Effects of Manganese on the Hypothalamic Regulation of Reproduction
Martina Azzopardi, Ashli Welcome, Clare Wilson
Contents
- Effects of Manganese on the Hypothalamic Regulation of Reproduction
Introduction
|
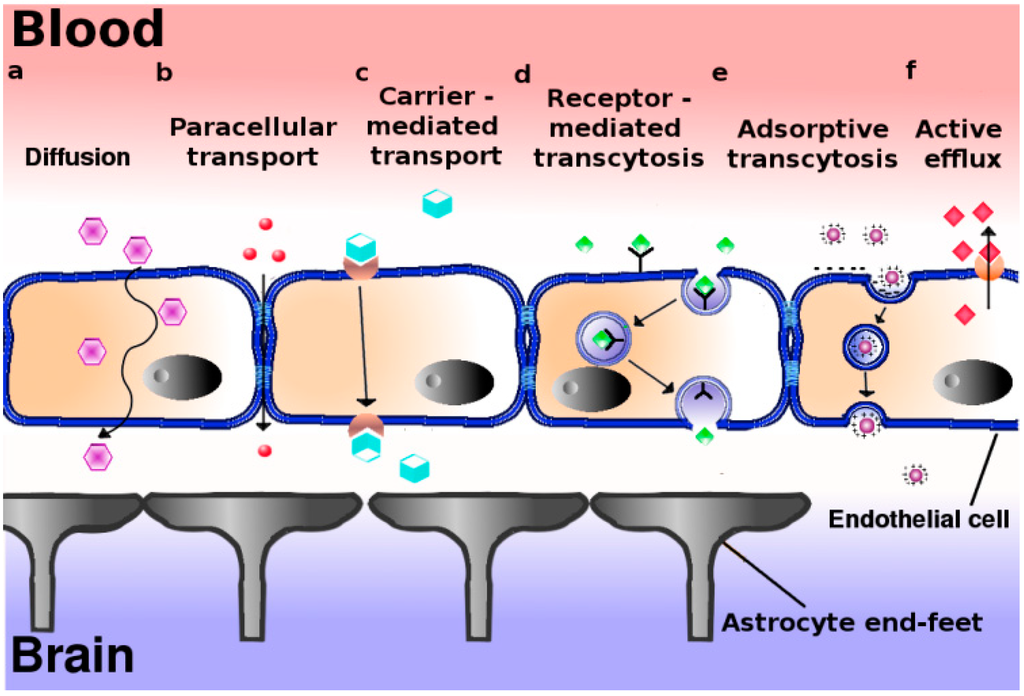
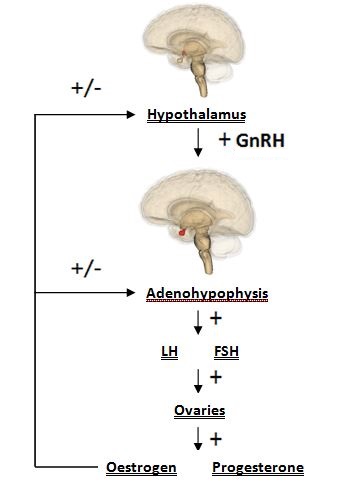
The Hypothalamus is a portion of the brain consisting of a number of small nuclei with a variety of functions; most importantly, it provides a link between the nervous system and endocrine system via the hypophysis. Manganese is a naturally-occurring metal required for normal mammalian reproductive function. It is able to cross the blood-brain barrier and accumulate in the hypothalamus, where it exerts its effect. It does so through several carriers in different oxidation states, specifically via Bornhorst:
* Divalent metal transporter 1 (DMT1)
* Transferrin receptors (TfR)
* Divalent metal/bicarbonate ion symporters ZIP8 and ZIP14
* Various calcium channels
* Solute carrier-39 (SLC39) family of zinc transporters
* Park9/ATP13A2
* Magnesium transporter hip14
* Transient receptor potential melastatin 7 (TRPM7) channels/transporters
* Homomeric purine receptors (P2X and P2Y)
* Citrate transporter
|
Manganese is able to directly influence neuroendocrine effect related to reproduction. Manganese crosses the blood-brain barrier more effectively, and is eliminated with greater difficulty, in younger animals; subsequently, it can exert its effect in the developing reproductive system readily. Hormones released by the hypothalamus are directly affected: namely, direct stimulation of the secretion of gonadotropin releasing hormone (GnRH). GnRH is a neuropeptide molecule (comprised of 10 amino acids) which is produced by the parvocellular cells of the hypothalamus. GnRH is an integral component of reproductive development through its direct stimulation of the production and secretion of gonadotropin hormones by hypophyseal cells: specifically, follicle stimulating hormone (FSH) and luteinizing hormone (LH).
