|
Size: 19980
Comment:
|
Size: 19982
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 12: | Line 12: |
| == 1.1 What is a neuron? == | === 1.1 What is a neuron? === |
Synpatic Vesicles
Contents
1. Introduction
In order for both animals and humans to maintain survival they must first have the ability to produce an appropriate response to external stimuli and situations. This, of course, is achieved by the central and peripheral nervous systems, composed of the brain and spinal chord and the peripheral nerves, respectively.
The nervous system is responsible for the control of the body and its responses, but also for the communication between its elements (Ludwig & Varacallo, 2019). Nerve cells are the cell type that are responsible for the transmission of electrical impulses to and from muscles and organs (Ludwig & Varacallo, 2019). There are millions of these nerve cells throughout the body and they are in essence responsible for the transfer of information throughout the body, to allow actions to be carried out whether they are motor or sensory, voluntary or involuntary, sympathetic or parasympathetic (Ludwig & Varacallo, 2019). This transfer of information between neurons and other cells is known as synaptic neurotransmission, which will be the essence of this review. Defects in neurotransmission can lead to an array of mental disorders in humans, ranging from autism spectrum disorder, to Alzheimer’s disease, and even addiction (van Spronsen & Hoogenraad, 2010)
1.1 What is a neuron?
The neuron is a specialized cell type that is structured to allow for the transmission of information. Neurons contain a nucleus enclosing their genetic information and other cell organelles that allow its function (Lodish, 2000). Neurons have unique characteristics in comparison with other cells of the body. Firstly, neurons are mostly incapable of division. This means that the brain at birth will contain more neurons than later in life (Huebner & Strittmatter, 2009) however, scientists have recently discovered neurogenesis in some parts of the brain. These networks of connectivity essentially get pruned over time to create highly intricate processing units (Piochon, Kano, & Hansel, 2016). Additionally, a neuron contains dendritic arbors in order to receive information, and axons with terminals to send information elsewhere. These specialized structures are essential in order for synaptic transmission to be carried out .
1.2 The synapse
Neurons can transmit information between one another in the form of an electrical or chemical synapse. An electrical synapse involves an almost direct connection between the two cells by means of gap junctions, allowing for extremely rapid transmission between cells (Purves & Williams, 2001). Chemical synapses, on the hand, do not physically connect, rather they contain a space between their endings known as the synaptic cleft (Purves & Williams, 2001). This space is approximately 20-40 nm, compared to the 3.5 nm space of electrical (Savtchenko & Rusakov, 2007).
Transmission of information throughout the nervous system is by means of electrical signals. However, in the case of chemical synapses this information is transmitted by chemicals known as neurotransmitters (NTs), which will initiate an electrical conduction (Purves & Williams, 2001). NTs are released from the presynaptic neuron into the synaptic cleft, and then bind to receptors on the postsynaptic cell surface and initiate a cascade of events leading to an appropriate electrical stimulation.
The first phase of a chemical synapse is the synthesis of NTs. Larger NTs (such as neuropeptides) are synthesized in the cell body (or soma) and are subsequently transported towards the axon terminal in a process known as axonal transport (Maday, Twelvetrees, Moughamian, & Holzbaur, 2014). Smaller NTs (such as those in the form of amines or amino acids) are usually synthesized at or in close proximity to the axon terminal (Purves & Williams, 2001)
The next phases involve the packaging and transporting of NTs. They are packaged into vesicles to be efficiently delivered from the soma and for appropriate compartmentalization at the axon terminal (Purves & Williams, 2001). Once an electrical signal reaches the presynaptic terminal, voltage gated Ca2+ channels embedded within the membrane are opened. Ca2+ ions flow into the cell and trigger vesicles to fuse with the cell membrane to release their NT contents (Purves & Williams, 2001). This is a process known as vesicular exocytosis and will be described in further detail below. The released NTs flood into the synaptic cleft and bind to the receptors on the surface of the postsynaptic neuron. There are many different types of these receptors, which will trigger a specific cascade of events in the postsynaptic cell to create a particular electrical signal. Once the transmission is complete, the correct removal of NTs from the synaptic cleft is an important component. This can be done in a number of ways; some will be degraded, some will be absorbed by the postsynaptic terminal and others can be recycled (Purves & Williams, 2001).
