Abnormalities in mitochondrial structure, causes and effects
Table of Contents
Contents
Introduction
To understand the basis of mitochondrial abnormalities and disorders, one must first understand the role and structure of the mitochondrion, and the relevant processes it is responsible for. A key aspect is the study of the genetic makeup and how the mitochondria is encoded with both nuclear DNA and also mitochondrial DNA, and the importance of maternal inheritance in regards to mtDNA. Knowledge of the genetic background is important if you are to then look at the various disorders and diseases associated with abnormalities in the mitochondria. It is no surprise that the majority of mitochondrial diseases affect tissues and organs which have the highest energy demands such as the heart, brain and muscles since the mitochondria is responsible for the production of ATP. It is essential to find more information around some of the most common and complicating diseases that arise from disorders in the mitochondria,as they are relatively incurable and hard to diagnose. It is also important to discover what are the causes - complex deficiencies, point mutations or mtDNA deletions are areas of major significance, especially when these are inherited by offspring. Diseases and disorders examined include MELAS syndrome, Kearns Sayre syndrome, Alzheimers, and Parkinson’s disease. Secondary disorders and conditions arise from such syndromes and diseases, such as diabetes, exercise intolerance, lactic acidosis and multiple organ failure.
The Mitochondrion
The mitochondrion is a double-membraned structure found in most eukaryotic cells. The mitochondria generates most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. hence the name ‘powerhouse of the cell’ given by Philip Siekevitz. The number of mitochondria present in a cell depends upon the metabolic requirements of that cell, and may range from a single large mitochondrion to thousands of the organelles. Mitochondria, which are found in nearly all eukaryotes, including plants, animals, fungi, and protists, are large enough to be observed with a light microscope.
2.1 Structure of the Mitochondrion
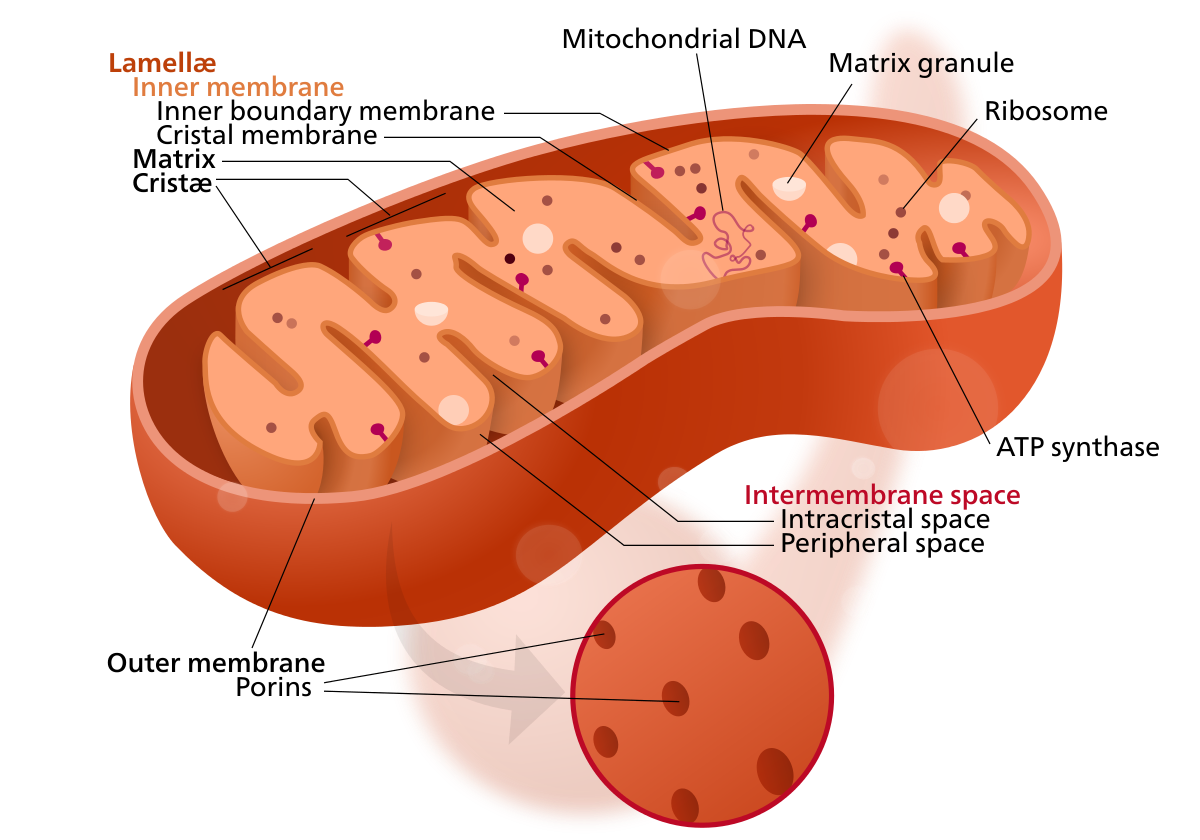
The organelle is composed of compartments that carry out specialized functions. Because of their double-membraned organization, there are five distinct parts to a mitochondrion. These 5 main compartments or regions are: the outer membrane, the intermembrane space, the inner membrane, and the cristae and matrix. These structures can be seen in figure 1. Figure 1 Structure of the mitochondrion
2.2 Role and processes of mitochondria
The central set of reactions involved in ATP production are collectively known as the citric acid cycle, or the Krebs cycle. Genes in the mitochondrial respiratory chain complex gene group provide instructions for proteins involved in oxidative phosphorylation, also called the respiratory chain. Oxidative phosphorylation is an important cellular process that uses oxygen and simple sugars – adenosine diphosphate (ADP) to create adenosine triphosphate (ATP), the cell's main energy source. Five protein complexes, made up of several proteins each, are involved in this process(Alston et al., 2017). The complexes are named complex I (NADH dehydrogenase), complex II (Succinate dehydrogenase), complex III (Ubiquinol cytochrome-c-oxidoreductase), complex IV (cytochrome-C-oxidase), and complex V (ATP synthase).
