily by CD8+ T-cells, thus escaping elimination.
1. Downregulation of MHC class I expression on tumor cells surface
The first obstacle is the downregulation of the MHC class I molecular expression on the tumor cell surface. One of the main problems facing any type of cancer treatment is the widespread heterogeneity of the primary tumors, which results in epigenetic and genetic modification at a colonel level. Heterogenicity is created by genetic modifications in a random process which can cause the downregulation of MHC-I tumor cells, thus masking themselves from the CD8+ T-cells. Therefore, the communication with the host immune system determines the capacity of a given tumor clone to survive. The process which mediated the interaction between MHC class I and CD8+ T cells take place in early stages in the primary tumors (Garrido et al, 2016).
2. Creation of an immunosuppressive microenvironment
Another hindrance is the creation of an immunosuppressive environment. As stated by Santarpia and Karachaliou (2015), adaptive immune response mainly IFN-γ-secreting T cells are utilized as a core function in tumor immune surveillance. However, tumors can escape this surveillance and maintain an immunosuppressive microenvironment through several mechanisms which include recruitment of regulatory cells and production of molecules which suppress antitumor T-cell responses.
Moreover, Baginska et al (2013) stated that hypoxic tumor cells can activate resistance mechanisms to create an immunosuppressive microenvironment. Due to their ability to produce cytokines such as tumor necrosis factor (TNF)-α and stromal cell-derived factor 1 (SDF-1), hypoxic tumor cells encourage the formation of bone marrow-derived CD45+ myeloid cells to tumor areas. The invasion of myeloid cells is a highly immunosuppressive factor for NK cells. Myeloid-derived suppressor cells (MDSCs) are one of the chief sections of the immune-suppressive network responsible for the damage of NK cell- and T cell-dependent anti-cancer immunity. It has been discussed that cancer-expanded MDSCs induce anergy of NK cells by inhibiting cytotoxicity, NKG2D expression, and IFN-γ production through membrane-bound transforming growth factor (TGF)-β.
3. Treg-mediated T-cell suppression
|
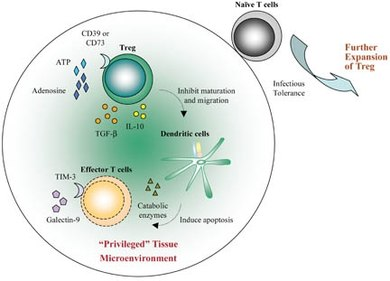
Figure 4: Treg migrate to the grafted tissue. Activated Treg convert ATP released by inflamed tissues to adenosine via the ectoenzymes CD39 and CD73. Local adenosine could contribute to the initial “privileged” microenvironment. Treg also secrete TGF-β and IL-10 which inhibit the maturation and migration of dendritic cells (DC). These “decommissioned” DC can secrete catabolic enzymes for depletion of essential amino acids (EAA) and, therefore, induce apoptosis of effector T cells. Moreover, the “privileged” tissues could release pro-apoptotic galectin-9 which binds to TIM-3 expressed by effector T cells such as Th1 and Th17. This further amplifies the immunosuppressive microenvironment. Each of these components within the graft can further reinforce the local anti-inflammatory state such that any naive T cell, migrating into this area, will be converted to induced Treg (iTreg) through infectious tolerance. The iTreg then expand and further suppress immunity in the “privileged” microenvironment.
Another barrier is the Treg-mediated T suppression. Schmidt et al (2012) stated that CD4+CD25highFoxp3+ regulatory T cells (Tregs) can repress other immune cells and therefore are key mediators of the peripheral self-tolerance. Conversely, Tregs prevent autoimmune diseases and allergy. They also inhibit immune reaction against tumors and pathogens. They can also prevent proliferation and cytokine production of CD4+CD25 conventional T cells (Tcons). When there is cell to cell contact, Tregs prevent TCR-induced proliferation and IL-2 transcription of Tcons. Moreover, Sojka, et al (2008) suggested that suppressive mechanisms can be separated into 3 categories: cell to cell contact, local secretion of inhibitory cytokines and local competition of growth factors. A noteworthy inhibitory pathway to enter the Treg field is the alteration of cyclic adenosine monophosphate(cAMP) levels in the target cells. A rise in the cAMP levels has been extensively associated with prevention of cellular proliferation and differentiation, even in lymphocytes which cause selective prevention of cytokine gene expression, which include IL-2and interferon-γ.