Physiological role of Mn in reproduction
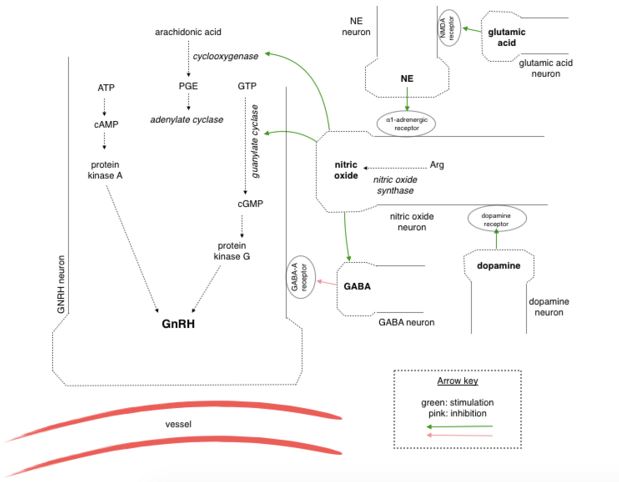
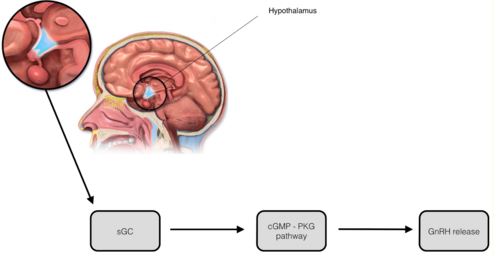
Manganese triggers GnRH release in rats by facilitating activation of soluble guanylyl cyclase (sGC), which subsequently stimulates the cGMP - protein kinase G pathway (cGMP-PKG). The cGMP-PKG pathway controls the release of GnRH in prepubertal female rats. Experiments found that the release of GnRH due to the effects of manganese was inhibited when a blocker of sGC (1H-1,2,4-oxadiazolo-4,3-quinoxalin-1, more commonly known as ODQ) was introducedPrestofillipo
|
Glutamic acid and catecholamines are important neurotransmitters in GnRH release. The former stimulates noradrenergic neuronal terminals resulting in release of norepinephedrine and subsequent activation of α1-adrenergic receptors located on specific neurons causing a rise in intracellular Ca2+ levels. This activates nitric oxide synthase leading to production of nitric oxide. Manganese has long been established as the favoured cofactor for guanylyl cyclase (GC) and it acts to increase the activity GC directly, or combined with cofactor nitric oxide (NO). The cGMP-PKG pathway is then activated, leading to the exocytosis of GnRH 2.
|
GnRH has a stimulating effect on the release of FSH and LH. FSH is responsible for follicle maturation, and LH triggers ovulation via the LH surge. In this way, manganese plays a key role in the basic processes of mammalian reproduction
Additionally, manganese is shown to have further effect on normal hormonal function through its involvement in maintaining physiological levels of sex hormone precursor. This effect of manganese on the hormones of the reproductive system can be attributed to its role as a cofactor to both mevalonate kinase and farnesyl pyrophosphate synthase. Both enzymes are involved in cholesterol synthesis via the mevalonate pathway. Cholesterol is a steroid hormone and specifically, a sex hormone precursor. Thus, a lack of cholesterol affects normal hormonal function as synthesis capacity for important reproduction biomolecules, such as progesterone and oestrogen, will be directly affected via an inhibited mevalonate pathway. https://upload.wikimedia.org/wikipedia/commons/0/08/Progesterone_biosynthesis.png https://upload.wikimedia.org/wikipedia/commons/thumb/b/b4/Mevalonate_pathway.svg/2000px-Mevalonate_pathway.svg.png
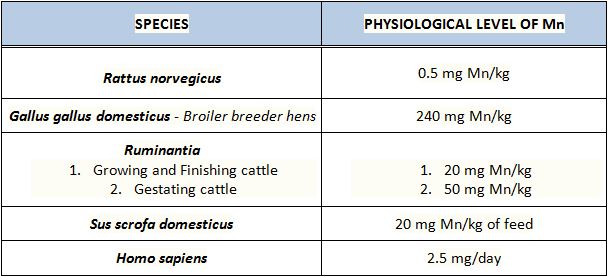
Typical Manganese Levels In Different Species
|
Pathogenicity: a summary of the effects of manganese in reproduction
Manganese is responsible for reproductive development, and so high levels trigger the early onset of puberty by increasing the expression of GnRH genes. Manganese is present in the plasma of mothers & the umbilical cord blood of premature and full-term babies. Limited information suggests that higher-than-usual amounts of Mn can cause birth defects Dart
Insufficient levels of manganese in young animals leads to abnormal skeletal development (bone stiffness, reduced bone strength, etc), delayed growth and impaired development. In adult animals this deficiency leads to decreased reproductive performance: conception is difficult, perinatal death is common, abortion may occur and oestrous cycles are irregular or absent. National Research Council. The lack, or irregularity of oestrous cycles occurs in cases of manganese deficiency as without manganese sGC is not activated, so the cGMP-PKG pathway is not stimulated, and ultimately GnRH is not produced. A lack of GnRH means that little/no FSH and LH are produced. As FSH and LH are responsible for the production of the key reproductive hormones, oestrogen and progesterone respectively. Furthermore, ovulation is triggered by the LH surge, so it stands to reason that if without LH there can be no ovulation, and no oestrous. In males suffering from a deficiency of manganese, symptoms include decreased sperm production, testicular degeneration, and a lack of libido.Prestifilipo
