Itt írjon a(z) Abnormal_mito-ról/ről
Abnormalities in mitochondrial structure, causes and effects
Table of Contents
Contents
Introduction
In this essay we will examine firstly the role and structure of the mitochondrion, and the relevant processes it is responsible for. A key aspect will be the study of the genetic makeup and how the mitochondria is encoded with both nuclear DNA and also mitochondrial DNA, and the importance of maternal inheritance in regards to mtDNA. Knowledge of the genetic background is important if you are to then look at the various disorders and diseases associated with abnormalities in the mitochondria. There is no surprise that the majority of mitochondrial diseases affect tissues and organs which have the highest energy demands such as the heart, brain and muscles since the mitochondria is responsible for the production of ATP Using various scientific papers and articles we will gather information on some of the most common and complicating diseases that arise from disorders in the mitochondria, and what are the causes - Complex deficiencies, Point mutations or mtDNA deletions are areas of major significance, especially when these are inherited by offspring. Diseases and disorders examined include MELAS syndrome, Kearns Sayre syndrome, Alzheimers, and Parkinson’s disease. Secondary disorders and conditions arise from such syndromes and diseases, such as diabetes, exercise intolerance, lactic acidosis and multiple organ failure.
The Mitochondrion
The mitochondrion is a double-membraned structure found in most eukaryotic cells. The mitochondria generates most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. hence the name ‘powerhouse of the cell’ given by Philip Siekevitz.
Structure of the Mitochondrion
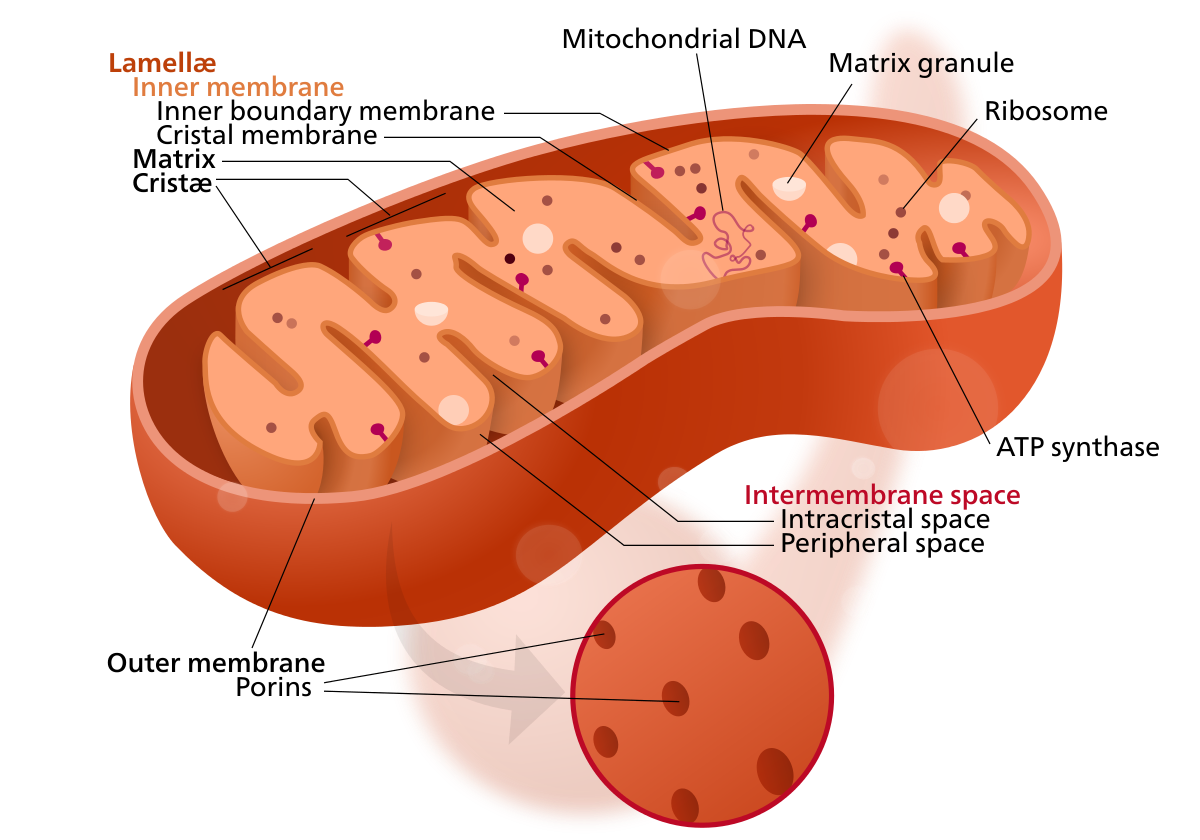
The organelle is composed of compartments that carry out specialized functions. Because of their double-membraned organization, there are five distinct parts to a mitochondrion. These 5 main compartments or regions are: the outer membrane, the intermembrane space, the inner membrane, and the cristae and matrix. These structures can be seen in figure 1.