Genetics of Mitochondrial disease
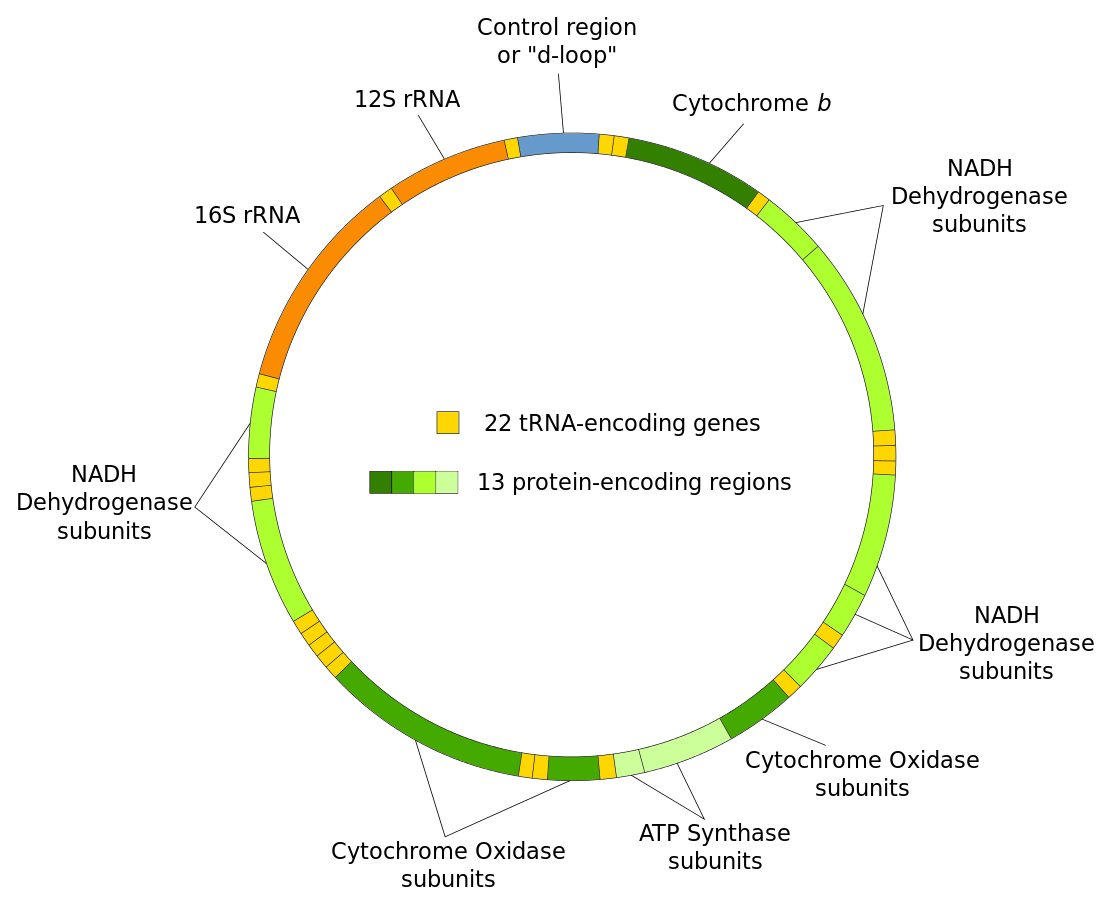
Although most of a eukaryotic organism genes are found in the nucleus a distinct amount of the genome is found within the mitochondria. Mitochondria contain small circular chromosomes that function in the mitochondrion’s task of producing energy in the form of ATP. Although the mitochondria genomes code for proteins necessary for their proper functioning they are not functionally autonomous, many genes required for the correct functioning of mitochondria are encoded on the nuclear genome. A variety of genes are found within mitochondrial genomes including respiratory complex genes and rRNA genes (see Figure 2). Mutations in these genes can cause disease. The size of the mitochondrial genome can vary dramatically depending on the organism for example the Mus musculus mitochondrial genome is 16kb, homo sapiens is 17kb and the biologically important Arabidopsis thaliana is 367kb.
|
Figure 2 Mitochondria genome |
3.1 Mitochondrial DNA
Each mitochondrial DNA is specific to carry out a particular set of functions associated with mitochondria, including the synthesis of molecules that are used for cellular respiration, units that code for the synthesis of tRNA of each amino acid, and DNA involved in the synthesis of rRNA that uses for protein synthesis. Human mtDNA is a 16569-kb circular, double stranded molecule, which contains 37 genes: 2rRNA genes 22tRNA genes and 13 structural genes encoding subunits of the mitochondrial respiratory chain,which is the ‘business end’ of the oxidative metabolism (Salvatore et al.,2005). Molecules of mtDNA range from about 5 µ.m in length (about 10 X 106 daltons or 15 X 103 base-pairs) in animals and chlamydomonas to about 30 µ.m (60 X 106d, 90 X 103 bp) in pea plants (Birky Jr et al., 1978).