2. Endocytosis and vesicle cycling
As described, chemical neurotransmission is one way of transmitting information between two cells. It occurs at the synapse, where NTs are released by vesicular exocytosis at the presynaptic membrane. Synaptic vesicles have a distinct uniform shape, are approximately 50nm in size and are composed of/contain a very distinct set of proteins. They are stimulated to release their contents in response to Ca2+ influx, a process that occurs on the order of milliseconds (Gad et al., 2000). Since signals can arrive at the axon terminal in very high frequency and strength, the vesicles containing NTs can become depleted very quickly. To adapt to this phenomenon, the body has developed a system of recycling NTs at the axon terminal through endocytosis.
The general steps of vesicle cycling by are as follows:
Step 1: Vesicles are loaded with NT through the action of NT transporters.
Step 2: Vesicles migrate towards what is called the ‘active zone’ where they will undergo certain reactions to prepare them for fusion with the cell membrane
Step 3: Ca2+ influx triggers vesicles to fuse with the plasma membrane
Step 4: Complete fusion with the cell membrane occurs
Step 5: Endocytosis via clathrin-coated vesicles occurs to reuptake NTs from the synaptic cleft (Gad et al., 2000).
2.1 In depth into NT release (steps 1-4)
As mentioned, NTs are released by the presynaptic neuron by means of exocytosis. But how do these vesicles make their way to the surface of the cell and undergo the process altogether? An article published in Science in 2003 by Hu et al. (Hu et al., 2003) demonstrates this process using original and clever methods. Prior to the release of this article, there existed the SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) hypothesis. SNARE proteins were thought to be present on the vesicular membrane and the presynaptic terminal membrane (v- and t- SNARES, respectively) and involved in the coordinated interaction between the two. This 2003 publication aimed to prove the hypothesis in a clear and reproducible manner. They did this by looking to determine if SNARE proteins could fuse entire cells.
Hu and colleagues genetically modified the DNA sequence by adding a specific promoter before the sequence coding for the SNARE proteins. This promoter would allow SNARE to be expressed specifically on the cell surface on the outside - that is in a “flipped” position. Since these modified SNARE proteins were also tagged with Green Fluorescent protein (GFP) their positioning on the cell membranes could be confirmed microscopically. They were then cultured together and the cells actually fused. This was proven because the nuclei of both cell types were made to be different colours. When the cells fused, the colours would mix and with this colour change, they could determine that fusion had actually occurred. In addition to the mixing of colours, they occasionally observed cells with multiple nuclei; also indicating the cells had fused.
Therefore, the hypothesis was correct because two cells were be able to entirely fused by the addition of SNARE proteins on their cell surface. In general, this article proved the importance of SNARE proteins in the membrane fusion of synaptic vesicles.
2.2 In depth into clathrin-mediated endocytosis (step 5)
In the last step, clathrin-mediated endocytosis is the main mechanism by which the presynaptic neuron internalizes a variety of molecules. Clathrin is a monomeric protein, which has a particular shape made up by three heavy and three light chains. This structure gives it biochemical properties that allow it to form a cage-like structure over the intracellular surface of the presynaptic neuron (Royle, 2006). As illustrated in Figure 2 the process of endocytosis begins with the assembly of clathrin at the inner membrane surface. This, along with other triggers, initiates an invagination of the membrane that will be coated in a cage structure of clathrin molecules. Complete fission occurs through the pinching of the membrane and the clathrin cage is disassembled to reach an uncoated intracellular vesicle containing recycled NTs (Gad et al., 2000).
