Effects of long term sleep deprivation
Cathrine N. Thorvaldsen, Iselin M. Isaksen & Ingvild Kive
Contents
Introduction
Sleep is a naturally recurring state. In a physiological sense, it is characterized by an altered unconsciousness with special brainwave patterns, parasympathetic dominance and decreased metabolic rate and temperature.
Sleep occurs in two stages. Rapid eye movement (REM) or paradoxical sleep accounts for 20-25% of total sleep in humans, and is characterized by paralyzed muscles and unregulated breathing, temperature and heart rate (Sleepdex). In this stage, dreaming can occur (Sjaastad et al., 2003). Non – REM occurs when an organism falls asleep and brain waves get slower and bigger. This stage can be divided into 3 stages; wakefulness, superficial sleep and slow wave sleep (SWS) known as deep sleep (Mastin, 2013).
The Reticular formation is one of the major regulators of sleep. It functions to filter information and adjust the activity level in upper brain areas. It produces serotonin that generates sleep and norepinephrine that causes wakening. When falling asleep sensory stimuli is at minimum, causing a decrease in stimulation of the reticular formation leading to gradual unconsciousness (Sjaastad et al., 2003). The thalamus and hypothalamus is also involved in the sleep – wake cycle due to its role in activation/inhibition of upper brain areas.
The sleep wake cycle is regulated by interplay of two processes; promoting wakening and sleep respectively. This is influenced by the circadian system, coordinating it to the external day – night cycle (Colten and Altevogt,2006).
Sleep patterns varies in different species according to sleep cycles, deepness and duration. In some species such as whales and dolphins, it has been observed that the hemispheres sleep in turn meaning that one hemisphere is always functioning. Architecture and need of sleep changes with increasing age (Sjaastad et al., 2003). We can find changes in for example initiation and maintenance of sleep and time spent in the different sleep stages (Colten and Altevogt, 2006). Young individuals seem to need more sleep than adults. In elderly, the need of sleep is further reduced (Sjaastad et al., 2003).
Failure to get enough sleep results in sleep deprivation. Sleep deprivation can be either acute or chronic. Acute sleep deprivation lasts for a short period of time (1 or 2 days) with a reduction of total sleep time or no sleep at all. Chronic sleep deprivation refers to a longer period of time with insufficient sleep. Not only duration, but also the quality of sleep are contributing factors of sleep deprivation (Cirelli, 2016). It is unclear why animals need sleep because it has not been proved that any physiological process depends on sleep to function normally. However, research proves that the individual needs sleep to stay healthy (Sjaastad et al., 2003). Scientific research shows that failure to obtain right amount and quality of sleep has negative consequences on the individual.
Effects of sleep deprivation
In the following section we will review some important effects of long term sleep deprivation. Mainly focusing on its impact on the thyroid gland, cardiovascular pathogenesis, pancreas and weight gain as well as cognitive functions.
Effects on the thyroid hormone economy
The most important hormones the thyroid gland secretes are T4 and T3. Hypothalamic-pituitary-thyroid axis (HPT) regulates T3 activity with some help from D1, D2 and D3 enzymes. Hypothalamus stimulates pituitary to both synthesise and also release thyroid-stimulation hormone (TSH) and also release thyrotrophin-releasing hormone (TRH) (Rodrigues et al., 2015).
The aims of these studies were to find the serum TSH and TH concentration in different stages of sleep deprivation. They also tested if a rebound period was enough for the hormonal changes to get back to normal. During their experiments they sleep deprived the rats for 24 and 96 hours with and without rebound period, and also sleep restricted them with and without rebound period (Rodrigues et al., 2015).
Their results showed that SD without any rebound period increased T3 values and BAT D2 activity, but also decreased T4 and TSH values. SD with a 24 hour rebound period normalized all values except TSH and T3 values. With a 96 hour rebound period most values got normalized except T3 and T4 values. It was also noticed that sleep restriction with no rebound period increased T3 values. An important finding was that TSH secretion got decreased and that T3 level got increased. The last mentioned may explain the lack of a hypothyroxinaemia-induced TSH response and maybe help in the inhibition of HPT axis. Stress from paradoxical SD increases concentration of corticosterone in the serum, which can explain the high BAT D2 and serum T3 levels and the low TSH. When the rats were sleep restricted the HPT axis normalized T4 and TSH secretion (Rodrigues et al., 2015).