Pathogenic effects as a result of deficiency or surplus of Mn are discussed in further detail according to which species is affected in the following paragraphs below.
Species-specific effects of Mn
Rodents - Rattus norvegicus
|
Due to manganese’s role as a cofactor to many enzymes it is fundamental to many biological reactions. Despite being a necessary trace element, elevated levels of manganese can negatively affect reproductive function, and deficiency can cause impairment of reproductive development.
Exposure of prepubertal female rats (Rattus norvegicus) to elevated levels of manganese results in premature puberty. This is because manganese triggers the release of puberty related hormones, specifically GnRH. An experiment found that female rat pups exposed postnatally to elevated levels of manganese had a precocious increase in GnRH gene expression in the hypothalamus Srivastka. Increased levels of manganese in prepubertal rats therefore provoke the secretion of GnRH from the medial basal hypothalamus. GnRH causes the release of LH and FSH, which in turn trigger ovulation and follicle release respectively.
Manganese restriction in pregnant mice has been found to cause delay in achievement of developmental milestones (e.g. opening of the eyes, descent of the testicles). Additionally, maternal manganese deficiency has been linked to a significant increase in pup death in the weeks preceding and following birth. Torrente
Broilers - Gallus gallus domesticus
Approximately 3-5% of ingested Mn is absorbed across the intestinal wall during digestion, and excess Mn is readily excreted via bile. Molecular mechanisms involved in Mn transport from the digestive tract include DMT1 - an electrogenic transporter located on duodenal enterocytes and transports divalent cations, like Mn2+, from the extracellular to the intracellular side.
Broilers generally suffer from a reduced ability to absorb Manganese. This may be due to the negative impact of intensive selective breeding methods incorporated by large-scale factory boiler farms; low priority is given to improving their weak digestive system when the focus is larger muscle mass and faster growth rate. Thus they require additional Mn supplementation, preferably organic form of Mn in the diet to meet nutritional requirements and promote positive reproductive performance. Insufficient manganese levels lead to a decrease in circulating P4, Estradiol (E2), LH and FSH, which ultimately affect the function of the hypothalamic-pituitary-gonadal axis. This may cause complications leading to malfunction of normal ovulatory processes, regression of the testes in roosters, and early death of chicks.Bai
|
However, excess amounts of manganese consequently cause the degeneration of dopaminergic neurons, which are also found in the hypothalamus. The mechanism by which Mn leads to selective destruction of these neurons is unknown however. Their neurotransmitter dopamine, through its different neuronal systems and receptor subtypes, plays a crucial role in the control of several aspects of sexual behaviour, specifically in the preparatory phase of sexual arousal (as well as motivation and reward).A,M.. Thus, it can be said that a decrease in the number of dopaminergic neurons may somewhat diminish the willingness to copulate, so having a detrimental effect on population numbers.