Charles G. Drake et al (2006) proposed that tumor cells or tumor- infiltrating macrophages secrete a chemokine along with some small cytokines namely CCL22 which chemoattracts CD4+CD25+ Tregs as seen in figure 4. However, it is also likely that the immunosuppressive milieu of the tumor which has a high concentration of TGF-β, induces tumor infiltrating CD4+ T cells which then become FoxP3+ Tregs. Regular Tregs or induced Tregs usually prevent antitumoral immune responses by inhibiting tumor-specific CD4+ cells and CTLs.
2. The side effect of checkpoint inhibitors
Immune checkpoints inhibitors improve immunological antitumor activity by breaking down tumor immune tolerance. One of the organs which is affected by the treatment is the thyroid. The effect by anti CTLA4-mAbs on the thyroid usually manifests as thyroiditis, which is associated with antithyrogloulin, anti-thyro peroxidase antibody positivity and hyperthyroidism (Salvatore et al, 2013). Even though checkpoint inhibitors are effective when it comes to the treatment of cancer, they might have substantial and recurrent side effects. The side effect usually include autoimmune diseases which include colitis, skin disorder and hepatitis. The pathway of CTLA-4 and PD-1 also encourage comparable effects. When treated with pembrolizumab there were less recurrent high-grade effect than when patients were treated with ipilimumab (Dyck et al, 2017).
References
Anderson, A. C.; Joller, N. and Kuchroo, V. K(2016). Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation . Immunity. 44: (5) 989– 1004.
Alegre, M. L.; Frauwirth, K. A. and Thompson, C. B.(2001):T-cell regulation by CD28 and CTLA‐4. Nat. Rev. Immunol.1: (3) 220– 228.
Ai, M. and Curran, M. A.(2015). Immune checkpoint combinations from mouse to man. Cancer Immunol. Immunother.64: (7) 885– 892
Ali, O. A.; Lewin, S. A.; Dranoff, G. and Mooney, D. J.(2016). Vaccines combined with immune checkpoint antibodies promote cytotoxic T‐cell activity and tumor eradication. Cancer Immunol. Res. 4: (2) 95– 100.
Alvarez, I. B.; Pasquinelli, V.; Jurado, J. O.; Abbate, E.; Musella, R. M.; la Barrera, S. S. and Garcia, V. E.(2010). Role played by the programmed death‐1‐programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J. Infect. Dis. 202: (4) 524– 532.
Baginska, J.; Viry, E.; Pagetti, J.; Medves, S.; Berchem, G.; Mousay,E.; Janji, B.(2013). The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Journal of Frontier Immunology.
Baptista, M. Z.; Sarian, L. O.; Derchain, S. F.; Pinto, G. A. and Vassallo, J. (2016). Prognostic significance of PD‐L1 and PD‐L2 in breast cancer. Hum. Pathol. 47: (1) 78– 84.
Bengsch, B.; Johnson, A. L.; Kurachi, M.; Odorizzi, P. M.; Pauken, K. E.; Attanasio, J.; Stelekati, E. et al (2016). Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD‐1 are an early driver of CD8(+) T cell exhaustion. Immunity. 45: (2) 358– 373.
Benson, D. M.; Bakan, C. E.; Mishra, A.; Hofmeister, C. C.; Efebera, Y.; Becknell, B.; Baiocchi, R. A. et al (2010). The PD‐1/PD‐L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT‐011, a novel monoclonal anti‐PD‐1 antibody. Blood. 116: (13) 2286– 2294.
Boussiotis V. A. (2017): Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England Journal Of Medicine 375: (18) 1767–1778
Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C. et al (2003). Identification of PVR (CD155) and Nectin‐2 (CD112) as cell surface ligands for the human DNAM‐1 (CD226) activating molecule. J. Exp. Med.198: (4) 557– 567.