Effect on immune system and its effect on cardiovascular pathogenesis
|
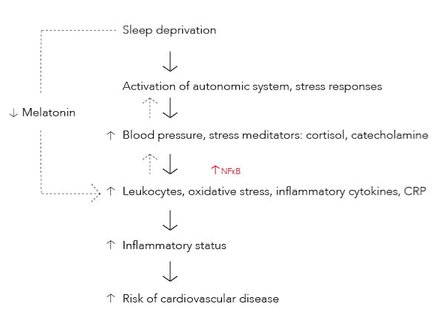
Sleep inhibits release of stress mediators into the bloodstream and is normally followed by a suppression of cortisol and catecholamine release. Therefore, sleep deprivation (SD) enhance catecholamine and cortisol levels. These stress mediators have a role in leukocyte mobilization. The increased release of norepinephrine, a catecholamine, induce nuclear factor – kappa B (NF-kB) activity which has an essential role in inflammatory processes due to its activation of pro – inflammatory cytokines (Fig. 1) (Faraut et al., 2011).
SD activates the sympathetic system resulting in an increase in blood pressure and a higher risk of developing hypertension (Fig. 1). Baroreflex sensitivity is a mechanism in the autonomic nervous system that detects and regulates changes in blood pressure (Matos et al., 2013) by increasing/decreasing heart rate and stroke volume, as well as vascular contraction/dilation (Sjaastad et al., 2003). Data shows that sleep loss causes a reduction in baroreceptor sensitivity, especially in males (Matos et al., 2013). This can impair the hearts ability to adapt to changes of blood pressure. It has been hypothesized that the increase in blood pressure provokes vascular shear stress. This may cause inflammation in the vascular wall and affect the endothelial cells to produce inflammatory mediators (Faraut et al., 2011).
Melatonin is known for its effect on sleep synchronization (Faraut et al., 2011). It has a circadian rhythm and its release is stimulated by darkness (night-time). Melatonin has a scavenger effect with an ability of binding free radicals. It is also able to regulate non- specific immune cells and cytokine production. Therefore, melatonin could regulate immune and inflammatory responses as well as reducing oxidative stress in inflamed tissues (Fig. 1). SD decreases melatonin secretion (Faraut et al., 2011). This may explain why some inflammatory markers are elevated in people with SD, which might increase the risk of getting cardiovascular diseases.
Epidemiological studies show a connection between the increasing amount of leukocytes and an increasing risk of getting cardiovascular disease, such as type 2 diabetes. SD seem to alter non – specific immune parameters by increasing leukocytes such as lymphocytes, neutrophils and monocytes (Fig. 1). In a study of total SD, young men and women experienced leukocytosis after 64 hours. Increased leukocyte levels can induce atherosclerosis, vascular injury and myocardial infarction. Monocytes and neutrophils have a proteolytic and oxidative ability to damage coronary arteries, and is therefore an important factor in the development of coronary heart disease (Faraut et al., 2011).
Tumor necrosis factor (TNF-a) and interleukins (IL) are pro – inflammatory cytokines, mainly released from monocytes under circadian rhythms. Pro – inflammatory cytokines is essential in inflammatory processes due to its ability to chemoattract and regulate inflammatory mediators. They stimulate the production of acute phase proteins such as C – reactive protein (CRP) (Faraut et al., 2011) that is responsible for protecting cells and tissues during inflammatory processes (Sjaastad et al., 2003). SD for 7 days increased TNF values in men, and secretion of IL – 6 in both healthy women and men. Both TNF –a and IL-6 is known to increase the risk of coronary heart disease. IL – 6 can be a predictor of atherogenesis, atherothrombosis and fibrin formation. After 10 days of SD there was found increased levels of CRP (Fig. 1). CRP is known for its direct role on atherogenesis (Faraut et al., 2011). It is produced by the liver at onset of injury or infection. Therefore, its increased values may indicate cardiovascular damage (Sjaastad et al., 2003).
SD seem to cause an increase in oxidative stress markers such as Myeloperoxidase (MPO) (Fig. 1). This enzyme is mainly released from neutrophils. It is known as a pro– atherogenic agent and may induce atherogenesis in the vascular wall (Faraut et al., 2011).
