The physiological challenges posed by rapid ascent to 6000m
By Connor Herst, Ethan Ryan and Alice Gofard
Contents
Introduction
Rapid ascent to 6000m is equivalent to flying to an airstrip in the Andes mountains from sea level. When moved to this height, there are several significant changes in conditions that the body has to deal with. At this height the air pressure may by about 380mmHg, compared to 760mmHg at sea level and the temperature will decrease to an average of around -10 degrees Celsius. The P02 will drop significantly from 160 to 80mmHg. The rapid ascent does not allow the body to acclimatise to these changes.
Changed conditions
Air pressure decreases non-linearly with altitude gain. At height, although the composition of air is the same, fewer moles of gas will actually be taken into the lungs with each breath. The partial pressure of a gas in a mixture is equal to the fraction of the mixture occupied by the gas multiplied by the total pressure of the mixture. As the total air pressure is decreased at altitude, this means the partial pressure of any specific gas e.g. oxygen will be reduced. For example at 6000m where the air pressure is around 380mmHg:
P02 = 0.209x380 = 79.42 mmHg at standard 273K dry air
After accounting for an increase in gas temperature to body temperature and a saturation of the air with water, inspired P02 becomes around 70mmHg as the water gives counts for a partial pressure of 47mmHg at body temperature.
PI02 =333x0.209=69.7mmHg at 310K moist air
This shows that the inspired partial pressure of oxygen is significantly lower than at sea level, where it is around 149mmHg. This will have a large affect on the alveolar and therefore arteriolar partial pressures of oxygen. A normal resting breathing rate for a person who is not acclimatised would not provide a high enough arteriolar partial pressure of oxygen to supply oxygen to the body. Therefore rapid ascent to 6000m will initially cause hypoxic hypoxia. (Brown and Groscott, 2012)
Hypoxia
Alveolar hypoxia can affect the pulmonary vascular resistance. At altitude, there will be a general hypoxia of all the alveoli of the lung. This stimulates a general vasoconstriction of the pulmonary arteries and it is thought this occurs due to the low Pa02 acting directly on the smooth muscles of the pulmonary arterioles. This will cause an increase in pulmonary vascular resistance, and an increase in pulmonary arterial pressure and pulmonary hypertension. (Naeije and Brimioulle,2001) This increases the hydrostatic pressure in the capillaries, forcing fluid out of the capillaries and into the alveoli, causing pulmonary oedema.
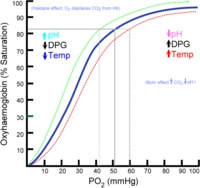 Figure 1
Figure 1
On a general cellular level, hypoxia will stimulate the production of hypoxia-inducible transcription factors (HIFs). HIFs consist of a dimer of an alpha subunit and a beta subunit. The HIFs positively regulate genes encoding angiogenic factors and erythropoietin, to promote the creation of new blood vessels and red blood cells to try to improve oxygen transport. It is a long term response for the proportion of blood cells and therefore haemoglobin in the blood to increase. In the red blood cells, it is thought hypoxia stimulates an increased production of 2,3 DPG which causes a right shift in the oxygen dissociation curve of haemoglobin, we can see this shift in the curve in figure 1. This will aid unloading of O2 where there is low PO2, but also make it oxygen loading onto haemoglobin in red blood cells less efficient in the lungs. (Wang and Semenza,1993)
On a general cellular level, hypoxia will stimulate the production of hypoxia-inducible transcription factors (HIFs). HIFs consist of a dimer of an alpha subunit and a beta subunit. The HIFs positively regulate genes encoding angiogenic factors and erythropoietin, to promote the creation of new blood vessels and red blood cells to try to improve oxygen transport. It is a long term response for the proportion of blood cells and therefore haemoglobin in the blood to increase. In the red blood cells, it is thought hypoxia stimulates an increased production of 2,3 DPG (figure 1) which causes a right shift in the oxygen dissociation curve of haemoglobin. This will aid unloading of O2 where there is low PO2, but also make it oxygen loading onto haemoglobin in red blood cells less efficient in the lungs.
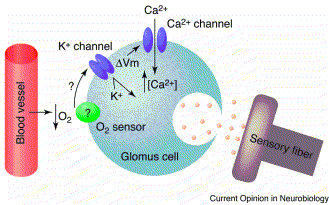 Figure 2
Figure 2
A decrease in arteriolar P02 will be detected by glomus cells (figure 2.), in a set of peripheral chemoreceptors located in the carotid bodies at the bifurcation of the common carotid. They can also detect changes in PC02 and pH. The fall in Pa02 will stimulate an inhibition of the potassium ion channel in the glomus cell. This causes cell depolarisation which leads to the entry of calcium into the cell, which is seen in figure 2, and the release of neurotransmitter.(Control of ventilation) This signal reaches the medulla and results in an increase in ventilation rate. In experiments, the ventilation rate has been shown to significantly increase as the alveolar P02 goes below 60mmHg, as will be the case in ascending rapidly to 6000m. This occurs as above 60mmHg, haemoglobin is 90% saturated with oxygen, so increasing ventilation rate would have little effect. However, below this level there is a steep decrease in the oxygen affinity of haemoglobin, so a sharp increase in ventilation is needed to keep P02 above the 60mmHg mark. This means that when a person rapidly ascends to 6000m, they will hyperventilate to raise their low P02 levels.