Callahan, M. K.; Postow, M. A. and Wolchok, J. D.(2016). Targeting T cell co‐receptors for cancer therapy. Immunity. 44: (5) 1069– 1078.
Carter L. L.; Fouser L. A.; Jussif J.; Fitz L.; Deng B.; Wood C. R.; Collins M.; Honjo T.; Freeman G. J.; Carreno B. M. (2002): PD‐1:PD‐L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL‐2. European Journal Of Immunology 32: (3) 634-643
C.G.; Jaffee, E.; Pardoll, D. M.(2006): Mechanisms of Immune Evasion by Tumors..Journal of advances in Immunology 90: 51-81
Chen L.; Dallas F. B. (2013): Molecular mechanisms of T cell co-stimulation and co-inhibition. Natureserch journal 13: (4) 227-242
Chemnitz, J. M.; Parry, R. V.; Nichols, K. E.; June, C. H. and Riley, J. L.(2004): SHP‐1 and SHP‐2 associate with immunoreceptor tyrosine‐based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol.173: (2) 945– 954.
Cheng, W. F.; Hung, C. F.; Lin, K. Y.; Ling, M; Juang, J; He, L; Lin, C. T.; Wu, T-C. (2003): CD8+ T cells, NK cells and IFN-g are important for control of tumor with downregulated MHC class I expression by DNA vaccination. Gene Therapy 10: (16) 1311–1320.
Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka‐Akita, H.; Fujioka, Y. et al(2012): Tumor‐infiltrating DCs suppress nucleic acid‐mediated innate immune responses through interactions between the receptor TIM‐3 and the alarmin HMGB1. Nat. Immunol.13: (9) 832– 842.
Curran, M. A.; Montalvo, W.; Yagita, H. and Allison, J. P.(2010): PD‐1 and CTLA‐4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 107: (9) 4275– 4280.
Da Silva, I. P.; Gallois, A.; Jimenez‐Baranda, S.; Khan, S.; Anderson, A. C.; Kuchroo, V. K.;Osman, I. et al.(2014): Reversal of NK‐cell exhaustion in advanced melanoma by Tim‐3 blockade. Cancer Immunol. Res. 2: (5) 410– 422.
Der Merwe, P. A.; Bodian, D. L.; Daenke, S.; Linsley, P. and Davis, S. J.(1997): CD80 (B7‐1) binds both CD28 and CTLA‐4 with a low affinity and very fast kinetics. J. Exp. Med. 185: (3) 393– 403.
Dunne, P. D.; McArt, D. G.; O'Reilly, P. G.; Coleman, H. G.; Allen, W. L.; Loughrey, M.; Schaeybroeck, S. et al .(2016) : Immune‐derived PD‐L1 gene expression defines a subgroup of stage II/III Colorectal Cancer patients with favorable prognosis who may be harmed by adjuvant chemotherapy. Cancer Immunol. Res. 4: (7) 582– 591.
Duraiswamy, J.; Kaluza, K. M.; Freeman, G. J. and Coukos, G. (2013): Dual blockade of PD‐1 and CTLA‐4 combined with tumor vaccine effectively restores T‐cell rejection function in tumors. Cancer Res. 73: (12) 3591– 3603.
Duraiswamy, J.; Freeman, G. J. and Coukos, G. (2013): Therapeutic PD‐1 pathway blockade augments with other modalities of immunotherapy T‐cell function to prevent immune decline in ovarian cancer. Cancer Res. 73: (23) 6900– 6912.
Dyck, L,; Mills, K.H.G. Immmune checkpoints and their inhibition in cancer and infectious diseases (2017). European journal of Immunology 47: (5)
Ebert, P. J.; Cheung, J.; Yang, Y., McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S. E. et al.(2016): MAP Kinase inhibition promotes T cell and anti‐tumor activity in combination with PD‐L1 checkpoint blockade. Immunity. 44: (3) 609– 621.
Elsas, A.; Hurwitz, A. A. and Allison, J. P.(1999): Combination immunotherapy of B16 melanoma using anti‐cytotoxic T lymphocyte‐associated antigen 4 (CTLA‐4) and granulocyte/macrophage colony‐stimulating factor (GM‐CSF)‐producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190: (3) 355– 366.
