|
Size: 12411
Comment:
|
Size: 13158
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 87: | Line 87: |
| When provided higher dietary levels of manganese, it is suggested that early onset of estrus is exhibited in sows. Those fed on higher concentrations of manganese had a greater tendency to undergo estrus earlier than those fed on lower concentrations. CITE. Estrus is stimulated via a surge in LH brought about through a rise in oestrogen provided by the developing ovarian follicles under regulation by gonadotropic hormones LH & FSH. Administration of pulsatile doses of GnRH has been shown to induce estrus in multiple species CITE and helps in the explanation as to why greater manganese levels are seen to bring about these effects; an increase in manganese allows for greater production and secretion of GnRH and ultimately, a rise in LH. |
Effects of Manganese on the Hypothalamic Regulation of Reproduction
Martina Azzopardi, Ashli Welcome, Clare Wilson
Contents
Introduction
|
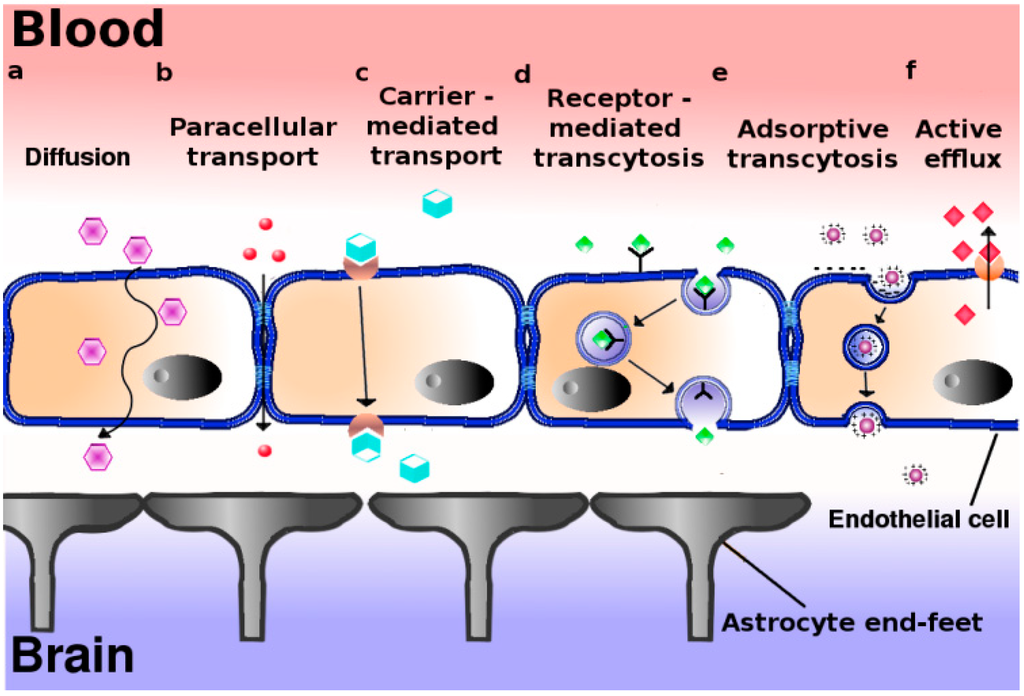
The hypothalamus is a portion of the brain consisting of a number of small nuclei with a variety of functions; most importantly, it provides a link between the nervous system and endocrine system via the hypophysis. Manganese is a naturally-occurring metal required for normal mammalian reproductive function. It is able to cross the blood-brain barrier and accumulate in the hypothalamus, where it exerts its effect. It does so through several carriers in different oxidation states, specifically via:
* Divalent metal transporter 1 (DMT1)
* Transferrin receptors (TfR)
* Divalent metal/bicarbonate ion symporters ZIP8 and ZIP14
* Various calcium channels
* Solute carrier-39 (SLC39) family of zinc transporters
* Park9/ATP13A2 * Magnesium transporter hip14
* Transient receptor potential melastatin 7 (TRPM7) channels/transporters
* Homomeric purine receptors (P2X and P2Y)
* Citrate transporter.
|
![]() End of edit conflict
End of edit conflict
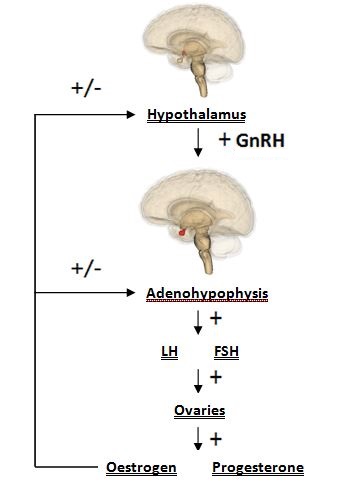
Manganese is able to directly influence neuroendocrine effect related to reproduction. Manganese crosses the blood-brain barrier more effectively, and is eliminated with greater difficulty, in younger animals; subsequently, it can exert its effect in the developing reproductive system readily. Hormones released by the hypothalamus are directly affected: namely, direct stimulation of the secretion of gonadotropin releasing hormone (GnRH). GnRH is a neuropeptide molecule (comprised of 10 amino acids) which is produced by the parvocellular cells of the hypothalamus. GnRH is an integral component of reproductive development through its direct stimulation of the production and secretion of gonadotropin hormones by hypophyseal cells: specifically, follicle stimulating hormone (FSH) and luteinizing hormone (LH). Both FSH and LH are essential for sexual maturation and typical reproductive processes.