3.2 Inheritance of mtDNA
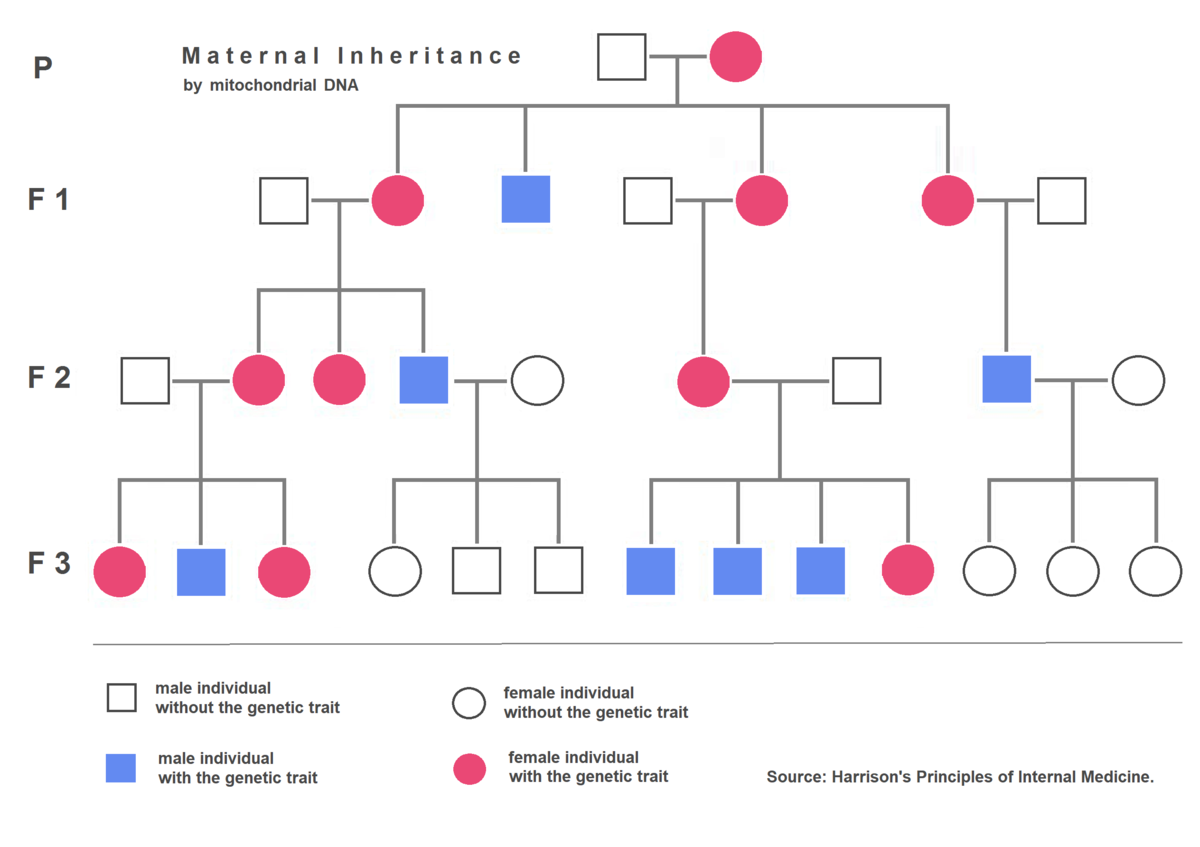
Organelle genes have a unique method of inheritance called uniparental inheritance. Which mean offspring only inherit organelle genes from one parent. In the case of mitochondria, the parent is the mother so therefore the inheritance follows a pattern called maternal inheritance. Therefore, the DNA found within the mitochondria is the same as the mother’s DNA the reason for this is that male and female parents do not contribute the same amount of cytoplasm to the zygote. The egg contributes most of the cytoplasm and therefore the mitochondria found within. In extremely rare cases there have been some evidence of paternal mitochondrial paternal inheritance has emerged. Maternal inheritance has some severe implications for mitochondrial diseases. If a mitochondrial mutation is found within a female, all the offspring will have the mutation whereas if a male contains the mutation and is crossed with a wild-type female none of the offspring will contain the mutation. i.e.: wild-type female x mutant male = wild-type offspring yet: mutant female x wild-type male= mutant offspring (See Figure 3).
|
Figure 3 Demonstration of mtDNA maternal inheritance |
3.3 Mutations in mtDNA
The mtDNA has a very high mutation rate, which results in three classes of clinically relevant mtDNA mutations: recently deleterious germline line mutations resulting in mitochondrial disease; ancient regional variants, a subset of which permitted humans to adapt to differences in their energetic environments; and somatic mutations that accumulate with age eroding mitochondrial energy production and providing the aging clock (C. Wallace, 2010).
3.3.1 Rearrangement mutations
Rearrangement mutations can either be de novo deletion mutations or maternally transmitted insertion mutations, which are unstable and generate deletion mutations in postmitotic cells (C. Wallace, 2010). For example, the mildest mtDNA rearrangement phenotype is maternally inherited Type II diabetes and deafness, These are thought to be caused by inheritance of a mtDNA duplication mutation.