No authors listed, American Association for cancer research: First‐line atezolizumab effective in bladder cancer. Cancer Discov. 2016. 8
Freeman, G. J.; Long, A. J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L. J. et al. (2000): Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192: (7) 1027– 1034
Garrido, F; Aptsiauri, N; Doorduijn, E. M.; Garcia Lora, A. M.; Thorbald van Hall (2016): The urgent need to recover MHC class I in cancers for effective immunotherapy. Current Opinion in Immunology 39: 44–51.
Garon, E. B.; Rizvi, N. A.; Hui, R.; Leighl, N.; Balmanoukian, A. S.; Eder, J. P.; Patnaik, A. et al.(2015): Pembrolizumab for the treatment of non‐small‐cell lung cancer. N. Engl. J. Med. 372: (21) 2018– 2028.
Grosso, J. F.; Kelleher, C. C.; Harris, T. J.; Maris, C. H.; Hipkiss, E. L.; Marzo, A.; Anders, R. et al.( 2007): LAG‐3 regulates CD8+ T cell accumulation and effector function in murine self‐ and tumor‐tolerance systems. J. Clin. Invest.117: (11) 3383– 3392.
Hodi, F. S.; O'Day, S. J.; McDermott, D. F.; Weber, R. W.; Sosman, J. A.; Haanen, J. B.; Gonzalez, R. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010. 363: (8) 711– 723.
Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D. and Triebel, F.(1995): CD4/major histocompatibility complex class II interaction analyzed with CD4– and lymphocyte activation gene‐3 (LAG‐3)‐Ig fusion proteins. Eur. J. Immunol. 25: (9) 2718– 2721.
Iraolagoitia, X. L.; Spallanzani, R. G.; Torres, N. I.; Araya, R. E.; Ziblat, A.; Domaica, C. I.; Sierra, J. M. et al.(2016): NK cells restrain spontaneous antitumor CD8+ T cell priming through PD‐1/PD‐L1 interactions with dendritic cells. J. Immunol. 197:(3) 953– 961.
Jago, C. B.; Yates, J.; Camara, N. O.; Lechler, R. I. and Lombardi, G.(2004): Differential expression of CTLA‐4 among T cell subsets. Clin. Exp. Immunol. 136: (3) 463–471.
Jung, H. I.; Jeong, D.; Ji, S.; Ahn, T. S.; Bae, S. H.; Chin, S.; Chung, J. C. et al.(2016): Overexpression of PD‐L1 and PD‐L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. Treat. 49: (1) 246– 254.
Kang, C. W.; Dutta, A.; Chang, L. Y.; Mahalingam, J.; Lin, Y. C.; Chiang, J. M.; Hsu, C. Y. et al.(2015): Apoptosis of tumor infiltrating effector TIM‐3+CD8+ T cells in colon cancer. Sci. Rep. 5: 15659
Kataoka, K.; Shiraishi, Y.; Takeda, Y. Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T. et al.(2016): Aberrant PD‐L1 expression through 3'‐UTR disruption in multiple cancers. Nature. 534: (7607) 402– 406
Krummel, M. F. and Allison, J. P.(1996): CTLA‐4 engagement inhibits IL‐2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med.183: (6) 2533– 2540.
Latchman, Y.; Wood, C. R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y. et al.(2001): PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat. Immunol. 2: (3) 261– 268.
Larkin, J.; Chiarion‐Sileni, V.; Gonzalez, R.; Grob, J. J.; Cowey, C. L.; Lao, C. D.; Schadendorf, D. et al.(2015): Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373: (1) 23– 34.
Leite, K. R.; Reis, S. T.; Junior, J. P.; Zerati, M.; Gomes Dde, O.; Camara‐Lopes, L. H. and Srougi, M.(2015): PD‐L1 expression in renal cell carcinoma clear cell type is related to unfavorable prognosis. Diagn. Pathol. 10: 189.
Linsley, P. S.; Brady, W.; Urnes, M.; Grosmaire, L. S.; Damle, N. K. and Ledbetter, J. A.(1991): CTLA‐4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 174: (3) 561– 569.