Broiler breeder hens were fed diets supplemented with different concentrations of Mn/kg as MnSO4 (inorganic form) or Mn proteinate (organic form). The most beneficial amount was found experimentally to be 240 mg/kg in Mn proteinate form since it promoted eggshell-breaking strength without affecting eggshell thickness Xie. Mn proteinate was favoured over the inorganic form since it is hypothesised that organic mineral complexes/ chelates could resist interference from dietary and nutritional factors from the digestive tract and directly reach the intestinal brush border, where it is hydrolysed and absorbed as ions into the blood. This results in higher bioavailability of the complexed/ chelated form than the inorganic form of the metal. Organic complexes/ chelates can also maintain their structural integrity in the digestive tract and arrive at absorptive sites in the small intestine as the original, intact molecules. Expression of FSH and GnRH-I genes was significantly increased with this amount of Mn supplement. Furthermore, inorganic Mn supplementation doubled GnRH-I compared to its organic counterpart. This could be possibly due to a decreased capacity of the organic form of Mn passing through the blood-brain barrier.
Ruminantia
Manganese deficiency in cattle, goats and sheep was found to detrimentally impair the oestrous cycle and reduce calf birth weight. It caused a delay in the first occurrence of the oestrus cycle, and more attempts were required to obtain a potentially successful conception. This is because optimal embryo development to the blastocyst stage is partially dependent on the presence of Mn during maturation of oocytes in vitro (in vitro maturation - IVM). Moreover, availability of Mn during oocyte maturation ensures ‘normal’ intracellular glutathione (GSH) content in cumulus oocyte-complexes (COCs) and protects DNA integrity of cumulus cells. Anchordoquy
Gestating Heifers
Nutritionally speaking it is recommended that both growing and finishing cattle intake approx 20mg Mn/kg daily via their diet. National Research Council This amount, although meeting the needs for growth and skeletal development, is not sufficient to meet the needs of normal reproduction. Mn deficiency in gestating heifers is often difficult to diagnose due to no apparent visual signs in the female herself. However, the signs can be observed in the calf, which is born suffering from impaired fetal growth and development. Experimental results suggest that feeding gestating heifers a diet of 50mg of Mn/kg of body weight is sufficient to overcome signs of Mn deficiency in calves Hansen
Swine - Sus scrofa domesticus
|
A less extensively investigated species, yet one with the potential to reveal a clearer picture of the role of Mn in hypothalamic regulation of reproduction, can be found in studies of swine.
Manganese deficiency in Sus scrofa domesticus can be characterised as affecting oestrus cycle, lactation and foetal resorption. CITE Foetal resorption has been demonstrated in many species to result from abnormal levels of progesterone. CITE Progesterone levels can be affected due to a lack of precursor production through a lack of manganese to work as a cofactor in the mevalonate pathway.
Female gilts fed on lower Mn concentrations have been shown to exhibit irregularity of oestrus cycle and even a complete absence of the indications of oestrus cycle occurring whatsoever. CITE As before this can be attributed to the affected mevalonate pathway and additionally, can be connected to the lack of stimulation of the cGMP/PKG pathway and hence, a reduction in GnRH secretion from the hypothalamus CITE A decrease in GnRH ultimately will affect the LH pulse during oestrus and consequently, ovulation.
The effect on lactation was demonstrated by poor udder development and significantly reduced milk production and secretion to an extent where this presented as an almost total absence of both. CITE Progesterone affects alveolar and lobe growth: higher levels prevent lactation before parturition and a drop in the hormone postpartum acts as the trigger for lactogenesis. Oestrogen also stimulates growth of the milk duct system (again, lobuloalveolar development) and, with a rise and fall in levels as with progesterone, triggers milk production postpartum.CITE Both of these hormones are affected through the mevalonate pathway through their precursor origin of cholesterol. Furthermore, FSH and LH levels which are affected through GnRH release can potentially alter physiological levels of both progesterone and oestrogen production. *can cite both Plumlee & Grummer for lactation effect*
When provided higher dietary levels of manganese, it is suggested that early onset of estrus is exhibited in sows. Those fed on higher concentrations of manganese had a greater tendency to undergo estrus earlier than those fed on lower concentrations. CITE. Estrus is stimulated via a surge in LH brought about through a rise in oestrogen provided by the developing ovarian follicles under regulation by gonadotropic hormones LH & FSH. Administration of pulsatile doses of GnRH has been shown to induce estrus in multiple species CITE and helps in the explanation as to why greater manganese levels are seen to bring about these effects; an increase in manganese allows for greater production and secretion of GnRH and ultimately, a rise in LH.
