Itt írjon a(z) inhalation_anestesiology-ról/ről
Mechanisms of actions and the effects on different organs of inhaled narcotics
Introduction
Inhaled narcotics refer to the application of administering agents that can block the sensation of pain, any response to noxious stimuli and cause sleepiness, which allows surgical operations to be carried out without the patient feeling any discomfort. The patient breathes in a mixture of chemicals, and the person will get in the state of unconsciousness. Many chemicals with such an effect have been discovered by scientists. In this essay, we would like to focus on isoflurane and sevoflurane that are very commonly used nowadays for induction and maintenance of general anaesthesia.
Contents
Mechanisms of actions
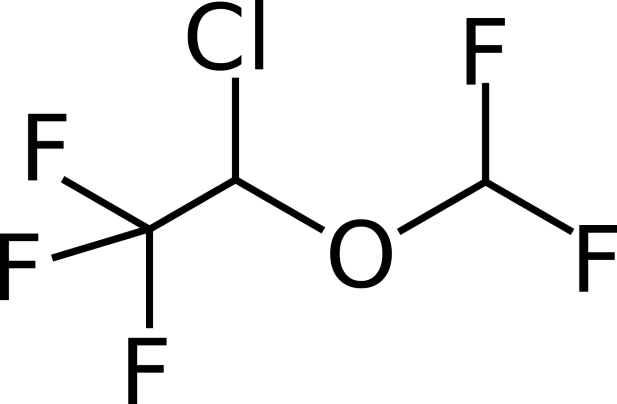
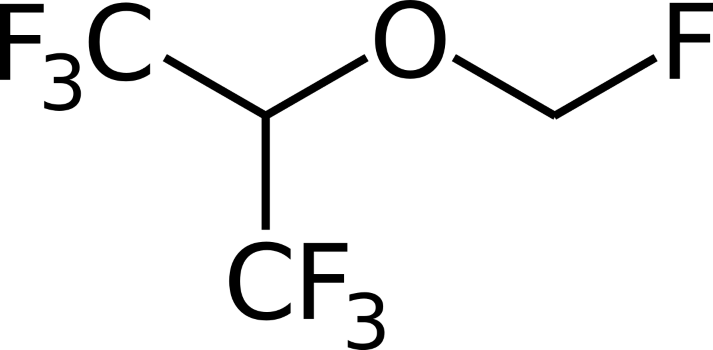
Just as a lot of other anaesthetic drugs, the exact mechanisms of actions of both chemicals that can introduce the state of immobilization have not been completely discovered. However, isoflurane (Figure 1) and sevoflurane (Figure 2) are known to affect the central nervous system to reduce pain sensation. Both are clear liquids. When it is placed in a vaporizer, it becomes a gas and is ready to be used. Both are in the ether group and their structures are substituted with halogen atoms such as chlorine and fluorine as can be seen in the picture.
|
|
|
Sevoflurane
Sevoflurane primarily works on one of the GABA receptors in the central nervous system, termed as GABA-A receptors. GABA stands for gamma aminobutyric acid.
|
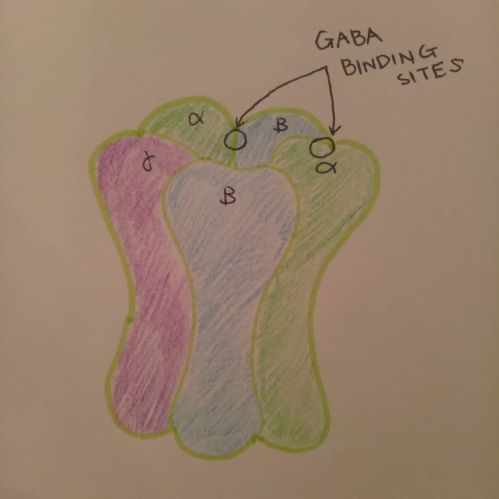
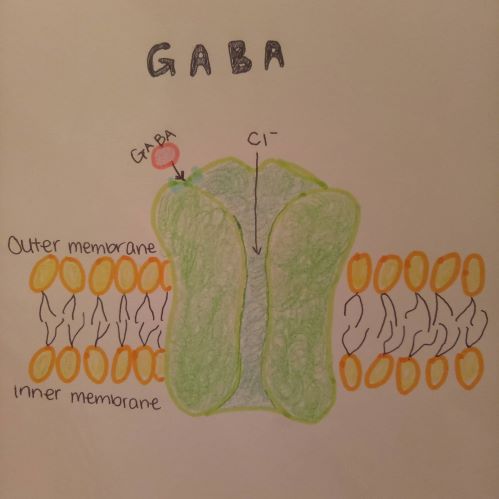
As learnt in class, GABA is the most common neurotransmitter inhibitor in the central nervous system. In Figure 3, a GABA-A receptor is shown. GABA-A receptor is a complex that consists of two alpha, two beta and one gamma subunits. These subunits penetrate the membrane, and the extracellular part of GABA-A receptor has the binding site for a couple of ligands including GABA. When GABA binds to the binding site, the receptor opens and Cl ion influx takes place, which is shown in Figure 4 below.
