ANTIPHOSPHOLIPID SYNDROME
INTRODUCTION
Anti-Phospholipid Syndrome (APS) is also known as Hughes syndrome; named so after one of the founders of this autoimmune disease; Hughes, Harris, and Gharavi in 1986. APS causes thrombosis (blood clotting) and some pregnancy-related complications. The presence of anti-phospholipid antibodies (aPL) in blood plasma, and one or more cases of venous or arterial thrombosis and/or pregnancy complications; such as miscarriages, pre-mature birth, and stillbirth, will classify a patient as being a sufferer from APS. (Wilson et al., 1999)
Primary APS is the occurrence of this syndrome without any other underlying diseases , or without evidence of any agent that could have induced production of aPL. Many of the cases of recurrent pregnancy loss in the past are now known to be part of APS upon detection of aPL.
Secondary APS occurs in association with other auto-immune diseases; such as systematic lupus erythematosus (SLE), certain infections, and drugs. . It does not mean that secondary APS differs from primary APS, as the clinical manifestations of APS in both cases can be identical.
Classification Criteria of APS (A definite diagnosis of APS requires the presence of at least one clinical criteria and one laboratory criteria.)
The Laboratory Criteria:
• Detection of IgG, IgM aCL or LAC in medium to high titers, in two different measurements at least six weeks apart. (Keeling, et al., 2012)
The Clinical Criteria:
• During pregnancy: one or more pregnancy loss after 10th gestational week or one or more episode of early delivery (because of severe placental insufficiency.)
• Thrombosis: at least one episode of thrombosis within artery, vein or small vessels within any organ or tissue. (Keeling et al., 2012)
Even if a patient has a laboratory criteria for APS in the absence of clinical criteria; he/she is considered to have APS even though they are not included under APS definition (because of the absence of clinical criteria)
Similarly, APS is characterized by many aPL, and therefore patients having a clinical criteria for APS but not a laboratory criteria, but have another auto-antibody which is found in APS, also have probable APS even though they are not considered having definite APS.
APS is highly related to other autoimmune diseases, especially systemic lupus erythematosus (SLE) where 20-35% of patients with this disease fulfill the criteria for APS. Clinical criteria of this disease occurs frequently but that does not mean that the patient with these criteria has this disease. The incidence of APS is very low.
ANTIPHOSPHOLIPID ANTIBODIES
Antibodies are known to help the body to ward of infections. However; in cases of APS, anti-phospholipid antibodies (aPL) are produced which act against those plasma proteins which bind to phospholipids; more specifically against the beta2-glycoprotein I; which is one of the main antigens for anti-phospholipid antibodies. (Galli et al., 1990) The main anti-phospholipid antibodies are;
(i) Lupus Anticoagulant (LA)
(ii) Anti-Cardiolipin Antibody (anti-aCL) of IgG or IgM isotype.
(iii) Anti-β2 – glycoprotein-I antibody (anti-β2GPI) of IgG or IgM isotype
LA inhibits agglutination in the presence of thrombin
Anti-β2GPI inhibits Factor Xa (Stuart-Power Factor) in the coagulation cascade.
The presence of LA is found by performing an in-vitro coagulation test whereas aCL and anti-β2GPI occurrence is found by by running ELISA assay tests.