There seem to be a connection between sleep duration and risk of getting coronary artery calcification. This connection was studied in a healthy population of 495 people and the result showed us that those with SD had a higher risk of getting coronary artery calcification (Faraut et al., 2011).
The changes of cardiovascular parameters showed differences according to sex. Some studies show that males seem to be more affected by sleep loss, and have a higher risk of getting cardiovascular diseases such as myocardial infarction and cardiovascular death. This phenomenon is still largely unknown, but there is hypothesized that woman have sex hormones that have a protective effect on the cardiovascular system (Matos et al., 2013).
In contrast, other studies have shown that women with SD have a higher risk of developing cardiovascular diseases. Three consecutive nights of SD resulted in an increase of LDL - cholesterol and both neutrophils and monocytes in women. In men, the neutrophil counts increased, but there was no change in amount of LDL - cholesterol. It has also been shown in some studies that there is an association between poor quality and sleep duration between elevated amounts of IL – 6, CRP and fibrinogen in women (Faraut et al., 2011). The data present in this article can seem contradictory to each other. There are a lot of aspects that needs to be considered when looking at sex differences, eg. type of disease investigated. The sex differences are widely researched but there is not any conclusion yet.
Effects on the pancreas
Sleep deprivation has been described as a risk factor for insulin resistance and diabetes. Chronic sleep deprivation has also been associated with obesity which again may lead to insulin resistance and diabetes. Aged individuals are more susceptible to impaired glucose tolerance and diabetes, and they often suffer from sleep disturbances like sleep loss (Naidoo et al., 2013).
It is well known that sleep deprivation induces UPR (unfolded protein response) in the brain. UPR functions to limit the accumulations of unfolded proteins in the endoplasmic reticulum. Increased levels of binding immunoglobulin protein (BiP) assists the adaptive UPR. One of the functions of UPR is to limit ER stress. Failure to do so leads to a maladaptive response which is characterized by activation of cell death pathways, where pro-apoptoic transcription factor C/EBP homologous protein (CHOP) is produced (Naidoo et al., 2013).
In a study (Naidoo et al., 2013) it was found that there was a response to sleep deprivation (acute, 6 hours) and aging in the pancreas. In the pancreas of young animals sleep deprivation was found to significantly increase the BiP expression, and induction of adaptive UPR was confirmed. This was not the case in aged mice. Instead they expressed significantly more CHOP at baseline which was further increased by sleep deprivation. ER stress and induction of maladaptive UPR was also observed in the endocrine cells of pancreas. This has an impact on the insulin secreting beta cells which play a major role in the glucose metabolism. The CHOP induction in the pancreatic tissue points to chronic sleep deprivation being a contributor to the loss or dysfunction of endocrine cells and that these effects may be worsened by normal aging (Naidoo et al., 2013).
The pancreas seemed to be able to function normally after acute sleep deprivation. After chronic sleep deprivation (20 hours a day for 8 days), young mice had decreased glucose levels and decreased fasting insulin levels. This suggests improved insulin sensitivity. The insulin levels were easily increased by glucose intake. Aged mice had increased fasting glucose levels following sleep deprivation, and after glucose intake they showed lower insulin levels. This indicates that peripheral insulin sensitivity is decreased in aged mice subjected to chronic sleep deprivation, and also that it leads to insufficient insulin secretion. Decreased insulin sensitivity and secretion may be contributing factors to worsened glycemic control. In a similar experiment done on humans indications of beta cell dysfunction were observed (Naidoo et al., 2013).
Effects on weight gain
In an experiment (Mendes de Oliveira et al., 2015) mice were sleep deprived for 21 hours each day followed by 3 hours of sleep, this lasted for 15 days. The point was to examine the late effects of sleep deprivation on a following period of a high fat diet. Mostly in relation to weight gain and insulin resistance. The mice were placed in four different groups (Mendes de Oliveira et al., 2015):
- Control group: sleep and food as they wish, normal diet.
- SD group: a sleep deprived group followed by 7 weeks on a normal diet.
- HFD group: a non-sleep deprived group followed by 6 weeks on a high fat diet.
- SD+HFD group: a sleep deprived group followed by one week of recovery and then 6 weeks on a high fat diet.