|
The Cl ion influx prevents to generate action potential, which in turn makes less excitation. Sevoflurane enhances the affinity of the receptors for GABA. The elevated affinity for GABA is observed in isoflurane as well. Even though there are fewer studies done for sevoflurane than isoflurane, it is safe to assume that they both work almost identically on the four transmembrane domains of GABA alpha subunits to achieve its interaction with neurotransmitters (Garcia et al, 2010). Moreover, sevoflurane also bears on presynaptic glutamatergic receptors. It is likely to cause less excitation in presynaptic neurons and elicit the inhibitory interneuron excitation. The inhibition may also be observed in several cascade processes that create glutamate excitation. Therefore, it successfully works on the presynaptic receptors without affecting the postsynaptic receptors. To our interest, sevoflurane may also work on leaky potassium channels. These K+ channels are pH sensitive, voltage-independent and located in the cerebral nuclei and motoneurons in the brain stem and spinal cord. Sevoflurane promotes the K+ channels to be more active. It then affects another ion channel, which elicits hyperpolarization. This system can depress the phrenic nerve motoneurons (Stucke et al, 2001).
Isoflurane
Isoflurane also has an impact on the glycine receptors and the GABA-A receptors. Similar to sevoflurane, it increases the affinity of the GABA-A receptors for GABA and enhances actions on the glycine receptor. However, in addition to these receptors, isoflurane is said to affect glutamate. Glutamate has three main G-protein ligand-gated channels and they are the NMDA receptors, the AMPA receptors and the Kinate receptors. Isoflurane can reduce the binding of MK-801 or the blocker of the NMDA receptor, which represents less receptor function. This may help produce the state of immobility. However, Sonner et al (2003) figured out that it does not seem to have any effects on the rest of the receptors. The sodium channel is a voltage-gated receptor. The presynaptic Na+ channel is inhibited by isoflurane, which results in a decline of neurotransmitter release including glutamate. This means a reduction of the action potential, and thus the nervous system is depressed (Herold et al, 2012). Although dosage and MAC are different for each chemical, it is clear that the two narcotics work mostly in the same way, probably because they are very similar in terms of structures.
Effects on the organs
Sevoflurane
As we discussed above in the mechanisms of actions of sevoflurane and isoflurane, sevoflurane functions by temporarily affecting the action of the central nervous system neurotransmitters. Once it is inhaled, sevoflurane can cause sleep and loss of sensation. It is a very modern halogenated narcotic, marking its first synthesis of sevoflurane by Ross C Terrel in 1979 and first use in 1995. Compared to older anesthetics, sevoflurane is only slightly soluble in water and sweet-smelling, which makes it suitable for induction of anesthesia for both children and adults. There are only a few published researches regarding nursing after the administering of sevoflurane. However, if the mother has recovered from anesthesia, she should be able to breastfeed her baby, as the amount of anesthetic agent in the milk is negligible (Dalal et al, 2014).
Central nervous system
Sevoflurane has a dose-dependent EEG (electroencephalography) and cerebrovascular depressant effects as well, which is parallel to those of isoflurane. It generates cerebrovasodilation, suppresses somatosensory-evoked potentials, by the same token it preserves the responsiveness of the cerebral blood flow to changes in arterial carbon dioxide tension. All of the inhalation agents decrease consumption of oxygen and the rate of metabolism. They decrease the mean arterial pressure as well, which causes the lower pressure of cerebral perfusion (Patel and Goa, 1996).