|
Figure 1 Structure of the mitochondrion |
Role and processes of mitochondria
The central set of reactions involved in ATP production are collectively known as the citric acid cycle, or the Krebs cycle. Genes in the mitochondrial respiratory chain complex gene group provide instructions for proteins involved in oxidative phosphorylation, also called the respiratory chain. Oxidative phosphorylation is an important cellular process that uses oxygen and simple sugars – Adenosine diphosphate (ADP) to create adenosine triphosphate (ATP), the cell's main energy source. Five protein complexes, made up of several proteins each, are involved in this process(Alston et al., 2017). The complexes are named complex I (NADH dehydrogenase), complex II (Succinate dehydrogenase), complex III (Ubiquinol–cytochrome c oxidoreductase), complex IV (cytochrome C oxidase), and complex V. (ATP synthase)
Genetics of Mitochondrial disease
Mitochondrial diseases
Mitochondrial diseases arise from mutations in either mtDNA or nuclear mitochondrial genes or they can arise from large scale deletions of mtDNA. Mitochondrial disorders are errors of metabolism caused by mutations in mitochondrial and nucleus genes that lead to impaired oxidative phosphorylation (Ito, Mori and Kagami, 2011).These abnormalities can cause severe syndromes as well as conditions affecting a multitude of tissues in the body, including the eyes, the heart, muscles, endocrine system and the central nervous system.
Kearns Sayre Syndrome: a mtDNA deletion disorder
Kearns Sayre Syndrome (KSS) is a mitochondrial myopathy and a clinical subtype of chronic progressive external ophthalmoplegia (CPEO).The syndrome is defined by the obligatory triad of onset before the age of 20 years, progressive external ophthalmoplegia, and pigmentary retinopathy (van Beynum et al., 2011). In most cases it is caused by single, large scale mitochondrial DNA deletions or mitochondrial DNA depletion (van Beynum et al., 2011). Single, large‐scale mtDNA deletions have a population frequency of 1.5/100 000 with three main associated phenotypes: chronic progressive external ophthalmoplegia, Kearns–Sayre syndrome, and Pearson syndrome (Alston et al., 2017). Chronic progressive ophthalmoplegia (CPEO) is the most common ocular manifestation of mitochondrial myopathies (Al-Enezi et al., 2008). It manifests in the ocular system in many ways eye movement paralysis (ophthalmoplegia), ptosis, oropharyngeal weakness, and proximal myopathy with exercise intolerance (Gustafason et al., 2019). Being a subtype of CPEO, the majority of these effects are also displayed by KSS. It is even believed that the number of tissues affected is higher than so far anticipated. (Finsterer et al, 2020)
Apart from the main three consistent features that characterize KSS (PEO, pigmentary retinopathy and presentation before 20 years of age), this syndrome often displays at least one of the following multisystem implications: cerebellar ataxia, heart block and elevated cerebrospinal fluid protein level.( Yu et al., 2016). Other features include hearing loss, dementia, cardiomyopathy and endocrine disorders, which highlights the impact of this mitochondrial syndrome. Cardiac manifestations in KSS are as high as 50% and sudden cardiac death reported in up to 20% of KSS cases.(Chawla et al.,2008). The most typical cardiac complications of the disease are conduction defects, which usually begin with left anterior fascicular block with or without right bundle branch block (RBBB), progressing sometimes rapidly to complete atrioventricular block. Other cardiac manifestations reported are first or second degree of AV block, QT prolongation, ventricular tachycardia, and cardiomyopathy (Van Beynum et al.,2011)
KSS is usually caused by deletions of mt-DNA, rather than a mutation of the mt-DNA. Due to this it is rarely inherited. single, large-scale mtDNA deletions (SLSMDs) have long been thought to occur sporadically in affected individuals or oocytes. (Gustafson et al, 2019). This suggests that mitochondrial disorders such as KSS develop somatically in the early embryo (Kapunga et al., 2015). Since KSS is caused by deletions of large portions of mitochondrial DNA (mtDNA), this results in the loss of genes involved in the electron chain transport and oxidative phosphorylation pathway, which has effects on energy production. A consistent and conspicuous finding in muscle biopsy samples from patients with KSS is the presence of a population of fibers lacking histochemically detectable COX (cytochrome-c oxidase). Because a proportion of these COX-deficient fibers shows no other morphological abnormality of mitochondria, it has been proposed that they might be precursors of ragged-red fibers and that COX deficiency could be a factor in the pathogenesis of KSS and other disorders(Mita et al. 1989).