3.3.2 Deleterious mtDNA base substitution mutations
Base substitution mutations can alter either polypeptide genes (polypeptide mutations) or rRNAs and tRNAs (protein synthesis mutations). Pathogenic polypeptide mutations encompass a broad spectrum of multisystem diseases including LHON (Wallace et al., 1988), Leigh syndrome (Holt et al.,1990), and mitochondrial myopathy (Andreu et al., 1999).
3.3.3 Ancient Adaptive Mutations and Disease Predisposition
Because of strict maternal inheritance, mtDNAs can only evolve by the sequential accumulation of mutations along radiating maternal lineages. If an mtDNA mutation arises that is beneficial in a particular environment, it and its descendants will increase in frequency in that environment (C.Wallace,2010). This results in the generation of a group of related mtDNA haplotypes (haplogroup) concentrated in a particular geographical region. Two-thirds to three-quarters of all African mtDNAs belong to macro haplogroup L. All European mtDNAs belong to macrohaplogroup N, encompassing the European haplogroups: H, I, J, Uk, T, U, V, W, and X. (Mishmar et al., 2003).
3.3.4 Somatic mtDNA Mutations in Age -Related Diseases
Mutations in the mtDNA have been observed to accumulate with age in a variety of postmitotic tissues in a wide range of species and in a spectrum of complex age-related diseases (Wallace, 2005), whilst tests have shown increasing the mtDNA mutation rate in mice increases their aging rate (Trifunovic et al.,2004), while decreasing the somatic mtDNA mutation rate by introducing catalase into the mitochondrial matrix extends mouse life span (Schriner et al., 2005). Therefore, the accumulation of somatic mtDNA mutations provides an aging clock that helps define an animal’s life span and contributes to the delayed-onset and progressive course of complex diseases (Wallace, 2005). Cancer is also an age-related disease, and both somatic and germline mtDNA mutations have been reported in cancers including renal adenocarcinoma, colon cancer cells, head and neck tumors.
Mitochondrial diseases
Mitochondrial diseases arise from mutations in either mtDNA or nuclear mitochondrial genes or they can arise from large scale deletions of mtDNA. Mitochondrial disorders are errors of metabolism caused by mutations in mitochondrial and nucleus genes that lead to impaired oxidative phosphorylation (Ito et al., 2011).These abnormalities can cause severe syndromes as well as conditions affecting a multitude of tissues in the body, including the eyes, the heart, muscles, endocrine system and the central nervous system.
4.1 Kearns Sayre Syndrome: a mtDNA deletion disorder
Kearns Sayre Syndrome (KSS) is a mitochondrial myopathy and a clinical subtype of chronic progressive external ophthalmoplegia (CPEO).The syndrome is defined by the obligatory triad of onset before the age of 20 years, progressive external ophthalmoplegia, and pigmentary retinopathy (van Beynum et al., 2011). In most cases it is caused by single, large scale mitochondrial DNA deletions or mitochondrial DNA depletion (van Beynum et al., 2011). Single, large‐scale mtDNA deletions have a population frequency of 1.5/100 000 with three main associated phenotypes: chronic progressive external ophthalmoplegia, Kearns–Sayre syndrome, and Pearson syndrome (Alston et al., 2017). Chronic progressive ophthalmoplegia (CPEO) is the most common ocular manifestation of mitochondrial myopathies (Al-Enezi et al., 2008). It manifests in the ocular system in many ways eye movement paralysis (ophthalmoplegia), ptosis, oropharyngeal weakness, and proximal myopathy with exercise intolerance (Gustafason et al., 2019). Being a subtype of CPEO, the majority of these effects are also displayed by KSS. It is even believed that the number of tissues affected is higher than so far anticipated (Finsterer et al., 2020).
Apart from the main three consistent features that characterize KSS (PEO, pigmentary retinopathy and presentation before 20 years of age), this syndrome often displays at least one of the following multisystem implications: cerebellar ataxia, heart block and elevated cerebrospinal fluid protein level (Yu et al., 2016). Other features include hearing loss, dementia, cardiomyopathy and endocrine disorders, which highlights the impact of this mitochondrial syndrome. Cardiac manifestations in KSS are as high as 50% and sudden cardiac death reported in up to 20% of KSS cases (Chawla et al., 2008). The most typical cardiac complications of the disease are conduction defects, which usually begin with left anterior fascicular block with or without right bundle branch block (RBBB), progressing sometimes rapidly to complete atrioventricular block. Other cardiac manifestations reported are first or second degree of AV block, QT prolongation, ventricular tachycardia, and cardiomyopathy (Van Beynum et al., 2011).
KSS is usually caused by deletions of mt-DNA, rather than a mutation of the mt-DNA. Due to this it is rarely inherited. single, large-scale mtDNA deletions (SLSMDs) have long been thought to occur sporadically in affected individuals or oocytes (Gustafson et al, 2019). This suggests that mitochondrial disorders such as KSS develop somatically in the early embryo (Kapunga et al., 2015). Since KSS is caused by deletions of large portions of mitochondrial DNA (mtDNA), this results in the loss of genes involved in the electron chain transport and oxidative phosphorylation pathway, which has effects on energy production. A consistent and conspicuous finding in muscle biopsy samples from patients with KSS is the presence of a population of fibers lacking histochemically detectable COX (cytochrome-c-oxidase). Because a proportion of these COX-deficient fibers shows no other morphological abnormality of mitochondria, it has been proposed that they might be precursors of ragged-red fibers and that COX deficiency could be a factor in the pathogenesis of KSS and other disorders (Mita et al., 1989).