Cardiovascular system
It is also a dose-dependent cardiovascular depressant similarly as desflurane and isoflurane. However, sevoflurane is not related with sympathoexcitatory activity upon induction or with the rapid increase of the inspired concentration level. Rivenes (2001) reported that sevoflurane may lessen the mean arterial pressure and systolic function in the left ventricle. However, it only showed a 15-16% decline of blood pressure while other halothanes decreased 25-26%. This decreasing effect of blood pressure is dose-dependent. Moreover, sevoflurane did not cause any arrhythmias that is usually induced by adrenaline or epinephrine. It can preserve cardiac output as well, which overall makes the heart rate stable throughout the surgical operation.
Respiratory system
All halogenated anaesthetics, including sevoflurane, depress the ventilation by decreasing the tidal volume of the lungs, this results in a slight increase in the respiratory rate. However, it cannot regulate the reduced alveolar ventilation, thus increased dead-space ventilation occur, consequently PaCO2 increases as well. All of these drugs expand the threshold of respiratory centres to CO2, sevoflurane also decreases the resistance of airways. The sensitivity of the upper airway is measured by using cuff inflation to provoke tracheal reflexes. Under the influence of sevoflurane, less irritation to the respiratory organs was observed than with another anesthetic agent as desflurane. The irritation consists of breath-holding, coughing, excitement and blood flow change to the applied area, which only occured at a very low rate.This is because sevoflurane has low irritation rate and inhibits hypoxic pulmonary vasoconstriction and smooth muscle contraction in the trachea (Klock et al, 2001). In addition, due to its sedative and dilator effect, sevoflurane is applied widely to deal with serious asthma. The anesthetic agent is absorbed through the respiratory wall, causing vasodilation of the area (Ruszkai et al, 2014).
Liver
General anesthetics are said to increase the risk of hepatitis. On the other hand, many studies had been claiming that newly developed anesthetics such as sevoflurane barely affects it. This is because sevoflurane reduces the hepatic flow and does not become trifluoroacetic acid or TFA protein complex that is produced through a cascade involving cytochrome P-450 2E1 (CYP2E1), whereas other halothanes undergo CYP2E1 process and is metabolized to TFA (Safari et al, 2014). However, a recent study by Nicoll et al (2012) states that sevoflurane develops acute liver damages and later ends up in chronic hepatitis. They tested the patients based on their antibodies, hepato-histology, and an anesthetic causality measurement. In the research, all the subjects showed greatly positive IgG antibodies against TFA and CYP2E1.Therefore, it should always be kept in mind that acute and chronic liver damages may occur as a result of application of sevoflurane.
Kidneys
Sevoflurane does not affect a seemingly healthy human without any kidney disorders or injuries. It does not have any impact on clearance of creatinine and glucose excretion that play a significant role in measuring the kidney function (Ongsio et al, 2017). In fact, it has several beneficial influences on the kidneys. Lee et al (2006) insist that sevoflurane reduces inflammation and necrosis of the tissues or cells. This specific character is able to deal with ischemic kidney injury that has no active treatment to this day.
Thyroid gland
It is known that general inhaled anesthetics have an impact on thyroid hormone levels. They may cause low T3 syndrome during and after the period of use. Low T3 syndrome, or euthyroid, is known to reduce T3 and T4 and elevate TSH level. As learnt in the thyroid hormone lecture, T4 is converted into T3 by 5’D enzyme. Sevoflurane, unlike other anesthetics, raises the amount of T3. It is significant because this can be a new treatment or alternative option for patients with thyroid hormone problems (Boztuğ and Aydoğdu, 2000).
Isoflurane
Isoflurane is a general inhalation anaesthetic drug used to induce and maintain general anaesthesia. Common side effects of isoflurane include slow or shallow breathing, low blood pressure, or abnormally fast or slow heart rate. Post-operatively, side effects of isoflurane include shivering, nausea, vomiting, and abdominal distention or gas (John and Cunha, 2018). There is an occasional risk of perioperative hyperkalemia and malignant hyperthermia. The use of isoflurane in obstetrical anaesthesia could not be installed yet because no sufficient data had been collected until this day. According to the US Food and Drug Administration, in some mice reproduction researches, embryo-fetal toxicity was observed. Pregnant mice that were exposed to 0.075% isoflurane showed an increased number of losses after implantation and those inhaling 3% isoflurane displayed a declined live-birth index as well as an increased post implantation loss. An possible explanation for this fact could be that isoflurane affects the development of the brain through NMDA or GABA receptor activity.