This has been further verified, in a publishment by (Finsterer et al,2020) a muscle biopsy from the left lateral vastus muscle in a patient diagnosed with KSS showed cytochrome-C-oxidase (COX)-negative fibers , glycogen depositions, fiber splitting, and ragged-red fibers.In muscle biopsy specimens, the mutant mtDNA accumulate preferentially in ragged red fibers. Ragged red fibers are typically negative for cytochrome oxidase activity (Al-Enezi et al., 2008). The correlation between KSS and absence of cytochrome-c oxidase suggests that the large scale single mtDNA deletion affects the Complex IV enzyme significantly. It most likely involves deletion of at least one of the three mtDNA structural subunit genes - MT-CO1, MT-CO2 or MT-CO3. (Alston et al, 2017) The extent of the deletion of mt-DNA varies in KSS but it is believed it can range from 1.3 to 10 kb, with the most common abnormality being a 4.9 kb deletion from the mitochondrial genome. (Kapunga et al, 2015).
MELAS Syndrome: a mtDNA mutation disorder
MELAS is abbreviated for mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes,which clearly highlights the vast amount of problematic conditions that can arise from this syndrome.It is characterized by nausea, vomiting, seizures, headaches, diabetes mellitus, short stature, muscle weakness, exercise intolerance, sensorineural hearing loss, myopathy, lactic acidosis, sudden neurological deficits - Sudden neurological deficits are called ‘stroke-like episodes’ (Ito, Mori and Kagami, 2011). MELAS is caused by mtDNA mutation, with over 30 mitochondrial DNA gene mutations have been reported to be associated with this syndrome (David et al.,2017).These mutations cause dysfunction to the oxidative phosphorylation process and energy production in the body. It is usually, but not invariably, associated with the A3243G point mutation in the mitochondrial DNA tRNALeu(UUR) gene (Sue et al.,1999), with some reports going as far as Approximately 80% of MELAS cases show mutation of mitochondrial DNA A3243G (Ito, Mori and Kagami, 2011).
The tRNA mutation in MELAS offers an alternative pathogenetic mechanism to other point mutations in mtDNA linked disorders, as the A--G transition at nucleotide 3243 may alter the recognition site for a protein responsible for transcription termination of rRNA genes . This transcription termination at this specific site is considered important in maintaining the appropriate steady-state ratios of mRNAs:rRNAs (Moraes et al., 1992). This shows that the mutant mtDNA of this condition can affect not only the energy production of the genome but also its genetic makeup. Due to it being caused by a mutation of mtDNA, this condition is one of the most common maternally inherited mitochondrial diseases. As it is inherited, it usually has an early onset with first onset of symptoms frequently between ages of two and ten years(Al-Enezi et al.,2008). The 3243A>G mutation results in impaired mitochondrial translation and protein synthesis including the mitochondrial electron transport chain complex subunits leading to impaired mitochondrial energy production (El-Hattab et al., 2015)
In a study shown by (Danhelovska et al.,2020), there is a significant impact of mitochondrial mutant DNA on complex 1 ( NADH dehydrogenase) as well as Complex IV (cytochrome-c-oxidase), leading to syndromes such as MELAS, Leigh’s syndrome and LHON(Leber’s hereditary optic neuropathy. In fact investigations have shown that Leigh’s syndrome is often manifested by a gene mutation - G13513A - commonly associated with MELAS.(Chol et al.,2003). This mutation shows a noticeable deficiency in complex 1 also. Clinically, Complex I deficiency represents a heterogeneous group of mitochondrial disorders with an early, neonatal onset of fatal lactic acidosis (Danhelovska et al., 2020).The fact that lactic acidosis is a common feature of Complex 1 strongly links it to MELAS syndrome (Alston et al., 2017). In cases of young children with early onset of encephalopathy, there was a strong correlation with both the A3243G mutation and abnormally high levels of ventricular lactate, coinciding with the cerebrospinal fluid lactate level as high as 4.8 mmol/L (normal = 0.6 to 2.2 mmol/L) (Kaufmann et al.,2004).