This has been further verified, in a publishment by (Finsterer et al., 2020), a muscle biopsy from the left lateral vastus muscle in a patient diagnosed with KSS showed cytochrome-C-oxidase (COX)-negative fibers , glycogen depositions, fiber splitting, and ragged-red fibers. In muscle biopsy specimens, the mutant mtDNA accumulate preferentially in ragged red fibers. Ragged red fibers are typically negative for cytochrome oxidase activity (Al-Enezi et al., 2008). The correlation between KSS and absence of cytochrome-c oxidase suggests that the large scale single mtDNA deletion affects the Complex IV enzyme significantly. It most likely involves deletion of at least one of the three mtDNA structural subunit genes - MT-CO1, MT-CO2 or MT-CO3 (Alston et al, 2017). The extent of the deletion of mt-DNA varies in KSS but it is believed it can range from 1.3 to 10 kb, with the most common abnormality being a 4.9 kb deletion from the mitochondrial genome (Kapunga et al, 2015).
4.2 MELAS Syndrome: a mtDNA mutation disorder
MELAS is abbreviated for mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes,which clearly highlights the vast amount of problematic conditions that can arise from this syndrome.It is characterized by nausea, vomiting, seizures, headaches, diabetes mellitus, short stature, muscle weakness, exercise intolerance, sensorineural hearing loss, myopathy, lactic acidosis, sudden neurological deficits - Sudden neurological deficits are called ‘stroke-like episodes’ (Ito et al., 2011). MELAS is caused by mtDNA mutation, with over 30 mitochondrial DNA gene mutations have been reported to be associated with this syndrome (David et al.,2017).These mutations cause dysfunction to the oxidative phosphorylation process and energy production in the body. It is usually, but not invariably, associated with the A3243G point mutation in the mitochondrial DNA tRNA (Leu-UUR) gene (Sue et al.,1999), with some reports going as far as Approximately 80% of MELAS cases show mutation of mitochondrial DNA A3243G (Ito et al., 2011).
The tRNA mutation in MELAS offers an alternative pathogenetic mechanism to other point mutations in mtDNA linked disorders, as the A--G transition at nucleotide 3243 may alter the recognition site for a protein responsible for transcription termination of rRNA genes . This transcription termination at this specific site is considered important in maintaining the appropriate steady-state ratios of mRNAs:rRNAs (Moraes et al., 1992). This shows that the mutant mtDNA of this condition can affect not only the energy production of the genome but also its genetic makeup. Due to it being caused by a mutation of mtDNA, this condition is one of the most common maternally inherited mitochondrial diseases. As it is inherited, it usually has an early onset with first onset of symptoms frequently between ages of two and ten years (Al-Enezi et al., 2008). The 3243A>G mutation results in impaired mitochondrial translation and protein synthesis including the mitochondrial electron transport chain complex subunits leading to impaired mitochondrial energy production (El-Hattab et al., 2015).
In a study shown by (Danhelovska et al., 2020), there was a significant impact of mitochondrial mutant DNA on complex 1 ( NADH dehydrogenase) as well as Complex IV (cytochrome-c-oxidase), leading to syndromes such as MELAS, Leigh’s syndrome and LHON(Leber’s hereditary optic neuropathy. In fact investigations have shown that Leigh’s syndrome is often manifested by a gene mutation - G13513A - commonly associated with MELAS.(Chol et al., 2003). This mutation shows a noticeable deficiency in complex 1 also. Clinically, Complex I deficiency represents a heterogeneous group of mitochondrial disorders with an early, neonatal onset of fatal lactic acidosis (Danhelovska et al., 2020).The fact that lactic acidosis is a common feature of Complex 1 strongly links it to MELAS syndrome (Alston et al., 2017). In cases of young children with early onset of encephalopathy, there was a strong correlation with both the A3243G mutation and abnormally high levels of ventricular lactate, coinciding with the cerebrospinal fluid lactate level as high as 4.8 mmol/L (normal = 0.6 to 2.2 mmol/L), (Kaufmann et al., 2004).
|
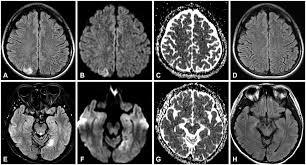
Figure 4 MRI of brain in MELAS patient |
The affected areas in neuroimaging are predominantly the temporal, parietal, and occipital lobes, and can be restricted to cortical areas or involve subcortical white matter. Neurological problems linked with MELAS include dementia, epilepsy, hearing loss, and recurrent headaches (El-Hattab et al., 2015). The high occurrence of stroke-like seizures caused by lesions in upper cortical areas and high cerebral lactate levels can be demonstrated in MRI scans that show both lesions and lactate peaks, which can be observed in Figure 4. Studies on the fibroblasts of patients with mitochondrial disorders such as MELAS and MERRF (myoclonic epilepsy and ragged red fibres) show an increase of secondary lysosomes with a possible explanation being that the defective mitochondria are rapidly degraded (M.James et al.,1996). This would also explain the cause of exercise intolerance that is so often seen in these disorders. There have also been studies to show MELAS can have cardiac complications such as hypertrophic cardiomyopathy,ventricular arrhythmias, sudden cardiac death, and conduction disorders (Brailova et al., 2020).
4.3 Alzheimer's disease
It is a very common form of dementia and affects millions of people across the world. Symptoms are severe memory loss, with episodic memory being particularly effected during the initial phases. Cases occur randomly, although inheritance of certain susceptibility genes enhances the risk. Alzheimer’s occurring in families represents the minority of cases and is caused by mutations in genes encoding for either the amyloid β-precursor protein , presenilin -1, or presenilin -2 (Baloyannis et al., 2011).