Central nervous system
It should be carefully used in young children under 3 years old and pregnant women because an extended (longer than 3 hours) or repeated exposure may have negative effects on fetal or infant’s brain development. Isoflurane has a smaller impact on cerebral vasodilation than halothane and it still lowers the metabolic oxygen consumption. Isoflurane is more often used with patients which have a higher intercranial pressure (Pawson and Forsyth, 2008). Studies were made to demonstrate that early age exposure to isoflurane could affect the mechanism by which it could disrupt brain circuit formation, additionally it has effects on neuronal survival, neurogenesis and formation of synapse. Zou et al (2011) found that early isoflurane exposure in mice resulted in a reduced stem cell pool and a decrease in the total number of dentate gyrus granule cells.
Cardiovascular system
On one side, isoflurane has positive effects as it is cardioprotective by preconditioning and postconditioning. However, on the other side, it has dose-dependent cardiovascular side effects similar to sevoflurane. As the concentration of isoflurane increases the heart rate, blood pressure, left ventricular systolic and diastolic function decreases (Yang et al, 2014). The purpose of the article was to characterize the hemodynamic effects of isoflurane with the use of rats. The results showed that high-dose isoflurane produced unfavorable hemodynamics. Old age and dehydration may predispose animals to the unfavorable hemodynamic effects of isoflurane. The optimal determination of the concentration for anaesthesia or preconditioning is crucial. Caution has to be taken for patients with coronary heart disease and it should not be applied as a sole agent of induction, if the patient has a ventricular dysfunction.
Respiratory system
Isoflurane provokes respiratory depression and reduces the ventilatory response to carbon dioxide. It irritates the upper airways, but it is a bronchodilator which is a beneficial side effect (Nolan, 2012). As all halogenated anaesthetics Isoflurane has the same effects as already described for sevoflurane.
Liver
In general, it diminishes the renal and hepatic blood flow and is known that patients can have postoperative hepatic dysfunction and hepatitis as consequence of the use of isoflurane. Isoflurane goes through a basic biotransformation. A large range of hepatotoxicity is known by using isoflurane and goes from transaminitis to fulminant hepatic failure. The study of Yingxian et al (2017) shows that the oxidative stress response is participating in the liver injury mechanism together with hepatic necrosis and apoptosis. The precise mechanism is not yet well known but the hypothesis of this study was to demonstrate that isoflurane induces liver injury by regulating the expression of insulin-like growth factor 1.
Kidneys
In rat model, researchers (Motayagheni et al, 2017) discovered that isoflurane contributes preconditioning renoprotective effects through anti-inflammatory and anti-apoptotic actions. Isoflurane shows a protective character against renal tubular necrosis and inflammation by inducing renal tubular CD73 and adenosine generation, which is reliant on transforming growth factor-beta 1.
Conclusion
In conclusion, it can be constated that several studies claim that sevoflurane and isoflurane work mostly on the GABA affinity to the receptors. However, it is not yet fully known how the mechanisms of actions interact with the central nervous system. As mentioned before, recent studies showed that indeed these inhaled narcotics can also contribute positive effects as preconditioning renoprotective effects through anti-inflammatory and anti-apoptotic actions. Though there are unfavorable side effects including shivering, nausea, vomiting, and abdominal distention or gas. Given that most of the effects are not severe, it is obvious why sevoflurane and isoflurane are very commonly used these days and administered to block nerves for anaesthesia. So far it is for example unknown how the use of isoflurane in early age exposure could affect the mechanism interrupting the brain circuit formation. Therefore, it will be very exciting to see what future researchers will discover in their studies about the mechanisms and effects of these inhaled narcotics.