The food intake among the groups was similar, but the caloric intake was higher in the HFD and SD+HFD mice (Mendes de Oliveira et al., 2015).
It was found that the HFD and SD+HFD mice gained more weight than the other groups. In addition, the SD+HFD mice gained weight faster than the HFD mice, they also gained quite a lot more. This points to a clear synergism between SD and a high fat diet. A high fat diet generally leads to an increase in some genes that are essential transcriptional regulators of adipogenesis. The increase of most of these genes was approximately twice as large in epididymal and subcutaneous fat pads of SD+HFD mice than HFD mice. Seemingly the period of sleep deprivation makes the adipose tissue predisposed to hypertrophy (Mendes de Oliveira et al., 2015).
Several observations were made supporting sleep deprivations role in insulin resistance (Mendes de Oliveira et al., 2015). Insulin resistance can occur if the insulin levels in the body are high for a long period of time. This causes the cells to be less sensitive to insulin. This leads to raised blood glucose levels, leading to an increased production of insulin further advancing the insulin resistance (The global diabetes community, 2016). There was observed an increase of approximately 50% of serum insulin after SD. The glucose and insulin tolerance tests were affected in the SD+HFD mice, showing that they had developed a weakened insulin sensitivity (Mendes de Oliveira et al., 2015).
In the adipose tissue the amount of proinflammatory cytokines (TNF-alpha and IL-6) was increased following SD. They are produced by adipose tissue in direct proportion to adiposity (Mendes de Oliveira et al., 2015), and are also closely linked to insulin resistance (The global diabetes community, 2016). In 33% of the SD+HFD mice there was an extensive macrophage infiltration in the epididymal fat. Macrophage infiltration is directly proportional to adiposity in mice and humans. It is the reason for the production of proinflammatory molecules in adipose tissue. Resistin levels were also increased in the adipose tissue after SD, also in non fat mice. It is linked with subclinical inflammation and insulin resistance and its effects can lead to cardiovascular disease, obesity and type 2 diabetes (Mendes de Oliveira et al., 2015).
Effects on memory
Contextual fear conditioning is a hippocampal dependent task. Here a rat will be put into a new environment, providing an aversive stimulus, and then it will be removed. When the rat is put back into the environment after some time, it will make a freezing response if it remembers the environment and the stimulus. Tone fear conditioning is a hippocampal independent task which is similar to contextual conditioning, but here a conditioned stimuli is also added. To separate context and tone conditioning, it is often used a preexposure trial to the test chamber without an unconditioned stimulus (Cursor, 2009).
Earlier studies show that sleep deprivation (SD) before training weakens the animals’ performance in hippocampal dependent tasks (cognitive processes.) This study’s aim was to do a research about the consequences of long term sleep deprivation in different moments of memory formation in the two fear conditions. During the experiments the rats were divided into different groups; tested immediately after SD, slept for 24h after SD before they got tested, slept for 96h after SD before they got tested (Rossi et al., 2014).
96 h of sleep deprivation after their training of aversive memory tasks weakened the recall of fear memory both in tone fear conditioning and contextual fear conditioning, but this got back to normal after sleep recovery. When SD took place right before their test, it was a weakening of reduction of the aversive memory in contextual fear conditioning only (Rossi et al., 2014).
Conclusion
Generally, sleep deprivation is a physiological stressor that causes “stress responses” in the individual. In this essay, we have reviewed some of the main effects. It alters thyroid gland economy, cardiovascular pathogenesis, pancreas and weight gain. It also affects cognitive processes like memory retrieval and acquisition, but not consolidation (Rossi et al., 2014).
Regarding cardiovascular effects, it can be stated that sleep loss does not cause cardiovascular disease directly, but increases the risk by altering parameters of the cardiovascular system.
Sleep deprivation can induce central hypothyroidism. The thyroid gland is able to adapt to this change by increasing the confirmation of T4 to T3. This results in an increased circulation of T3 levels. In conclusion it can be said that sleep deprivation alters HPT physiology and thyroid hormone economy (Rodrigues et al., 2015).
Both aging and sleep deprivation put stress on the pancreas. In a chronic setting, the combination may lead to defects in insulin secretion that contribute to the diabetogenic effects of sleep loss. The observed ER stress in the beta cells of pancreas indicates that sleep deprivation might have consequences for endocrine function. In conclusion it can be said that sleep deprivation significantly affects the insulin sensitivity, and if it is chronic it may lead to impaired insulin secretion (Naidoo et al., 2013).