The presence of aPL are detected in blood serum by performing an in vitro bio-assay for LA; and by running an ELISA assay for antibodies intereactiong with cardioliolipin and/or -β2 – glycoprotein-I . β2-glycoprotein I, also called apolipoprotein H (Apo-H), is a multifunctional apolipoprotein. β2-glycoprotein I (β2GPI) is the most important antigen for antiphospholipid antibodies. Structurally; it is a phospholipid bindoing protein present in the blood plasma with anti-coagulant properties. In Vitro it binds to anionic phospholipids producing an apparent anticoagulant effect by occupying the pro-coagulant binding sites. (Groot et al., 2012)
Not all patients with anti-β2GPI antibodies show clinical symptoms that are related to APS. Several reports indicate that anti-β2GPI antibodies with lupus anticoagulant (LA) activity are clinically of much importance. Most patients with LA caused by anti-β2GPI antibodies suffer from thrombosis as a result of recognition of the first domain of β2GPI by these antibodies
DIAGNOSIS OF APS
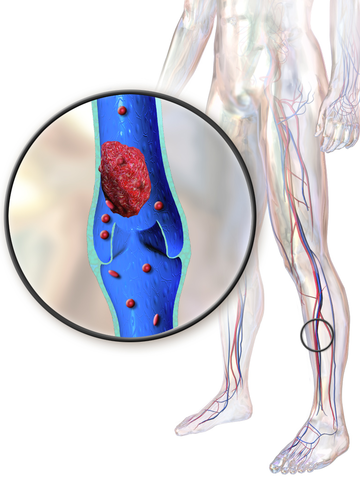
Fig 1. Deep Vein Thrombosis
Diagnosis of APS requires the presence of thrombosis or pregnancy morbidity, but APS patients can have disorders in various body systems as well:
• Venous Thrombosis: Venous thrombosis normally presents itself with DVT (Deep Vein Thrombosis) in the lower extremities. In some unusual cases, DVT can be seen in the upper extremities, intracranial veins, inferior and superior vena cava, hepatic veins, portal vein, renal vein, and retinal vein. (Refer to Figure 1)
• Arterial Thrombosis: Arterial thrombosis is not as common as venous thrombosis. It occurs in a variety of different symptoms in patients with primary APS. 50% of patients with arterial thrombosis experience transient ischemic attack or stroke and 23% of the patients experience myocardial infarction.
• Cardiac Disorders: Arterial occlusion may be either thrombotic or embolic. Valvular thickening, vegetations, regurgitation, premature coronary disease, myocardial infarction, dilated diffuse cardiomyopathy, congestive heart failure, pericardial effusion, and pulmonary hypertension can be seen in patients with APS. These arterial occlusion can be thrombotic or embolic.
• Neurological Disorders: Primary thrombosis and embolic occlusion of cerebral arteries cause cerebral infarction. Patients may have strokes and TIA (Transient Ischemic Attack), and aPL; particularly lupus anticoagulants (LAC).
• Obstetrical Disorders: Pregnancy complications can only be seen in aPL patients. APS continuously causes pre-embryonic and embryonic miscarriages, and fetal demise. Also, complications like eclampsia, intrauterine growth retardation, oligohydramninos, HELLP syndrome, and premature birth, systemic and pulmonary hypertension, high rate of subsequent venous or arterial thrombosis can be seen during pregnancy. Of all hereditary and acquired thrombophilias, APS is the most common thrombotic defect leading to fetal wastage.
• Dermatological Disorders: Dermatological manifestations are normally the first indication of APS. The most common feature of APS is non-inflammatory vascular thrombosis. APS patients develop, cutaneous ulcerations and necrosis, purpura, painful skin nodules, and subungual splinter hemorrhages.
• Pulmonary: Pulmonary micro-thrombosis is a very frequent symptom of APS. This symptom of APS is very severe and has a high mortality rate.
• Abdominal Manifestations: The most common effect of APS in the abdomen is the effect on the liver. After the effect on the liver, thrombotic events ensue. Different branches of the intestinal vasculature are affected as a result. Other manifestations of large- and small-vessel thrombosis include hepatic infarction, pancreatitis, intestinal ischemia and infarction, colonic ulceration, and giant gastric ulcerations.
• Renal: Renal artery stenosis and/or malignant hypertension, renal infarction, renal vein thrombosis, thrombotic microangiopathy,and increased allograft vascular thrombosis can be seen as a manifestation of APS.
• Retinal Disorders: Venous and arterial thrombosis is a common symptom of APS. Optic neuropathy and cilioretinal artery occlusions can be seen as well.
(Saigal et al., 2010)
CAUSES OF ANTIPHOSPHOLIPID SYNDROME
• Several of the clinical manifestations of APS (mainly thrombosis) can accompany several cancers. It is possible that aPL are the explanation of some of these conditions. The most common malignant diseases associated with secondary APS are lymphoma, leukemia, other cancers of the blood, and carcinomas (Cancer that begins in the skin or in tissues that line or cover body organs) of the lung, ovary, kidney, cervix and prostate.