References
Boztuğ, N.; Aydoğdu, T. (2000): Effect of sevoflurane on thyroid hormone levels. European Journal of Anaesthesiology 17: (19) 127
Dalal, P. G.; Bosak, J.; Berlin, C. (2014): Safety of the breast‐feeding infant after maternal anesthesia. Paediatric Anaesthesia 24: (4) 359-371
Garcia, P. S.; Kolesky, S. E.; Jenkins, A. (2010): General anesthetic actions on GABA(A) receptors. Current neuropharmacology 8: (1) 2–9
Herold, K.; Hemmings Jr. (2012): Sodium Channels as Targets for Volatile Anesthetics. Frontiers in Pharmacology 3: (50)
Khan, K. S.; Hayes, I.; Buggy, D. J. (2014): Pharmacology of anaesthetic agents II: inhalation anaesthetic agents, Continuing Education in Anaesthesia Critical Care & Pain 14: (3) 106–111
Klock, P.; Czeslick, E.; Klafta, J.; Ovassapian, A.; Moss, J. (2001): The Effect of Sevoflurane and Desflurane on Upper Airway Reactivity. Anesthesiology 94: (6) 963-967
Lee, T.; Kim, M.; Jan, M.; Emala, C. (2006): Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. American Journal of Physiology-Renal Physiology 291: (1) 67-78
Motayagheni, N.; Phan, S.; Eshraghi, C.; Nozari. A.; Atala, A. (2017): A Review of Anesthetic Effects on Renal Function. Potential Organ Protection. American Journal of Nephrology 46: (5) 380-389
Nicoll, A.; Moore, D.; Njoku, D.; Hockey, B. (2012): Repeated exposure to modern volatile anaesthetics may cause chronic hepatitis as well as acute liver injury. BMJ case reports
Nolan, J. P. (2012): Anaesthesia and neuromuscular block. In: Bennett, P. N.; Brown, M. J.; Sharma, P. editors. Clinical Pharmacology 11: 295-310
Ong Sio, L.; Dela Cruz, R.; Bautista, A. F. (2017): Sevoflurane and renal function: a meta-analysis of randomized trials. Medical gas research 7: (3) 186–193
Patel, S. S.; Goa, K. L. (1996): Sevoflurane. Drugs 4: 658-700
Pawson, P.; Forsyth, S. (2008): Anesthetic agents. Small Animal Clinical Pharmacology 2: 83-112
Rivenes, S.; Lewin, M.; Stayer, S.; Bent, S.; Schoenig, H.; McKenzie, E. (2001): Cardiovascular Effects of Sevoflurane, Isoflurane, Halothane, and Fentanyl–Midazolam in Children with Congenital Heart Disease: An Echocardiographic Study of Myocardial Contractility and Hemodynamics. Anesthesiology 94: (2) 223-229
Safari, S.; Motavaf, M.; Seyed Siamdoust, S. A.; Alavian, S. M. (2014): Hepatotoxicity of halogenated inhalational anesthetics. Iranian Red Crescent medical journal 16: (9)
Sonner, J.; Antognini, J.; Dutton, R.; Flood, P.; Gray, A.; Harris, R. (2003): Inhaled Anesthetics and Immobility: Mechanisms, Mysteries, and Minimum Alveolar Anesthetic Concentration. Anesthesia and Analgesia 97: (3) 718-40
Stucke, A. G.; Stuth, E. A.; Tonkovic, V.; Tonkovic, M.; Hopp, F. A.; Kampin, J.; Zuperku, E. (2001): Effects of Sevoflurane on Excitatory Neurotransmission to Medullary Expiratory Neurons and on Phrenic Nerve Activity in a Decerebrate Dog Model. Anesthesiology 95: (2) 485-491
Yang, C. F.; Yu-Chih Chen, M.; Chen, T. I.; Cheng, C. F. (2014): Dose-dependent effects of isoflurane on cardiovascular function in rats. Tzu Chi Medical Journal 26: (3) 119-122
Yingxian, Z.; Xiaoyu, X.; Guowei, L.; Juyuan, B.; Wenying, Z.; Shaopeng, Z. (2017): Isoflurane anesthesia induces liver injury by regulating the expression of insulin-like growth factor 1. Experimental and Therapeutic Medicine13: (4) 1608–1613
Zou, X.; Liu, F.; Zhang, X. (2011): Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol 33: (5) 592–597
Websites
US Food and Drug Administration, (2017): Forane (isoflurane, USP) liquid for inhalation. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017624s041lbl.pdf Accessed: 22 April 2019.
Figures
Figure 1 - Mills, B. (2006): Chemical structure of the anaesthetic sevoflurane. Wikimedia commons
Figure 2 - Mills, B. (2006): Chemical structure of the anaesthetic isoflurane. Wikimedia commons
Figure 3 - Drawn by 3550/E
Figure 4 - Drawn by 3550/E