Although the brain is 2% of the body weight, it receives 15% of cardiac output and accounts for 20% of total body oxygen consumption. The energy requirement is largely down to the neural demand to maintain ion gradients across the plasma membrane that is critical for the generation of action potentials. If there is a loss of oxygen or glucose even only for a short period time it will result in neural death.
Mitochondria are essential for neuronal function because the limited glycolytic capacity of these cells makes them highly dependent on aerobic oxidative phosphorylation for their energy needs this results in free radicle formation, if the amount of free radicals produced overwhelms the neural ability to neutralise them, oxidative stress occurs, followed by mitochondrial abnormality and neural damage(Moreira et al.,2010). It is proposed that mitochondrial abnormality and dysfunction would cause the onset of Alzheimer’s disease (Baloyannis et al.,2011). Neurons showing increased oxidative damage also have a significant increase in mtDNA. It was also found that a lot of the mtDNA was mainly localised in the neuronal cytoplasm.The mitochondrial abnormalities seen in the brains of people with Alzheimer’s are backed up by animal studies. It is reported that mitochondrial dysfunction is an early event in mice bearing the human Swedish and London mutations and thee mitochondrial defects like Alzheimer’s accumulate with age (P.T. Francis, 2005).
4.4 Parkinson's disease
As well as their major role in energy metabolism, mitochondria are involved in several cellular processes, such as the regulation of calcium homeostasis, stress response and cell death pathways.
Mitochondrial abnormalities result in cellular damage and are linked to aging and neurodegeneration. It is suggested that mitochondrial abnormalities play a part in the pathogenesis of Parkinson's disease, starting in the early 1980s with the observation that an inhibitor of complex I of the electron transport chain can induce Parkinson’s (Kaufmann et al., 2004).
It is the most common movement disorder and the second most common neurodegenerative disease after Alzheimer's disease.
It comes about from the loss of dopaminergic neurons in the substantia nigra pars compacta leading to a dopamine loss in the striatum. The consequence is the inability of the basal ganglia to function, accounts for serval motor symptoms such as tremors , other symptoms may also develop, sleep disturbances, depression and cognitive impairment (Surmeier et al., 2011). Patients with mutations in the gene encoding mitochondrial DNA polymerase (POLG) accumulate multiple mtDNA mutations and develop Parkinson’s. In older Parkinson's patients, clonally expanded mtDNA deletions have been observed and they cause defects in the respiratory chain (Moreira et al., 2010).
4.5 Prognosis of Mitochondrial diseases
The general message in prognosis of mitochondrial disorders is not a positive one, with most patients having to contend with the condition for the duration of their lifetime. It is important to make a distinction between pediatric and adult cases, the former being usually more dramatically affected and severe. In the last years many efforts have been made to find more effective treatments if not a real cure to improve both quality of life and survival rate in affected children, the purpose being to try to guarantee fairly normal lives at least in selected cases. Many children, however, still have to face major disabilities and a poor prognosis, survival rate ranging between few months and teenage years (Moggio et al., 2014). This is extremely apparent in cases of young children with mitochondrial disorders that cause cardiomyopathy, due to extreme deficiencies in cytochrome c oxidase, with one investigation by showing that eight children, all with a cytochrome-c oxidase deficiency died before the age of 13 years (Holmgren, 2003).
4.6 Possible treatment methods of Mitochondrial diseases
Due to the nature of the disorders, arising from deletions and/or mutations of mtDNA , they are in most cases impossible to cure. Patients with mitochondrial disorders still suffer from delays in diagnosis. General physicians are often not familiar with these disorders. The diagnosis of mitochondrial diseases is challenging because of wide variations in phenotypic expression and variable penetrance (David et al.,2017). For example, KSS syndrome is not an easy diagnosis to make in the early phase of the disease. Some of the patients demonstrate Pearson’s syndrome initially, whilst others might have associated Leigh disease (Van Beynum et al., 2011). However, even if the correct diagnosis is made, the adequate therapy could be challenging. However there are measures which can help patients live a more normal life by regulating some of the problematic issues which arise from mitochondrial disorders e.g. diabetes, visual problems, hearing impairment and stroke like episodes.
In fact it was shown in patients with MELAS syndrome, including the 13513G > A mutation, that L-arginine infusion during the acute phase of the stroke-like episode may reduce acute symptoms, and oral supplementation with L-arginine and/or L-citrulline may prevent further stroke-like episodes (Danhelovska et al., 2020) Many practitioners and scientists have trialled treatment of mitochondrial diseases with compounds often found throughout the enzyme complexes and mitochondria, however other experiments have shown treatment with co-enzyme Q10 plus vitamins K3 and C, riboflavin, thiamine, and niacin, that co-enzyme Q10 plus vitamin therapy does not significantly improve mitochondrial oxidative metabolism in patients with mitochondrial disease in general. Any clinical benefit that may follow from short-term administration appears slight (Matthews et al., 1993).