A decrease in arteriolar P02 will be detected by glomus cells, in a set of peripheral chemoreceptors located in the carotid bodies at the bifurcation of the common carotid. They can also detect changes in PC02 and pH. The fall in Pa02 will stimulate an inhibition of the potassium ion channel in the glomus cell. This causes cell depolarisation which leads to the entry of calcium into the cell, and the release of neurotransmitter.(Control of ventilation) This signal reaches the medulla and results in an increase in ventilation rate. In experiments, the ventilation rate has been shown to significantly increase as the alveolar P02 goes below 60mmHg, as will be the case in ascending rapidly to 6000m. This occurs as above 60mmHg, haemoglobin is 90% saturated with oxygen, so increasing ventilation rate would have little effect. However, below this level there is a steep decrease in the oxygen affinity of haemoglobin, so a sharp increase in ventilation is needed to keep P02 above the 60mmHg mark. This means that when a person rapidly ascends to 6000m, they will hyperventilate to raise their low P02 levels. (Lopez-Barneo, Ortega-Saenz, Pardal, Pascual and Piruat,2008)
Hypoxia will also have cardiovascular effects. Locally in the tissues, the low P02 in the arteries will cause hyperaemia via local vasodilation. This could cause a deadly decrease in blood pressure, if it were not for the primary chemoreceptor response. In this response, the general hypoxia is detected by the peripheral chemoreceptors in the aortic and carotid bodies which cause an increase in sympathetic vasoconstrictor tone. This compensates for the hypoxia stimulation, to maintain blood pressure to maintain sufficient blood supply to the coronary arteries and brain. The secondary chemoreceptor response is also caused by the carotid body chemoreceptors. They stimulate the vasomotor centre to cause venoconstriction of the splanchnic bed, vasodilation of arterioles to vital tissues and vasoconstriction of arterioles to non-vital tissues. This causes an increase in venous return and therefore an increase in cardiac output which increases blood and therefore oxygen supply to the vital tissues, despite the decreased oxygen content of the blood due to altitude. (Bärtsch, Simon, Gibbs,2007)
Hypocapnia
However this response only considers P02. Initially the subject at altitude will have a PaC02 of 40mmHg, as this is the normal partial pressure in the alveoli as a result of normal ventilation and cell metabolism. When the subject begins to hyperventilate due to hypoxia, there will be a resultant decrease in PaC02.(Laffey,2002). PaC02 is a very important marker in itself to the sensors involved in the control of breathing, but PaC02 also affects blood pH, another important variable which must be controlled.(Rhoades and Bell, 2008). Experiments varying alveolar PC02 and measuring ventilation rate have shown that a decrease in PaC02 provides a large stimulus to decrease ventilation rate. (West) It has also been shown that changes in PaC02 is sensed and responded to mainly by the central nervous system, as the peripheral sensors can be removed and the same responses to PaC02 change are seen. Chemosensitive areas have been found in the ventral surface of the medulla and by the XII cranial nerve root. They respond to changes in PaC02 and pH, as has been shown in experiments applying solutions which are acidic or have high PC02 to the chemosensitive areas where they increase ventilation rate within a couple of seconds. As the chemoreceptors are located in the medulla, they are covered with brain extracellular fluid and separated from the capillaries by the blood brain barrier. (Nattie, E. 1999) They must therefore respond to changes in the chemical composition of the cerebral-spinal fluid which they sit in as opposed to directly responding to blood composition like peripheral chemoreceptors. The blood brain barrier tightly controls the passage of solutes across it regulate the composition of the CSF and to help prevent infection and toxins reaching the brain. As the CSF is actively produced by the ventricles, and not simple a filtrate of the blood, it can have special properties, and has low levels of protein, HCO3- ,K+ and Ca2+ ions, in addition to high levels of sodium and chloride ions. (Irani, 2009) Changes in pH cannot directly transmit from the capillaries to the CSF as the blood brain barrier is impermeable to HCO3- and H+, however it is permeable to C02. Therefore changes in the pH of the CSF are mediated by changes in C02 concentration as it can diffuse across the barrier. It has been shown experimentally that it is change in pH that the chemoreceptors respond to, changing the PC02 while maintaining constant pH using buffers will give no response. Therefore the decrease in PaC02 will cause C02 to diffuse from the CSF into the blood in the capillaries. This removal of CO2 will drive an increased binding of HCO3- to H+ to give carbon dioxide and water. (Rhoades and Bell, 2008) This removal of H+ will in turn increase the pH and cause alkylosis of the CSF. This stimulates the chemoreceptors to cause a decrease in ventilation rate. The low protein concentration of the CSF means that the only major buffer is HCO3-. This means the pH in the CSF is relatively sensitive to changes in PaC02. The transport of C02 into the blood from the CSF, and its reaction with water to form carbonic acid which dissociates allows HC03- in the CSF to act as a buffer for blood pH. This takes place before the kidneys have time to respond by excreting HCO3- to decrease blood pH, which usually takes a couple of days of exposure. Therefore the decrease in PaC02 has a braking effect on the increased ventilation rate stimulated by low Pa02. There is a balance reached between the two variables.