This process is extremely complex and still under investigation. It involves a high level of coordination between biochemical processes and between a number of different proteins.
As an example, the role of synaptojanin (SYNJ) will be discussed in further detail. SYNJ was discovered to be involved in the process of clathrin-mediated endocytosis due to its co-localization with clathrin. It is a key protein in the process because it contains domains that help release the clathrin cage from the internalized vesicle. This occurs by dephosphorylating phosphatidylinositol (4,5) bisphosphates (PtdIns(4,5)P2) soon after endocytosis to destabilize the interactions between clathrin and the vesicle membrane so latter can be released (Lemmon, 2001). Additionally, SYNJ contains a PRD domain, and this is known to help recruit other proteins to the site of clathrin-mediated endocytosis.
The first of these recruited proteins is endophilin. Endophilin is involved in forming the clathrin coat during endocytosis and it helps localize synaptojanin to the clathrin-coated vesicle membrane (and therefore they work in a coordinated manner) (Milosevic et al., 2011). Endophilin and SYNJ apparently work through a common mechanism because mutations in one or the other produce identical phenotypes.
Another protein that interacts with SYNJ is amphiphysin. Amphiphysin has binding sites for clathrin and is a multilinker protein of clathrin complexes. It binds both endophilin and SYNJ simultaneously without any competition, indicating they have non-overlapping binding sites (Verstreken et al., 2003).
Another recruited protein, Dynamin, is a large GTPase, which is responsible for the process of pinching-off the clathrin-coated pit, and releasing the vesicle (Soda et al., 2012). Although it does not directly interact with SYNJ, it is recruited to the system through its interaction with endophilin and amphiphysin.
Lastly, SYNJ has been found to interact with auxilin, another protein involved in clathrin-mediated endocytosis. Auxilin is responsible for the disassembly and chaperoning of clathrin and subsequent coat destabilization. The mode of action of auxilin is still unknown but it may tether clathrin to the actin cytoskeleton, promoting disassembly of the coat and/or vesicular movement within the cell (Lemmon, 2001).
Through explanation of the role of SYNJ in clathrin-mediated endocytosis, we can demonstrate the level of complexity that is involved in this process. It is not simply a handful of proteins participating, but more of an orchestra of many interactions and enzymatic reactions.
2.3 An alternative to clathrin-mediated endocytosis
Endocytosis and exocytosis can occur in many different ways depending on the neuronal communication that needs to take place.
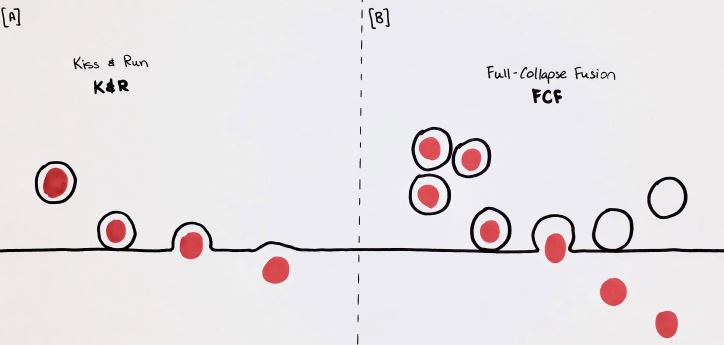
With regard to exocytosis, vesicles have always been thought to go through a full collapse fusion (FCF). This involved the complete fusion of the vesicular membrane with that of the cell in order to release its contents. The synthesis of NTs and vesicles are energetically expensive processes and simply releasing NTs and membrane into the synaptic cleft and cell membrane would be wasteful. Although clathrin-mediated endocytosis does recover much of the NTs, another process to increase the efficiency of the cell was discovered recently. This process is called ‘kiss-and-run” (K&R) vesicle fusion, illustrated in Figure 3 and it involves a transient fusion of the vesicle to the cell surface, and subsequent retrieval in order to recycle both vesicular membrane and NTs (Zhang, Li, & Tsien, 2009).