Sleep deprivation can induce prolonged physiological impairments that contribute to obesity. It is also a triggering factor to adipose tissue mass gain when it is associated with a subsequent period of a high fat diet. A previous period of sleep deprivation can potentiate obesity, insulin resistance and type 2 diabetes arising from a high fat diet. It is not yet known how long the period of sleep deprivation should be to have an impact on the adipose tissue, neither for how long the reverberating effects can last (Mendes de Oliveira et al., 2015).
References
Cirelli C. (2016): Sleep insufficiency: Definition, consequences, and management. URL: http://www.uptodate.com/contents/sleep-insufficiency-definition-consequences-and-management.
Colten H. R. and Altevogt B. M. (2006): Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington DC, The National Academies Press. (39-43)p. URL: http://www.ncbi.nlm.nih.gov/books/NBK19960/pdf/Bookshelf_NBK19960.pdf.
Cursor P.; Rusty N. R.; Browman K. E. (2009): Cued and Contextual Fear Conditioning for Rodents. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 2. URL: http://www.ncbi.nlm.nih.gov/books/NBK5223/.
Faraut B.; Boudjeltia K. Z.; Vanhamme L.; Kerkhofs M. (2011): Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Medicine Reviews16. (137-149)p.
Matos G.; Tenório N. M.; Bergamaschi C. T.; Campos R. R.; Cintra F.; Tufik S.; Andersen M. L. (2013). More than hormones: Sex differences in cardiovascular parameters after sleep loss in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry 44. (34–38)p.
Mendes de Oliveira E.; Visniauskas B.; Sandri S.; Migliorini S.; Andersen M. L.; Tufik S.; Fock R. A.; Chagas J. R.; Campa A. (2015): Late Effects of Sleep Restriction: Potentiating Weight Gain and Insulin Resistance Arising from a High-Fat Diet in Mice. Obesity 23: (2). (391-398)p.
Naidoo N.; Davis J. G.; Zhu J.; Yabumoto M.; Singletary K.; Brown M.; Galante R.; Agarwal B.; Baur J. A. (2013): Aging and sleep deprivation induce the unfolded protein response in the pancreas:implications for metabolism. Aging Cell 13. (131-141)p.
Rodrigues N. C.; Santos da Cruz N.; Nascimento C. D.; Rodrigues da Conceicao R.; Matos da Silva A. C.; Olivares L. E.; Marassi M. P. (2014): Sleep deprivation alters thyroid hormone economy in rats. Experimental Physiology 100.2. (193-202)p.
Rossi V. C.; Tiba P. A.; Moreira K. D.; Ferreira T. L.; Oliveira M. G.; Suchecki D. (2014): Effects of sleep deprivation on different phases of memory in rat: dissociation between contextual and tone fear conditioning tasks. Frontiers in Behavioral Neuroscience volume 8, article 389. (1-10)p.
Books
Sjaastad Ø. V.; Hove K.; Sand O. (2003): Physiology Of Domestic Animals. Oslo, Scandinavian Veterinary Press. (137-138)p.
Lecture Notes
Veterinary physiology, Szent Istvan University. (2009): Endocrinology. (177 and 202)p. http://www.vetphysiol.hu/eng/lecturenotes.php. Accessed in the academic year 2015/2016
Veterinary physiology, Szent Istvan University. (2014): Neurophysiology. (325-338)p. http://www.vetphysiol.hu/eng/lecturenotes.php. Accessed in the academic year 2015/2016.
Websites
Mastin L. (2013): TYPES AND STAGES OF SLEEP NON-REM (NREM) SLEEP. URL: http://www.howsleepworks.com/types_nonrem.html. Accessed: 19. March 2016
Sleepdex. Stages of Sleep. URL: http://www.sleepdex.org/stages.htm. Accessed: 19. March 2016.
The global diabetes community, (2016): Insulin Resistance. URL: http://www.diabetes.co.uk/insulin-resistance.html. Accessed: 20. March 2016.
Figures
Figure 1: Self made, Indesign (April 2016). Based on Fig. 1 in Faraut et al., 2011.