Thermoregulatory response
At 6000m, it is likely to be much colder than at sea level, at an average of around -10 degrees Celsius. This temperature drop will elicit a thermoregulatory response. Heat will be mainly lost as radiation and conduction from the skin. Therefore changing decreasing the skin temperature will decrease the heat lost via radiation and conduction. The skin temperature is decreased by the constriction of cutaneous vessels supplying the skin to divert blood flow away from the skin to hold heat in the centre of the body. There is also a local response on cutaneous vessels to the cold as they constrict when they sense cold. The rate of heat loss of the skin can be reduced by erector pilli, smooth muscle, contracting to cause the hairs on the skin to stand up. This traps a layer of warm air around the skin, decreasing the rate of heat loss. These adaptations minimize the heat lost by the body. The hypothalamus is the thermoregulatory centre and receives sensory temperature information from various sources including itself and skin temperature receptors. The posterior hypothalamus is responsible for responding to signals indicating the cold. It has been shown that a core temperature of 36.8°C is the threshold level at which vasoconstriction is stimulated. A temperature of 36°C stimulated non-shivering thermogenesis and below 35.5 °C stimulates shivering. Non-shivering thermogenesis is mainly controlled by thyroid hormones and secretions of noradrenalin and adrenaline which stimulate thermogenesis via the sympathetic nervous system. There are various metabolic mechanisms of thermogenesis, for example the use of uncouplers so heat is produced from oxidative phosphorylation, leakage of pumps and futile cycles. Shivering consists of frequent contractions of the skeletal muscles, which accelerates the rate of respiration, which is an exothermic reaction, giving out heat. It also happens while a person is stationary as opposed to exercise where more heat is lost by convection. (Guyton, A.C. 1996)
Conclusion
As has been discussed, there are a variety of environmental factors that change suddenly upon rapid ascent to 6000m, and these changes threaten homeostasis in the body. The body does adapt to the conditions, but at this height they may not compensate well or quickly enough for the body to go without side effects such as mountain sickness.
Bibliography
- Peter Bärtsch,MD; J.Simon R.Gibbs, MD, FRCP (2007) Effect of Altitude on the Heart and the Lungs
- Guang L.Wang and Gregg L. Semenza(1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia
- Rhoades and Bell, 2008. Medical Physiology: Principles for Clinical Medicine, P388
- Guang L.Wang and Gregg L. Semenza(1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia
- Rhoades and Bell, 2008. Medical Physiology: Principles for Clinical Medicine, P388
- Control of ventilation. Control of breathing (8)
- Brown, J.P.R., Groscott, P,W,M,. (2012) Humans at Altitude: Physiology and Pathophysiology
Body Temperature, Temperature Regualation and Fever, 911-922. Textbook of Medical Physiology, W.B. SaundersCompany,Philadelphia.
- Laffey, J,G,. The New England Journal of Medicine: Hypocapnea
- Rhoades and Bell, 2008. Medical Physiology: Principles for Clinical Medicine: P387
- West, J,B,. Oxford Journals: Human responses to extreme altitudes
- Nattie, E. 1999. CO2, brainstem chemoreceptors and breathing
- Irani, D,N. 2009. Properties and Composition of normal cerebrospinal fluid. p69
- J. Lo´pez-Barneo, P. Ortega-Sa´enz, R. Pardal, A. Pascual and J.I. Piruat (2008) Carotid body oxygen sensing (P1387)
- Naeije and Brimioulle,2001. Physiology in medicine: importance of hypoxic pulmonary vasoconstriction in maintaining arterial oxygenation during acute respiratory failure
Sources
http://icb.oxfordjournals.org/content/46/1/25.full.pdf+html)
http://ceaccp.oxfordjournals.org/content/early/2012/09/04/bjaceaccp.mks047.extract#
http://rfumsphysiology.pbworks.com/w/page/12566765/Control%20of%20Breathing
http://en.wikipedia.org/wiki/Oxygen%E2%80%93haemoglobin_dissociation_curve
http://www.anaesthesia.med.usyd.edu.au/resources/lectures/acidbase_mjb/description.html
Figures