Massi, D.; Brusa, D.; Merelli, B.; Ciano, M.; Audrito, V.; Serra, S.; Buonincontri, R. et al.(2014): PD‐L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann. Oncol. 25: (12) 2433– 2442.
Masteller, E. L.; Chuang, E.; Mullen, A. C.; Reiner, S. L. and Thompson, C. B.(2000): Structural analysis of CTLA‐4 function in vivo. J. Immunol. 164: (10) 5319– 5327.
Ngiow, S. F.; Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M. W. and Smyth, M. J.(2011): Anti‐TIM3 antibody promotes T cell IFN‐gamma‐mediated antitumor immunity and suppresses established tumors. Cancer Res. 71: (10) 3540– 3551.
Norris, S.; Coleman, A.; Kuri‐Cervantes, L.; Bower, M.; Nelson, M. and Goodier, M. R.(2012): PD‐1 expression on natural killer cells and CD8(+) T cells during chronic HIV‐1 infection. Viral Immunol. 25: (4) 329– 332.
Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L. and Boussiotis, V. A.(2012): Selective effects of PD‐1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5: (230) ra46.
Pianko M. J.; Liu Y.; Bagchi S.;Lesokhin A. M.(2017): Immune checkpoint blockade for hematologic malignancies: a review. Stem Cell Investigation Journal 4: (32)
Sakuishi, K.; Apetoh, L.; Sullivan, J. M.; Blazar, B. R.; Kuchroo, V. K. and Anderson, A. C.(2010): Targeting Tim‐3 and PD‐1 pathways to reverse T cell exhaustion and restore anti‐tumor immunity. J. Exp. Med. 207: (10) 2187– 2194.
Salvatore, M. C.; Barnabei, A; Marchetti, P; De Vecchis, L; Salvatori, R ; Torino, F.(2013): Endocrine Side Effects Induced by Immune Checkpoint Inhibitors. The Journal of Clinical Endocrinology & Metabolism, Volume 98: (4) 1361–1375.
Santarpia, M; Karachaliou, N. (2015):Tumor immune microenvironment characterization and response to anti-PD-1 therapy. Cancer Biology Medicine 12: (2) 74–78.
Schneider, H.; Downey, J.; Smith, A.; Zinselmeyer, B. H.; Rush, C.; Brewer, J. M.; Wei, B. et al.(2006): Reversal of the TCR stop signal by CTLA‐4. Science 313: (5795) 1972– 1975.
Scharping, N. E.; Menk, A. V.; Moreci, R. S.; Whetstone, R. D.; Dadey, R. E.; Watkins, S. C.; Ferris, R. L. et al.(2016): The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 45: (2) 374– 388.
Schmidt, A; Oberle, N; Krammer, P. H. (2012): Molecular mechanisms of Treg-mediated T cell suppression. Journal of Frontiers in Immunology 3: 51.
Seidel J. A.; Otsuka A.; Kabashima K. (2018): Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Journal of Frontiers In Oncology 8: 86.
Sheppard, K. A.; Fitz, L. J.; Lee, J. M.; Benander, C.; George, J. A.; Wooters, J.; Qiu, Y. et al.(2004): PD‐1 inhibits T‐cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 574: (1-3) 37– 41.
Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M. et al.(2016): PD‐1 blockade boosts radiofrequency ablation‐elicited adaptive immune responses against tumor. Clin. Cancer Res. 22: (5) 1173– 1184.
Sojka, D. K.; Huang, Yu‐Hui; Fowell, D.J.(2008): Mechanisms of regulatory T‐cell suppression – a diverse arsenal for a moving target. Immunology Volume 124: (1).
Stanietsky, N.; Rovis, T. L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J. et al.(2013): Mouse TIGIT inhibits NK‐cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 43: (8) 2138– 2150.
Ribas, A. and Flaherty, K. T.(2015): Gauging the long‐term benefits of ipilimumab in melanoma. J. Clin. Oncol. 33: (17) 1865– 1866.
Robert, C.; Thomas, L.; Bondarenko, I.; O'Day, S.; Weber, J.; Garbe, C.; Lebbe, C. et al.(2011): Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364: (26) 2517– 2526.