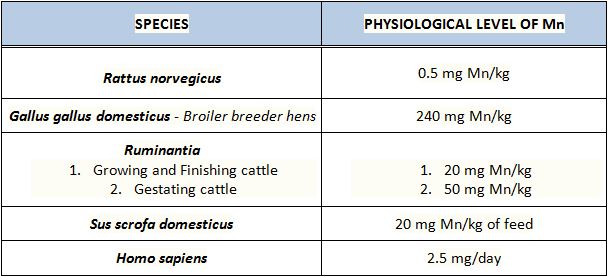
Typical manganese levels in different species
|
Pathogenicity: a summary of the effects of manganese in reproduction
Manganese is responsible for reproductive development, and so high levels trigger the early onset of puberty by increasing the expression of GnRH genes. Manganese is present in the plasma of mothers & the umbilical cord blood of premature and full-term babies. Limited information suggests that higher-than-usual amounts of Mn can cause birth defects.
Insufficient levels of manganese in young animals leads to abnormal skeletal development (bone stiffness, reduced bone strength, etc), retarded growth, and delayed development. In adult animals this deficiency leads to decreased reproductive performance: conception is difficult, perinatal death is common, abortion may occur, and oestrous cycles are irregular or absent http://www.nap.edu/read/9791/chapter/7#66. The lack, or irregularity of oestrous cycles occurs in cases of manganese deficiency as without manganese sGC is not activated, so the cGMP-PKG pathway is not stimulated, and ultimately GnRH is not produced. A lack of GnRH means that little/no FSH and LH are produced. As FSH and LH are responsible for the production of the key reproductive hormones, oestrogen and progesterone respectively. Furthermore, ovulation is triggered by the LH surge, so it stands to reason that if without LH there can be no ovulation, and no oestrous. In males suffering from a deficiency of manganese, symptoms include decreased sperm production, testicular degeneration, and a lack of libido. http://www.odon.uba.ar/uacad/fisiologia/docs/nuevos/acute.pdf
Pathogenic effects as a result of deficiency or surplus of Mn are discussed in further detail according to which species is affected in the following paragraphs below.
= = Species-specific effects of manganese = =
Rodents - Rattus norvegicus
|
Due to manganese’s role as a cofactor to many enzymes it is fundamental to many biological reactions. Despite being a necessary trace element, elevated levels of manganese can negatively affect reproductive function, and deficiency can cause impairment of reproductive development. Exposure of prepubertal female rats (Rattus norvegicus) to elevated levels of manganese results in premature puberty. This is because manganese triggers the release of puberty related hormones, specifically GnRH. An experiment found that female rat pups exposed postnatally to elevated levels of manganese had a precocious increase in GnRH gene expression in the hypothalamus http://www.ncbi.nlm.nih.gov/pubmed/23997110. Increased levels of manganese in prepubertal rats therefore provoke the secretion of GnRH from the medial basal hypothalamus. GnRH causes the release of LH and FSH, which in turn trigger ovulation and follicle release respectively. Manganese restriction in pregnant mice has been found to cause delay in achievement of developmental milestones (e.g. opening of the eyes, descent of the testicles). Additionally, maternal manganese deficiency has been linked to a significant increase in pup death in the weeks preceding and following birth. http://www.ncbi.nlm.nih.gov/pubmed/11943509
Broilers - Gallus gallus domesticus
Approximately 3-5% of ingested Mn is absorbed across the intestinal wall during digestion, and excess Mn is readily excreted via bile. Molecular mechanisms involved in Mn transport from the digestive tract include DMT1 - an electrogenic transporter located on duodenal enterocytes and transports divalent cations, like Mn2+, from the extracellular to the intracellular side. http://journals.cambridge.org/download.php?file=%2FBJN%2FBJN108_02%2FS0007114511005629a.pdf&code=221401c413e2cfd814e1b68b258da7cd
Broilers generally suffer from a reduced ability to absorb Manganese. This may be due to the negative impact of intensive selective breeding methods incorporated by large-scale factory boiler farms; low priority is given to improving their weak digestive system when the focus is larger muscle mass and faster growth rate. Thus they require additional Mn supplementation, preferably organic form of Mn in the diet to meet nutritional requirements and promote positive reproductive performance. Insufficient manganese levels lead to a decrease in circulating P4, Estradiol (E2), LH and FSH, which ultimately affect the function of the hypothalamic-pituitary-gonadal axis. This may cause complications leading to malfunction of normal ovulatory processes, regression of the testes in roosters, and early death of chicks. http://journals.cambridge.org/download.php?file=%2FBJN%2FBJN108_02%2FS0007114511005629a.pdf&code=221401c413e2cfd814e1b68b258da7cd
|
However, excess amounts of manganese consequently cause the degeneration of dopaminergic neurons, which are also found in the hypothalamus. The mechanism by which Mn leads to selective destruction of these neurons is unknown however. Their neurotransmitter dopamine, through its different neuronal systems and receptor subtypes, plays a crucial role in the control of several aspects of sexual behaviour, specifically in the preparatory phase of sexual arousal (as well as motivation and reward). http://www.ncbi.nlm.nih.gov/pubmed/7770195. Thus, it can be said that a decrease in the number of dopaminergic neurons may somewhat diminish the willingness to copulate, so having a detrimental effect on population numbers.