• Several drugs can cause the appearance of aPL. These include, procainamide, phenothiazines, ethosuximide, phenytoin, quinine, chlorothiazide, hydralazine and interferon-alpha. Some of these drugs also induce autoantibodies and a lupus-like disease (Lupus is a chronic inflammatory disease that causes your body’s immune system to attack itself.)
• Many infectious agents can induce aPL, mainly aCL. These infectious agents can be hepatitis C virus, varicella virus (causes chickenpox), parvo virus B19 (causes anemia and fever), human immunodeficiency virus (causes AIDS), cytomegalo virus (causes infectious mononucleosis) and several bacteria. Mostly these infectious agents do not cause APS as they induce aPL (do not bind β2GPI and thus do not cause thrombosis). However, in some of the cases of APS, infections overrule the clinical manifestations. (Favaloro et al. 2008)
TREATMENT
Because of the unreliability connected with assays tests in the detection of the presence of antiphospholipid antibodies, the best possible treatment for the antiphospholipd syndrome is uncertain. Since it is associated with thrombophilic disturbance and extensive microangiopathy the logical solution would be to focus on the prevention of thrombosis with anticoagulatory treatment. To achieve that the patient needs intesne observation and the specific therapy includes the use of intravenous heparin, corticosteroids, and possibly plasma exchange with or without intravenous immunoglobulin. Another important factor is to take into account the piatient's current clinical status and medical history which an individualized treatment should be based on. Clinical studies have shown that the combination of heparin and aspirin during pregnancy is an effective treatment for women with APS. Other drugs that could be proven useful are low-dose aspirin and hydroxychloroquine. (Erkan D, et al. 2014) (Sangle et al. 2011) '
COAGULATION CASCADE
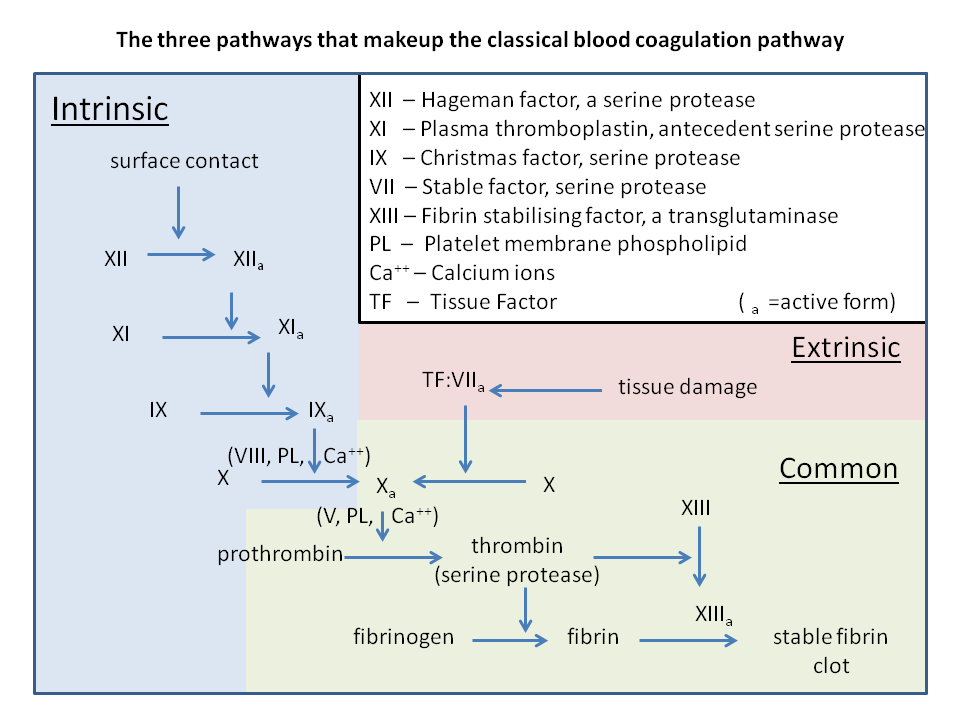
Fig 2. Coagulation Cascade
Blood coagulation initiates from the moment an injury to the endothelial cell lining of a vessel is received. First platelet activation occurs (consisting of primary and secondary activation); followed by the coagulation cascade; which includes the contact activation pathway (intrinsic pathway) and the tissue factor pathway (extrinsic pathway); which both finally lead to fibrin formation. (Refer to Figure 2)
Primary Aggregation: In case of an injury to the endothelial blood vessel lining, thrombocytes (platelets) bind with specific receptors to the negative charges of the injured collagenous fibers of endothelial cells. Endothelial cells produce a protein called the von Willebrand factor. It is activated by the exposed sub-endothelial collagen fibers. This exerts a positive effect on primary aggregation, by increasing its action. The now activated von Willebrand factor connects the injured surface with the thrombocytes.