Current scientific literature is lacking in studies to point to a direct link between the effect on reproduction directly shown to be of hypothalamic origin in swine. However, a valid path of research can be proposed given the direct effect of manganese on the oestrus cycle and lactation; similarly guided experiments to those conducted in rats and poultry could potentially elucidate on similar hormonal implications via affected hypothalamic regulation in this species.
Humans - Homo sapiens
Infants and young children do not have fully developed manganese excretion mechanisms, and their gastrointestinal tracts also absorb manganese more readily than that of an adult. Additionally, manganese crosses the blood-brain barrier more effectively in children. The culmination of the aforementioned factors renders children susceptible to overexposure to manganese. This sensitivity of infants and children to high levels of manganese has high nutritional and medical relevance as many rice- and soybean-based infant formulas have a high manganese concentration (unlike breast milk which contains a very little manganese). Oulhote.
Maternal consumption of manganese supplemented water in Bangladesh was found to significantly reduce the risk of spontaneous abortion. The principal cause of spontaneous abortion is placental oxidative stress. The risk is greatest at 8-12 weeks of pregnancy due to a sudden burst of oxidative stress from the mitochondria rich placenta in order to establish maternal blood flow. In order to combat this oxidative stress, an adequate amount of antioxidants are required to detoxify placental superoxide ions produced during oxidative stress (and in doing so prevent oxidative stress related tissue damage which can lead to foetal death). Manganese superoxide dismutase (MnSOD), pictured below, is one such antioxidant. MnSOD may play an essential role in foetal protection in malnourished/ undernourished women suffering from a zinc deficiency as they would have reduced zinc superoxide dismutase (ZnSOD), and so would be more sensitive to the devastating effects of oxidative stress. Supplementation of manganese to such women would allow ZnSOD to be replaced with MnSOD, thus preventing spontaneous abortion. Rahman.
Manganese deficiency is also involved in (pre-eclampsia) []. One of the major factors in the development of pre-eclampsia is oxidative stress in the placenta. A lack of manganese leads to a decreased amount of MnSOD, and so its antioxidant effect is impaired.
|
Other Species
Paracentrotus lividus
Maternal exposure to elevated manganese levels was found to cause offspring mutations, especially in the arms of larvae in sea urchins. The larval arms appeared to be shorter than average, or malformed. Migliaccio
Nyctereutes procyonoides
In Ussuri raccoon dogs, it was found that the number of pups born alive, and the number of pups weaned alive were highest in groups with adequate manganese levels. Increased levels of manganese resulted in the decreased litter size. Bao
Conclusion: applications in the veterinary field
Extreme levels of manganese, both high and low, have a catastrophic effect on the development and functioning of the reproductive system across species. That being said, at appropriate levels manganese can be very beneficial in a variety of medical situations. One such situation is in bovine (IVF) where the addition of an adequate amount of manganese to the IVF medium improves the health of the oocytes and results in more cleaved embryos Anchordoquy.
Further use for manganese in IVF can be seen in the (cryopreservation) of sperm. Sperm used in IVF is often stored in liquid nitrogen to preserve it, however this negatively impacts the motility of the sperm and often results in protein leakage. The addition of manganese to the semen resulted in improved quality of the semen once thawed (i.e. improved motility, decreased hypo-osmotic swelling, and decreased protein leakage) Ranjna. During sperm metabolism, reactive oxygen species (ROS) are generated and used for the maturation and capacitation of sperm. However, frozen sperm are very sensitive to lipid peroxidation caused by ROS, and so antioxidants (like MnSOD) have a protective function and so improve the overall quality of the sperm.
Another application of manganese is the improvement of eggshell quality. It has been found that manganese (in an appropriate amounts) increased the egg shell’s resistance to breakage without affecting its thickness. This indicates that the supplementation of manganese could improve the durability and strength of eggs without negatively impacting the ability of hatchlings to break through the shell (as would be the case if the thickness of the shell was increased).
References
* [1] Bornhorst J., Wehe C. A., Hügel S., Karst U., Galla H-J. & Schwerte T, (JBC Papers, 2012). Impact of Manganese on and Transfer across Blood-Brain and Blood-Cerebrospinal Fluid Barrier in Vitro, DOI 10.1074/jbc.M112.344093, (5) 7-15
* [2] Prestifilippo, J., Fernandez-Solari, J., De Laurentiis, A., Mohn, C., de la Cal, C., Reynoso, R., Dees, W. and Rettori, V. (2008). "Acute Effect of Manganese on Hypothalamic Luteinizing Hormone Releasing Hormone Secretion in Adult Male Rats: Involvement of Specific Neurotransmitter Systems" Toxicological Sciences, 105(2), pp.295-302.