Broiler breeder hens were fed diets supplemented with different concentrations of Mn/kg as MnSO4 (inorganic form) or Mn proteinate (organic form). The most beneficial amount was found experimentally to be 240 mg/kg in Mn proteinate form since it promoted eggshell-breaking strength without affecting eggshell thickness http://ps.oxfordjournals.org/content/93/4/959.long Mn proteinate was favoured over the inorganic form since it is hypothesised that organic mineral complexes/ chelates could resist interference from dietary and nutritional factors from the digestive tract and directly reach the intestinal brush border, where it is hydrolysed and absorbed as ions into the blood. This results in higher bioavailability of the complexed/ chelated form than the inorganic form of the metal. Organic complexes/ chelates can also maintain their structural integrity in the digestive tract and arrive at absorptive sites in the small intestine as the original, intact molecules http://journals.cambridge.org/download.php?file=%2FBJN%2FBJN108_02%2FS0007114511005629a.pdf&code=221401c413e2cfd814e1b68b258da7cd Expression of FSH and GnRH-I genes was significantly increased with this amount of Mn supplement. Furthermore, inorganic Mn supplementation doubled GnRH-I compared to its organic counterpart. This could be possibly due to a decreased capacity of the organic form of Mn passing through the blood-brain barrier.
Swine - Sus scrofa domesticus
A less extensively investigated species, yet one with the potential to reveal a clearer picture of the role of Mn in hypothalamic regulation of reproduction, can be found in studies of swine.
Manganese deficiency in Sus scrofa domesticus can be characterised as affecting oestrus cycle, lactation and foetal resorption. CITE Foetal resorption has been demonstrated in many species to result from abnormal levels of progesterone. CITE Progesterone levels can be affected due to a lack of precursor production through a lack of manganese to work as a cofactor in the mevalonate pathway.
Female gilts fed on lower Mn concentrations have been shown to exhibit irregularity of oestrus cycle and even a complete absence of the indications of oestrus cycle occurring whatsoever. CITE As before this can be attributed to the affected mevalonate pathway and additionally, can be connected to the lack of stimulation of the cGMP/PKG pathway and hence, a reduction in GnRH secretion from the hypothalamus CITE A decrease in GnRH ultimately will affect the LH pulse during oestrus and consequently, ovulation.
The effect on lactation was demonstrated by poor udder development and significantly reduced milk production and secretion to an extent where this presented as an almost total absence of both. CITE Progesterone affects alveolar and lobe growth: higher levels prevent lactation before parturition and a drop in the hormone postpartum acts as the trigger for lactogenesis. Oestrogen also stimulates growth of the milk duct system (again, lobuloalveolar development) and, with a rise and fall in levels as with progesterone, triggers milk production postpartum.CITE Both of these hormones are affected through the mevalonate pathway through their precursor origin of cholesterol. Furthermore, FSH and LH levels which are affected through GnRH release can potentially alter physiological levels of both progesterone and oestrogen production. *can cite both Plumlee & Grummer for lactation effect* When provided higher dietary levels of manganese, it is suggested that early onset of estrus is exhibited in sows. Those fed on higher concentrations of manganese had a greater tendency to undergo estrus earlier than those fed on lower concentrations. CITE. Estrus is stimulated via a surge in LH brought about through a rise in oestrogen provided by the developing ovarian follicles under regulation by gonadotropic hormones LH & FSH. Administration of pulsatile doses of GnRH has been shown to induce estrus in multiple species CITE and helps in the explanation as to why greater manganese levels are seen to bring about these effects; an increase in manganese allows for greater production and secretion of GnRH and ultimately, a rise in LH.