Secondary Activation: Filopodia formation occurs via the actin and microtubule system of the platelets and thanks to this, formation of white thrombus occurs. White thrombus consists of platelet and collagen fiber connections thanks to the filopodia. In the presence of an injury, the inhibiting action of prostacyclins (PGI2) and nitrogen monoxide (NO) produced by the endothelia, on the factors responsible for aggregation, stops. This prevents thrombocyte aggregation. The thrombocytes then release serotonin, TXA2, adenosine-diphosphates, and other stimulating factors; which cause an increase in thrombocyte presence; hence increasing aggregation.
Central Cascade: At the same time of platelet activation, the activation of the cascade enzyme-system in the blood plasma starts, which in the end leads to a fibrin net formation. This is the basis og coagulation i.e. fibrin net formation from fibrinogen of the blood plasma. Coagulation occurs either because of an external tissue injury (extrinsic way) or by the injury of the internal intima (intrinsic way).
The central cascade activates Factor Xa (Stuart-Power; activated) which is an important piece in the prothrombin-thrombin conversion. Thrombin(activated factor II) enables fibrinogen(factor I) to be converted into a fibrin-net(activated factor I), which is further stabilized by Factors XIII – XIIIa (Laki Lorand Factor)
Extrinsic Way: Known as Tissue Factor Pathway. When plasma enters the tissues it activates Factor VII (Proconvertin); which becomes convertin. The now activated factor VII and the tissue Factor III (thrombokinase), activate the Factor X (Stuart-Prower factor).
Intrinsic Way: Known as Contact Activation Pathway. The plasma factor XII (Hageman factor) connects to the surface of the injured intima, and binds to a macromolecular protein (kininogen) and to the enzyme kallikrein; which it activates. Thus, in the presence of kininogen and kallikrein, the Factor XII activates its own self. The active XII factor activate the factor XI (Plasma Thromboplastin Antecedent; PTA) and this leads to an activation complex, first activating Factor IX (Christmas Factor), followed by Factor X (Stuart-Prower) activation. However; activation of Factor X, apart from the presence of Factor IXa; also need Factor IV (Calcium), Factor VIII (Anti-Haemophilic), and TF3.
Common Pathway of Extrinsic and Intrinsic Ways: Activated factor X in the presence of Ca, the activated V and TF3 produced by the platelets, stimulates the prothrombin-thrombin transformation. Thrombin turns the plasma fibrinogen to fibrin net. XIII factor activated by thrombin stabilizes the fibrin net.
MECHANISM OF THE ANTIPHOSPHOLIPID SYNDROME AND ITS EFFECTS ON THE COAGULATION CASCADE
APS is an auto-immune disorder; since one’s own antibodies are targeting self-cells and tissues; which, under normal circumstances, antibodies would not target. The different anti-phopholipid antibodies mentioned previously; attack phospholipids; more specifically β2-glycoportein I (β2GPI). Its action with negatively charged phospholipids, heparin and dextran shows that it plays a role in the coagulation cascade.
β2GPI and Coagulation Factors
The presence of β2GPI causes a slower action of thrombin in the coagulation pathway; by binding to Factor XI; thus inhibiting activation of the Factor by thrombin and Factor XIIa.
However; it is interesting to note that studies have shown that cleaved β2GPI by plasmin; will result in a negative feedback action which opposes its inhibition of Factor XI activation process.