Alzheimer's s does not have a cure as such. However there is an improved Understanding of the disease and the development of effective treatments are essential not only to cure but also eventually to prevent or postpone the onset of the symptoms in the patients. The traditional cures used to treat the Alzheimers are so far the cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) and memantine that block the N-methyl-D-aspartate (NMDA) receptor and the excess of glutamate activity. NMDA receptors and acetylcholine (Ach) are fundamental in memory and learning (Francis, 2005). These treatments only improve the learning and memory functions, without really slowing down the progression of the disease. Because mitochondria play a role in the development of alzheimers they should be taken in to mind as a cure for it. However many different compounds that affected the mitochondria have been tried without a successful result. Although in the last few years alzheimers has been looked at as more of a multi-function disease. The mitochondria being used as a therapeutic factor has been reconsidered and could be used in combination with other drugs (Baloyannis et al., 2011).
A study in Parkinson's patients showed that oral administration of co-enzyme Q10 was well tolerated and caused a trend towards increased complex I activity (Shults, 2020). Co-enzyme Q shuttles electrons from complexes I/II to complex III of the electron transport chain. Also, ubiquinol functions as antioxidant, prevents lipid peroxidation in most subcellular membranes and also protects mitochondrial membrane proteins and DNA from free radical oxidative damage (Dallner et al., 1995). Another randomized, double‐blind trial of co-enzyme Q10 in early Parkinsons diseases showed that co-enmyme Q shouldn’t be ignored or discounted as a treatment for parkinsons (Surmeier et al., 2011). But large trials are still needed to test the long term effects of co-enzyme Q10 intake.
Conclusion
It is increasingly recognised that mitochondrial disorders are more common than previously thought, with one in 4,300 adults in the UK diagnosed with a mitochondrial disorder (David et al., 2017). The advent of improved diagnostic techniques has led to a global increase in patients diagnosed with mitochondrial disorders. The appearance and diagnosis of mitochondrial disorders are becoming increasingly more evident as the symptoms that are characteristic of such disorders have been identified (e.g the triad of conditions that are present in all cases of KSS). It is clear that many of these mitochondrial disorders such as Leigh's syndrome, MELAS, and Leber's hereditary optic neuropathy have complicated and detrimental implications on individuals, in particular when they manifest at a young age, resulting in a short life expectancy and difficult prognosis. Developing an effective treatment for mitochondrial disease is an enormous challenge that is dependent on the integration of clinical understanding of disease progression, molecular genetic mechanisms, and neuropathological features in mitochondrial disease (Alston et al.,2017). Using recombinant gene technologies to repair or replace damaged/mutated genes to ensure such mutations and consequential disorders are not passed on to the offspring of the patient is of the utmost importance in this field of medicine.
References
- Al-Enezi, M., Al-Saleh, H. and Nasser, M. (2008): Mitochondrial disorders with significant ophthalmic manifestations. Middle East African Journal of Ophthalmology, 15:(2) 81.
- Alston, C., Rocha, M., Lax, N., Turnbull, D. and Taylor, R. (2016): The genetics and pathology of mitochondrial disease. The Journal of Pathology, 241: (2) 236-250.
- Andreu AL, Bruno C, Shanske S, Shtilbans A, Hirano M, Krishna S, Hayward L, Systrom DS, Brown RH Jr, Di Mauro (1998): Missense mutation in the mtDNA cytochrome b gene in a patient with myopathy. Neurology 51:1444–1447.
- Baloyannis, S. (2011): Mitochondria Are Related to Synaptic Pathology in Alzheimer's Disease. International Journal of Alzheimer's Disease 2:21-73.
- Birky Jr, C.W. (1978): Transmission genetics of mitochondria and chloroplasts. Annual review of genetics, 12:(1) 471-512.
- Brailova, M., Clerfond, G., Trésorier, R., Minet-Quinard, R., Durif, J., Massoullié, G., Pereira, B., Sapin, V., Eschalier, R. and Bouvier, D. (2020): Inherited Metabolic Diseases and Cardiac Pathology in Adults: Diagnosis and Prevalence in a Cardio Metabo Study. Journal of Clinical Medicine 9:(3) 694.
- Chawla, S., Coku, J., Forbes, T. and Kannan, S. (2007): Kearns-Sayre Syndrome Presenting as Complete Heart Block. Pediatric Cardiology, 29:(3) 659-662.
- Chol, M.(2003): The Mitochondrial DNA G13513A MELAS mutation in the NADH Dehydrogenase 5 Gene is A frequent cause of Leigh-Like Syndrome with Isolated Complex I Deficiency. BMJ Journals 40:(3) 188–191.
- C T Moraes, E. (1992): The Mitochondrial Trna(Leu(UUR)) Mutation In Mitochondrial Encephalomyopathy, Lactic Acidosis, And Stroke-like Episodes (MELAS): Genetic, Biochemical, And Morphological Correlations In Skeletal Muscle. American Journal of Human Genetics 50(5): 934–949.
- C. Wallace, D., 2010. Mitochondrial DNA Mutations In Disease And Aging. Annual Review of Genetics 39: 347-355.
- Dallner, F., G., Ernster, L., Scherstén, T. and Soussi, B. (1995): Coenzymes Q9 and Q10 in skeletal and cardiac muscle in tumour-bearing exercising rats. European Journal of Cancer, 31:(5) 760-765.
- Danhelovska, T., Kolarova, H., Zeman, J., Hansikova, H., Vaneckova, M., Lambert, L., Kucerova-Vidrova, V., Berankova, K., Honzik, T. and Tesarova, M. (2020): Multisystem mitochondrial diseases due to mutations in mtDNA-encoded subunits of complex I. BMC Pediatrics 20(1).
- David, J., Okiro, J., Murphy, K. and Elamin, M. (2017): Uncommon mutation in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS). BMJ Case Reports 20:121-133.