The main focus of a 2009 study was to determine if K&R vesicle reuse was present in small nerve terminals of the mammalian brain and to understand how this process is occurring. Zhang et al. used extremely interesting and unique methods to understand this process.
To do this, they loaded quantum dots (Qdots) (small particles that can be visualized microscopically) into vesicles so that they could observe the movement of the vesicular contents over time. The Qdots were visualized in individual vesicles and based on how they moved they could watch if FCF or K&R was occurring and under what conditions. Additionally, they could also see if the vesicles were refilling.
They found that K&R does indeed take place at small nerve terminals of the mammalian brain. The readily releasable pool (RRP) is a group of vesicles that are close to the presynaptic membrane and are somewhat “primed” for the arrival of an action potential. The RRP is usually the group of vesicles that are used upon short term but frequent neuronal stimulation.
When these neurons were stimulated, K&R was more frequent at first, but then as the stimulation of neurons continued over time, FCF became the more dominant process. This showed that the RRP primarily uses K&R while the longer-term stimulation uses the FCF.
In all, these researchers showed firstly that the use of Qdot is an effective way of visualizing these different processes of vesicle fusion, and secondly that K&R appears to be the dominant method upon initial high frequency stimulation. They showed that both K&R and FCF processes of vesicle fusion take place but that one occurs more than the other depending on the type of stimulus and the conditions of the vesicles themselves.
3. Conclusion
In conclusion, synaptic transmission occurs predominantly through a chemical synapse. Chemical synapses are those containing a relatively large synaptic cleft between cells where NTs are released. NTs are released by the presynaptic cell by means of exocytosis, diffuse across the synaptic cleft then bind to specific receptors on the presynaptic cell and subsequently initiate an electrical signal. NTs are contained within synaptic vesicles and can become depleted quickly with high levels of stimulation and one such way that neurons manage this, is by recycling vesicles and NTs. Synaptic vesicle recycling is an extremely complex process and involves the coordinated interplay of several proteins and molecules. One of these is the SNARE complex, which is known to help vesicles fuse with the presynaptic cell membrane.
Clathrin-mediated endocytosis is the classic example and general scheme of how this process works. The process involves several proteins, one of which is synaptojanin. This protein has been shown to be involved in the shedding of the clathrin coat to complete the endocytic process.
Recent studies however, have shown clathrin is not the only method of vesicle recycling. Researchers have recently shown (Zhang et al., 2009) the occurrence of kiss-and-run as a mechanism of recycling vesicles more rapidly, which is required for certain types of presynaptic stimulation.
Endocytosis and vesicle recycling is an important element of synaptic neurotransmission; without it the process would be much slower and less efficient. Research is still ongoing in the field and it will be interesting to see what new elements will be discovered and how this may play a role in neurological diseases.
4. Figures
Figure 1. Chemical synapse as depicted by (Purves & Williams, 2001).
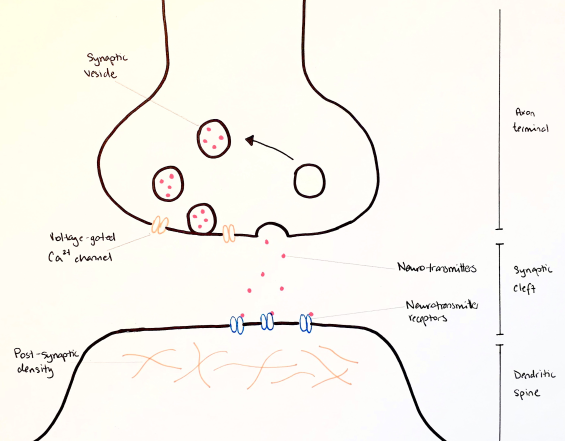
Figure 2. Clathrin-mediated endocytosis, as described by (Gad et al., 2000).