|
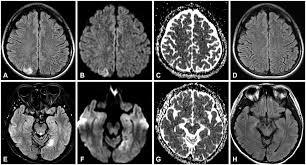
Figure 4 MRI of brain in MELAS patient |
The affected areas in neuroimaging are predominantly the temporal, parietal, and occipital lobes, and can be restricted to cortical areas or involve subcortical white matter. Neurological problems linked with MELAS include dementia, epilepsy, hearing loss, and recurrent headaches (El-Hattab et al., 2015). The high occurrence of stroke-like seizures caused by lesions in upper cortical areas and high cerebral lactate levels can be demonstrated in MRI scans that show both lesions and lactate peaks, which can be observed in Figure 4. Studies on the fibroblasts of patients with mitochondrial disorders such as MELAS and MERRF (myoclonic epilepsy and ragged red fibres) show an increase of secondary lysosomes with a possible explanation being the defective mitochondria are rapidly degraded (M.James et al.,1996). This would also explain the cause of exercise intolerance that is so often seen in these disorders. There have also been studies to show MELAS can have cardiac complications such as hypertrophic cardiomyopathy,ventricular arrhythmias, sudden cardiac death, and conduction disorders (Brailova et al.,2020).
Alzheimer's disease
Parkinson's disease
Prognosis of Mitochondrial diseases
The general message in prognosis of mitochondrial disorders is not a positive one, with most patients having to contend with the condition for the duration of their lifetime. It is important to make a distinction between pediatric and adult cases, the former being usually more dramatically affected and severe. In the last years many efforts have been made to find more effective treatments if not a real cure to improve both quality of life and survival rate in affected children, the purpose being to try to guarantee fairly normal lives at least in selected cases. Many children, however, still have to face major disabilities and a poor prognosis, survival rate ranging between few months and teenage years (Moggio et al., 2014). This is extremely apparent in cases of young children with mitochondrial disorders that cause cardiomyopathy, due to extreme deficiencies in cytochrome c oxidase, with one investigation by Holmgren (2003) showing that eight children, all with cytochrome-c oxidase deficiency died before the age of 13 years.
Possible treatment methods of Mitochondrial diseases
Conclusion
It is increasingly recognised that mitochondrial disorders are more common than previously thought, with one in 4,300 adults in the UK diagnosed with a mitochondrial disorder (David et al., 2017). The advent of improved diagnostic techniques has led to a global increase in patients diagnosed with mitochondrial disorders. The appearance and diagnosis of mitochondrial disorders are becoming increasingly more evident as the symptoms that are characteristic of such disorders have been identified (e.g the triad of conditions that are present in all cases of KSS). It is clear that many of these mitochondrial disorders such as Leigh's syndrome, MELAS, and Leber's hereditary optic neuropathy have complicated and detrimental implications on individuals, in particular when they manifest at a young age, resulting in a short life expectancy and difficult prognosis.Developing an effective treatment for mitochondrial disease is an enormous challenge that is dependent on the integration of clinical understanding of disease progression, molecular genetic mechanisms, and neuropathological features in mitochondrial disease (Alston et al.,2017). One area of focus should be using recombinant gene technologies to ensure such mutations are not passed on to the offspring of the patient.
References
- Al-Enezi, M., Al-Saleh, H. and Nasser, M., 2008. Mitochondrial disorders with significant ophthalmic manifestations. Middle East African Journal of Ophthalmology, 15(2), p.81.
- Alston, C., Rocha, M., Lax, N., Turnbull, D. and Taylor, R., 2016. The genetics and pathology of mitochondrial disease. The Journal of Pathology, 241(2), pp.236-250.
Brailova, M., Clerfond, G., Trésorier, R., Minet-Quinard, R., Durif, J., Massoullié, G., Pereira, B., Sapin, V., Eschalier, R. and Bouvier, D., 2020. Inherited Metabolic Diseases and Cardiac Pathology in Adults: Diagnosis and Prevalence in a CardioMetabo Study. Journal of Clinical Medicine, 9(3), p.694.
- Chawla, S., Coku, J., Forbes, T. and Kannan, S., 2007. Kearns-Sayre Syndrome Presenting as Complete Heart Block. Pediatric Cardiology, 29(3), pp.659-662.