Thus, since β2GPI is that which is mainly acted upon by anti-phospholipid antibodies in the case of ALS sufferers, the disruption of this part of the coagulation cascade may be an important factor in such cases. (Miyakis et al. 2004)
The relation between 2GPI production and the coagulation cascade was found out by experiments performed on mice lacking β2GPI; who exhibited flawed thrombin production. (Sheng et al. 2001)
Effect on Platelet Formation
Increased platelet activity is observed in patients with APS. (Joseph JE et al. 2001) Thus a correlation between anti-phospholipid antibody presence and increase platelet activation plays a role in the increase of thrombosis occurrence.
CONCLUSION
APS, is a major concern of many medical specialties due to the vast complications associated with it. There are now enough indications that patients with APS and thrombosis suffer from it frequently and are in need of preventive treatment. The long-term anticogulatory treatment seems to present the lowest risk of recurrent thrombosis in these patients. It is proven that LA-inducing anti-β2GPI antibodies are correlated with the presence of thrombrosis. Currently the question and the goal is to find out whether these antibodies are associated with recurrent fetal loss. More investigation is also necessary to find other possible populations of antiphospholipid antibodies that are also clinically relevant.
REFERENCES
David Keeling, Ian Mackie, Gary W. Moore, Ian A. Greer, Michael Greaves, and British Committee for Standards in Haematology (2012): Guidelines on the investigation and management of antiphospohlipd syndrome. British Journal of Haematology. 157:(1) 47-58
Erkan D, et al, (2014): 14th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends, Autoimmun Rev
Joseph JE, Harrison P, Mackie IJ, et al. (2001): Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001;115:451–9.
Galli M., Comfurius P., Maassen C., Hemker H.C., De Baets M.H., Van Breda-Vriesman P.J., Barbui T., Zwaal R.F. & Bevers E.M. (1990): Anticardiolipin antibodies (ACA) are directed not to cardiolipin but to a plasma cofactor.
Miyakis, S., Giannakopoulos, B. & Krilis, S. A. (2004): Beta-2 glycoprotein1: function in health and disease. Thrombosis Research: vascular obstruction, hemorrhage and hemostasis, 114 (5/6), 335-346.
N.A. Sangle, K.J. Smoch (2011): Antiphospholipid antibody syndrome. Arch Pathol Lab Med—Vol 135, September 2011
P.G. de Groot, J.C.M. Meijers, R.T. Urbanus (2012): Recent developments in our understanding of the antiphospholipid syndrome
Renu Saigal, Amit Kansal, Manoop Mittal, Yadvinder Singh, Hari Ram, (2010): Antiphospholipid antibody syndrome.
R.C.W. Wong, E.J. Favaloro (2008): Clinical features, diagnosis, and management of the antiphospholipid syndrome. Seminars in Thrombosis and Hemostasis 2008; 34(3): 295-304
Sheng Y, Reddel SW, Herzog H, Wang YX, Brighton T, France MP, et al. (2001): Impaired thrombin generation in h2-glycoprotein I null mice. J Biol Chem;276:13817— 21.
Wilson, W.A., Gharavi, A.E., Koike, T., et al. (1999): International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome; report of an international workshop. Arthritis Rheum 43, 1309
OTHER REFERENCES
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2012.09037.x/pdf
http://onlinelibrary.wiley.com/doi/10.1111/j.1751-553X.2012.01414.x/pdf
http://www.japi.org/march_2010/Article_08.pdf
http://www.bcshguidelines.com/documents/antiphospholipids_2012.pdf
https://www.orpha.net/data/patho/Pro/en/Antiphospholipid-FRenPro5517v01.pdf
http://ro.uow.edu.au/cgi/viewcontent.cgi?article=1205&context=smhpapers
https://www.orpha.net/data/patho/Pro/en/Antiphospholipid-FRenPro5517v01.pdf
http://bmhlibrary.info/10403256.pdf
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1809064/
http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2141.2001.03101.x/pdf
http://www.archivesofpathology.org/doi/pdf/10.5858/2010-0325-RSR.1
PICTURE REFERENCES
Figure 1: Deep Vein Thrombosis http://commons.wikimedia.org/wiki/Category:Deep_vein_thrombosis#mediaviewer/File:Blausen_0290_DeepVeinThrombosis.png
Figure 2: Coagulation Cascade http://commons.wikimedia.org/wiki/File:Classical_blood_coagulation_pathway.png
All from WikiCommons