Robert, C.; Ribas, A.; Wolchok, J. D.; Hodi, F. S.; Hamid, O.; Kefford, R.; Weber, J. S. et al.(2014): Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet 384: (9948) 1109– 1117.
Takahashi, T.; Tagami, T.; Yamazaki, S.; Uede, T.; Shimizu, J.; Sakaguchi, N.; Mak, T. W. et al.(2000): Immunologic self‐tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte‐associated antigen 4. J. Exp. Med. 192: (2) 303– 310.
Topalian, S. L.; Drake, C. G. and Pardoll, D. M.(2012): Targeting the PD‐1/B7‐H1(PD‐L1) pathway to activate anti‐tumor immunity. Curr. Opin. Immunol. 24: (2) 207– 212.
Topalian, S. L.C. G.; Drew M.P. (2015): Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell Journal ,Volume 27: (4) 450-461
Twyman‐SaintsVictor, C.; Rech, A. J.; Maity, A.; Rengan, R.; Pauken, K. E.; Stelekati, E.; Benci, J. L. et al.(2015): Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 520: (7547) 373– 377.
Pauken, K. E. and Wherry, E. J.(2015): Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36: (4) 265– 276.
Peggs, K. S.; Quezada, S. A.; Chambers, C. A.; Korman, A. J. and Allison, J. P.(2009): Blockade of CTLA‐4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti‐CTLA‐4 antibodies. J. Exp. Med. 206: (8) 1717– 1725.
Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H. et al.(2012): PD‐1 blockade enhances T‐cell migration to tumors by elevating IFN‐gamma inducible chemokines. Cancer Res. 72: (20) 5209– 5218.
Quezada S. A.; Simpson T. R.; Peggs K. S.; Merghoub T.; Vider J.; Fan X.; Blasberg R.; Yagita H.; Muranski P.; Antony P. A.; Restifo N. P.; Allison J. P. (2010): Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. Journal of Eperimental Medicine 207: (3) 637.
Wing, K.; Onishi, Y.; Prieto‐Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T. et al.(2008): CTLA‐4 control over Foxp3+ regulatory T cell function. Science. 322: (5899) 271– 275.
Woods, D. M.; Sodre, A. L.; Villagra, A.; Sarnaik, A.; Sotomayor, E. M. and Weber, J.(2015): HDAC inhibition upregulates PD‐1 ligands in melanoma and augments immunotherapy with PD‐1 blockade. Cancer Immunol. Res. 3: (12) 1375– 1385.
Woo, S. R.; Turnis, M. E.; Goldberg, M. V.; Bankoti, J.; Selby, M.; Nirschl, C. J.; Bettini, M. L. et al.(2012): Immune inhibitory molecules LAG‐3 and PD‐1 synergistically regulate T‐cell function to promote tumoral immune escape. Cancer Res. 72: (4) 917– 927.
Zhang, Y.; Kang, S.; Shen, J.; He, J.; Jiang, L.; Wang, W.; Guo, Z. et al.(2015): Prognostic significance of programmed cell death 1 (PD‐1) or PD‐1 ligand 1 (PD‐L1) expression in epithelial‐originated cancer: a meta‐analysis. Medicine (Baltimore). 94: (6) e515.
Zheng, H.; Zhao, W.; Yan, C.; Watson, C. C.; Massengill, M.; Xie, M.; Massengill, C. et al. (2016): HDAC inhibitors enhance T‐cell chemokine expression and augment response to PD‐1 immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 22: (16) 4119– 4132.
Zhou, Q.; Munger, M. E.; Veenstra, R. G.; Weigel, B. J.; Hirashima, M.; Munn, D. H.; Murphy, W. J. et al.(2011): Coexpression of Tim‐3 and PD‐1 identifies a CD8+ T‐cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 117: (17) 4501– 4510.
Figures
Figure 1: Tumor Microenvironment.jpg. Source: Wikimedia Commons.
Figure 2:The different immune checkpoints and their mechanisms. Source: Wikipedia
Figure 3: Effect of PD-L1 inhibitors. Source:National Cancer Institute ( owned by Terese Winslow LLC).
Figure 4: Infectious Tolerance. Source: Wikipedia