Chol, M., 2003. The Mitochondrial DNA G13513A MELAS Mutation In The NADH Dehydrogenase 5 Gene Is A Frequent Cause Of Leigh-Like Syndrome With Isolated Complex I Deficiency. [online] BMJ Journals. Available at: <https://jmg.bmj.com/content/40/3/188.short
C T Moraes, E., 1992. The Mitochondrial Trna(Leu(UUR)) Mutation In Mitochondrial Encephalomyopathy, Lactic Acidosis, And Strokelike Episodes (MELAS): Genetic, Biochemical, And Morphological Correlations In Skeletal Muscle.. [online] PubMed Central (PMC). Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1682620/
- Danhelovska, T., Kolarova, H., Zeman, J., Hansikova, H., Vaneckova, M., Lambert, L., Kucerova-Vidrova, V., Berankova, K., Honzik, T. and Tesarova, M., 2020. Multisystem mitochondrial diseases due to mutations in mtDNA-encoded subunits of complex I. BMC Pediatrics, 20(1).
- David, J., Okiro, J., Murphy, K. and Elamin, M., 2017. Uncommon mutation in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS). BMJ Case Reports, p.bcr2016218133.
- El-Hattab, A., Adesina, A., Jones, J. and Scaglia, F., 2015. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Molecular Genetics and Metabolism, 116(1-2), pp.4-12.
- Finsterer, J., Winklehner, M., Stöllberger, C. and Hummel, T., 2020. Unusual Phenotype and Disease Trajectory in Kearns–Sayre Syndrome. Case Reports in Neurological Medicine, 2020, pp.1-6.
Gustafson, M., McCormick, E., Perera, L., Longley, M., Bai, R., Kong, J., Dulik, M., Shen, L., Goldstein, A., McCormack, S., Laskin, B., Leroy, B., Ortiz-Gonzalez, X., Ellington, M., Copeland, W. and Falk, M.,(2019). Mitochondrial single-stranded DNA binding protein novel de novo SSBP1 mutation in a child with single large-scale mtDNA deletion (SLSMD) clinically manifesting as Pearson, Kearns-Sayre, and Leigh syndromes. PLOS ONE, 14(9), p.e0221829.
- Holmgren, D., 2003. Cardiomyopathy in children with mitochondrial disease Clinical course and cardiological findings. European Heart Journal, 24(3), pp.280-288.
- Ito, H., Mori, K. and Kagami, S., 2011. Neuroimaging of stroke-like episodes in MELAS. Brain and Development, 33(4), pp.283-288.
- JAMES, A., WEI, Y., PANG, C. and MURPHY, M., 1996. Altered mitochondrial function in fibroblasts containing MELAS or MERRF mitochondrial DNA mutations. Biochemical Journal, 318(2), pp.401-407.
- Kabunga, P., Lau, A., Phan, K., Puranik, R., Liang, C., Davis, R., Sue, C. and Sy, R., 2015. Systematic review of cardiac electrical disease in Kearns–Sayre syndrome and mitochondrial cytopathy. International Journal of Cardiology, 181, pp.303-310.
Kaufmann, P., Shungu, D., Sano, M., Jhung, S., Engelstad, K., Mitsis, E., Mao, X., Shanske, S., Hirano, M., DiMauro, S. and De Vivo, D., 2004. Cerebral lactic acidosis correlates with neurological impairment in MELAS. Neurology, 62(8), pp.1297-1302.
- Matthews, P., Ford, B., Dandurand, R., Eidelman, D., O'Connor, D., Sherwin, A., Karpati, G., Andermann, F. and Arnold, D., 1993. Coenzyme Q10 with multiple vitamins is generally ineffective in treatment of mitochondrial disease. Neurology, 43(5), pp.884-884.
Maurizio Moggio, M., 2014. Mitochondrial Disease Heterogeneity:A Prognostic Challenge. [online] PubMed Central (PMC). Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4299169/
Mita, S., Schmidt, B., Schon, E., DiMauro, S. and Bonilla, E., (1989). Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proceedings of the National Academy of Sciences, 86(23), pp.9509-9513.
Sue, C., Bruno, C., Andreu, A., Cargan, A., Mendell, J., Tsao, C., Luquette, M., Paolicchi, J., Shanske, S., DiMauro, S. and De Vivo, D., 1999. Infantile encephalopathy associated with the MELAS A3243G mutation. The Journal of Pediatrics, 134(6), pp.696-700.
- van Beynum, I., Morava, E., Taher, M., Rodenburg, R., Karteszi, J., Toth, K. and Szabados, E.,( 2011). Cardiac Arrest in Kearns–Sayre Syndrome. JIMD Reports, pp.7-10.
- Yu, N., Zhang, Y., Zhang, K., Xie, Y., Lin, X. and Di, Q., 2016. MELAS and Kearns–Sayre overlap syndrome due to the mtDNA m. A3243G mutation and large-scale mtDNA deletions. eNeurologicalSci, 4, pp.15-18.