[3] Dart R. C. (Lippincott Williams & Wilkins, 2004), Medical Toxicology, ISBN 0781728452, 9780781728454
<<Anchor (5)>> Subcommittee on Beef Cattle Nutrition; Committee on Animal Nutrition; Board on Agriculture; National Research Council, Nutrient Requirements of Beef Cattle (2000), ISBN 978-0-309-38813-9
- Lee, B., Hiney, J., Pine, M., Srivastava, V. and Dees, W. (2007). Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. The Journal of Physiology, 578(3), pp.765-772.
[4]Hansen SL, e. (2016). Feeding a low manganese diet to heifers during gestation impairs fetal growth and development. - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17033018 [Accessed 23 Apr. 2016].
[5] Srivastava VK, e. (2016). Early life manganese exposure upregulates tumor-associated genes in the hypothalamus of female rats: relationship to manganese-induced precocious p... - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23997110 [Accessed 23 Apr. 2016].
[6]Torrente M, e. (2016). Effects of prenatal exposure to manganese on postnatal development and behavior in mice: influence of maternal restraint. - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11943509 [Accessed 23 Apr. 2016].
[7]A, M. (2016). Dopamine and sexual behavior. - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7770195 [Accessed 23 Apr. 2016].
[8]Oulhote, Y., Mergler, D. and Bouchard, M. (2014). Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environmental Health, 13(1), p.87.
[9]Rahman, S., Åkesson, A., Kippler, M., Grandér, M., Hamadani, J., Streatfield, P., Persson, L., Arifeen, S. and Vahter, M. (2013). Elevated Manganese Concentrations in Drinking Water May Be Beneficial for Fetal Survival. PLoS ONE, 8(9), p.e74119.
[10]Prestifilippo, J., Fernandez-Solari, J., De Laurentiis, A., Mohn, C., de la Cal, C., Reynoso, R., Dees, W. and Rettori, V. (2008). Acute Effect of Manganese on Hypothalamic Luteinizing Hormone Releasing Hormone Secretion in Adult Male Rats: Involvement of Specific Neurotransmitter Systems. Toxicological Sciences, 105(2), pp.295-302.
[11]Anchordoquy JP, e. (2016). Influence of manganese on apoptosis and glutathione content of cumulus cells during in vitro maturation in bovine oocytes. - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24154966 [Accessed 23 Apr. 2016].
[12]Ranjna S Cheema, G. (2009). Manganese provides antioxidant protection for sperm cryopreservation that may offer new consideration for clinical fertility. Oxidative Medicine and Cellular Longevity, [online] 2(3), p.152. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2763241/ [Accessed 23 Apr. 2016].
[13]Migliaccio, O., Castellano, I., Cirino, P., Romano, G. and Palumbo, A. (2015). Maternal Exposure to Cadmium and Manganese Impairs Reproduction and Progeny Fitness in the Sea Urchin Paracentrotus lividus. PLOS ONE, 10(6), p.e0131815.
[14]Bao K, e. (2016). Effect of supplementation of organic manganese on reproductive performance of female Ussuri raccoon dogs (Nyctereutes procyonoides) during the bree... - PubMed - NCBI. [online] Ncbi.nlm.nih.gov. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25082102 [Accessed 23 Apr. 2016].
[15]National Research Council (U.S.)., (1996). Nutrient Requirements of Beef Cattle (Nutrient requirements of domestic animals). National Academies Press.
[16]Bai, S., Lu, L., Wang, R., Xi, L., Zhang, L. and Luo, X. (2011). Manganese source affects manganese transport and gene expression of divalent metal transporter 1 in the small intestine of broilers. British Journal of Nutrition, 108(02), pp.267-276.
[17]Xie, J., Tian, C., Zhu, Y., Zhang, L., Lu, L. and Luo, X. (2014). Effects of inorganic and organic manganese supplementation on gonadotropin-releasing hormone-I and follicle-stimulating hormone expression and reproductive performance of broiler breeder hens. Poultry Science, 93(4), pp.959-969.
[18]Anchordoquy, J., Anchordoquy, J., Sirini, M., Mattioli, G., Picco, S. and Furnus, C. (2013). Effect of Different Manganese Concentrations during in vitro Maturation of Bovine Oocytes on DNA Integrity of Cumulus Cells and Subsequent Embryo Development. Reproduction in Domestic Animals, 48(6), pp.905-911.