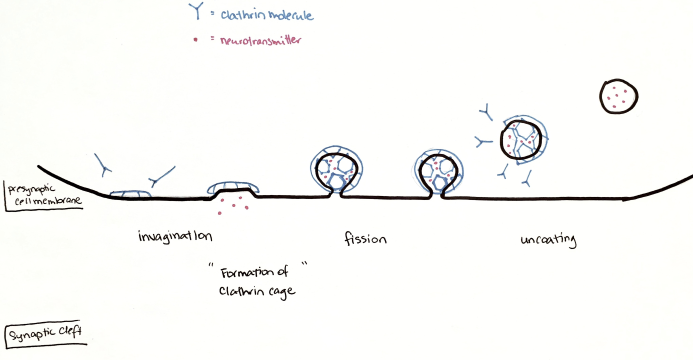
Figure 3. Illustration of K&R (A) and FCF (B), as described by (Zhang et al., 2009).
5. References
Gad, H., Ringstad, N., Low, P., Kjaerulff, O., Gustafsson, J., Wenk, M., . . . Brodin, L. (2000). Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron, 27(2), 301-312.
Hu, C., Ahmed, M., Melia, T. J., Sollner, T. H., Mayer, T., & Rothman, J. E. (2003). Fusion of cells by flipped SNAREs. Science, 300(5626), 1745-1749. doi:10.1126/science.1084909
Huebner, E. A., & Strittmatter, S. M. (2009). Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ, 48, 339-351. doi:10.1007/400_2009_19
Lemmon, S. K. (2001). Clathrin uncoating: Auxilin comes to life. Curr Biol, 11(2), R49-52.
Lodish, H. F. (2000). Molecular cell biology (4th ed.). New York: W.H. Freeman.
Ludwig, P. E., & Varacallo, M. (2019). Neuroanatomy, Central Nervous System (CNS) StatPearls. Treasure Island (FL).
Maday, S., Twelvetrees, A. E., Moughamian, A. J., & Holzbaur, E. L. (2014). Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron, 84(2), 292-309. doi:10.1016/j.neuron.2014.10.019
Milosevic, I., Giovedi, S., Lou, X., Raimondi, A., Collesi, C., Shen, H., . . . De Camilli, P. (2011). Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron, 72(4), 587-601. doi:10.1016/j.neuron.2011.08.029
Piochon, C., Kano, M., & Hansel, C. (2016). LTD-like molecular pathways in developmental synaptic pruning. Nat Neurosci, 19(10), 1299-1310. doi:10.1038/nn.4389
Purves, D., & Williams, S. M. (2001). Neuroscience (2nd ed.). Sunderland, Mass.: Sinauer Associates.
Royle, S. J. (2006). The cellular functions of clathrin. Cell Mol Life Sci, 63(16), 1823-1832. doi:10.1007/s00018-005-5587-0
Savtchenko, L. P., & Rusakov, D. A. (2007). The optimal height of the synaptic cleft. Proc Natl Acad Sci U S A, 104(6), 1823-1828. doi:10.1073/pnas.0606636104
Soda, K., Balkin, D. M., Ferguson, S. M., Paradise, S., Milosevic, I., Giovedi, S., . . . Ishibe, S. (2012). Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest, 122(12), 4401-4411. doi:10.1172/JCI65289
van Spronsen, M., & Hoogenraad, C. C. (2010). Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep, 10(3), 207-214. doi:10.1007/s11910-010-0104-8
Verstreken, P., Koh, T. W., Schulze, K. L., Zhai, R. G., Hiesinger, P. R., Zhou, Y., . . . Bellen, H. J. (2003). Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron, 40(4), 733-748.
Zhang, Q., Li, Y., & Tsien, R. W. (2009). The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science, 323(5920), 1448-1453. doi:10.1126/science.1167373