- El-Hattab, A., Adesina, A., Jones, J. and Scaglia, F. (2015): MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Molecular Genetics and Metabolism 116:(1-2) 4-12.
- Finsterer, J., Winklehner, M., Stöllberger, C. and Hummel, T. (2020): Unusual Phenotype and Disease Trajectory in Kearns–Sayre Syndrome. Case Reports in Neurological Medicine 2:11-16.
- Francis, P. T. (2005). The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectrum 10: 6–9.
- Gustafson, M., Mc Cormick, E., Perera, L., Longley, M., Bai, R., Kong, J., Dulik, M., Shen, L., Goldstein, A., Mc Cormack, S., Laskin, B., Leroy, B., Ortiz-Gonzalez, X., Ellington, M., Copeland, W. and Falk, M. (2019): Mitochondrial single-stranded DNA binding protein novel de novo SSBP1 mutation in a child with single large-scale mtDNA deletion (SLSMD) clinically manifesting as Pearson, Kearns-Sayre, and Leigh syndromes. PLOS ONE 14:(9)122-129.
- Holt J, Harding AE, Morgan-Hughes JA. (1988): Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 331:717–719.
- Holmgren, D. (2003): Cardiomyopathy in children with mitochondrial disease Clinical course and cardiological findings. European Heart Journal 24:(3)280-288.
- Ito, H., Mori, K. and Kagami, S. (2011): Neuroimaging of stroke-like episodes in MELAS. Brain and Development, 33:(4) 283-288.
- James, A., Wei, Y., Pang, C. and Murphy, M. (1996): Altered mitochondrial function in fibroblasts containing MELAS or MERRF mitochondrial DNA mutations. Biochemical Journal, 318:(2) 401-407.
- Kabunga, P., Lau, A., Phan, K., Puranik, R., Liang, C., Davis, R., Sue, C. and Sy, R. (2015): Systematic review of cardiac electrical disease in Kearns–Sayre syndrome and mitochondrial cytopathy. International Journal of Cardiology, 181: 303-310.
- Kaufmann, P., Shungu, D., Sano, M., Jhung, S., Engelstad, K., Mitsis, E., Mao, X., Shanske, S., Hirano, M., Di Mauro, S. and De Vivo, D. (2004): Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology 62:(8) 1297-1302.
- Matthews, P., Ford, B., Dandurand, R., Eidelman, D., O'Connor, D., Sherwin, A., Karpati, G., Andermann, F. and Arnold, D. (1993): Coenzyme Q10 with multiple vitamins is generally ineffective in treatment of mitochondrial disease. Neurology 43:(5) 884-884.
- Maurizio Moggio, M. (2014): Mitochondrial Disease Heterogeneity: A Prognostic Challenge. Acta Myologica. 33(2): 86–93.
- Mita, S., Schmidt, B., Schon, E., Di Mauro, S. and Bonilla, E., 1989. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proceedings of the National Academy of Sciences 86: (23) 9509-9513.
- Mishmar D, Ruiz-Pesini EE, Golik P, Macaulay V, Clark AG, Hosseini S, Brandon M, Easley K, Chen E, Brown MD, others. (2003): Natural selection shaped regional mtDNA variation in humans. Proceedings of the National Academy of Sciences of the United States of America 100:171–176.
- Moreira, P., Carvalho, C., Zhu, X., Smith, M. and Perry, G.(2010): Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1802:(1) 2-10.
Salvatore Di Mauro & Guido Davidzon (2005): Mitochondrial DNA and disease, Annals of Medicine, 37: (3) 222-232.
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, others (2005): Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308:1909–1911.
- Sue, C., Bruno, C., Andreu, A., Cargan, A., Mendell, J., Tsao, C., Luquette, M., Paolicchi, J., Shanske, S., Di Mauro, S. and De Vivo, D. (1999): Infantile encephalopathy associated with the MELAS A3243G mutation. The Journal of Pediatrics 134: (6) 696-700.
- Surmeier D., Guzman, J., Sanchez-Padilla J. and Schumacker, P. (2011): The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson's disease. Neuroscience 198:221-231.
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT,Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R. (2004): Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429:417–423.
- van Beynum, I., Morava, E., Taher, M., Rodenburg, R., Karteszi, J., Toth, K. and Szabados, E. (2011): Cardiac Arrest in Kearns–Sayre Syndrome. JIMD Reports 7-10.
- Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, ElsasLJ, Nikoskelainen EK. (1988): Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242:1427–1430.
- Wallace DC. (2005): A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer. Annual Review of Genetics 39:359–407.
- Yu, N., Zhang, Y., Zhang, K., Xie, Y., Lin, X. and Di, Q. (2016): MELAS and Kearns–Sayre overlap syndrome due to the mtDNA m. A3243G mutation and large-scale mtDNA deletions. Neurological Science 4: 15-18.
Figures
Figure 1: Taken from Wikimedia Commons: Available at https://commons.wikimedia.org/wiki/File:Mitochondrion_structure.svg.
Figure 2: Taken from Wikimedia Commons: Available at https://commons.wikimedia.org/wiki/File:Mitochondrial_DNA_en.svg
Figure 3: Taken from Wikimedia Commons: Available at https://upload.wikimedia.org/wikipedia/commons/7/76/Maternal_Inheritance_-_mitochondrial_DNA.png
Figure 4: Taken from Wikimedia Commons: Available at https://commons.wikimedia.org/wiki/File:Brain_MRIs_of_Stroke-Like_Lesions_typical_of_MELAS.jpg