Itt írjon a(z) AlcoholEffectsonNeurons-ról/ről
Alcohol Effects on Neurons
Contents
Introduction
Many people are aware that it is dangerous to consume alcohol during pregnancy due to the harmful effects of ethanol as an intoxicant . They accept what they are told and in this essay one point we aim to discuss is the specific detrimental effects of fatal alcohol exposure to the foetus. The effect of alcohol on foetus can be referred to as foetal alcohol spectrum disorder (FASD). Numerous research has been carried out on the effects of FASD on the cerebral cortex , studies have shown that when exposed rv to alcohol developing cortical neurons are subjected to an increase rate of apoptosis.
Congnitive process that have a supervisory control over impulsive behaviour also have an ability to shift the attention of a human to complement the situation. They are controlled by the action of the Prefrontal cortex (PFC). These congnitive processes can be exemplified in poor decision making and in loss of inhibitory control over behaviour. A change in responce to drinking may be caused by functional changes of the PFC of both abstinent and non-abstitent alcohol. Chronic ethanol exposure can be the cause of changes in the PFC that are related to cognitive impairements,impulsivity and maladaptive decision making. The grey and white volume of the PFC are also altered. Following deficits of alcohol exposure to the body , the vunerability to relapse and disability to the development of effective treatment to for chronic relapsing disorder are increased.
Chronic alcohol exposure may alter behavioural and synaptic plasticity of the rodent prefrontal cortex. The NMDA receptor (NMDAR) is necessary for several forms of neuronal plasicity.It is a mediated glutamatergic neurotranssion, fluctuations of this in the pre-frontal limbic circuits maybe an indication of psychostimulants addiciton and also have a role in alcohol dependence. Acute alcohol exposure inhibits NMDAR, while chronic alcohol exposure increases the synaptic expression of NR2B subunits in the NMDAR. These subunits have been associated with synaptic plasticity and and alterations of learning and memory. A mouse model experiment was developed to show the implications of chronic alcohol exposure on medial PFC (mPFC) plasticity.
Chronic alcohol exposure can also be seen to disrupt dopamine receptor activity and the cognitive function of the medial prefrontal cortex. Dopamine controls the neuronetwork on which decision making is dependent upon and cognitive control. An optimal balance between D1 and D2-like DA receptors is required for the efficient activation of neural networks in the PFC. The DA neurotransmission in the PFC is a regulator of the cognitive function. Alterations in D2 expression in the dorsolateral PFC may be due to the loss of cognitive ability in alcohols. If the D2 receptors are decreased there is an increase in alcohol consumption in animal models. Susceptibility of developing alcoholism in humans is is a result of high D2 receptor levels.
Monoamineoxidase (MAO) has a huge impact on the neurotransmitter of the brain. It role in the brain was largely documented in studies regarding depressive disorders and it is only recently studies have identified the MAO is also impacted by Alcoholism. The impact on neurotransmitter is due to its by-products hydrogen peroxide. We aim to show how increased Ethanol can Influence MOA and synthesise its detrimental by-products.
Alcohol effects on Dopamine
A hypothesis was developed that assumed Chronic alcohol distrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. During the course of this investigation male-long-evan rats were utilised as the subject of experimentation. This animal colony was housed in polycarbonate cages and subjected to a 12hr light/dark cycle. A chronic intermirrent ethanol exposure module was developed as the first step to developing this hypothesis. Initiation of this treatment began when the rats were between 92 and 107 days old (Davidson et al 2014). Chronic intermittent ethanol (CIE) was induced. The rats were exposed to ethanol vapour or air in the inhalation chambers for 14h/d for 15 consectutive days with 10hr periods of withdrawal separating each exposure (Davidson et al 2014). The concentrations of ethanol in each chamber were measured on a day to day to day basis. Blood samples were taken from the tail vein in each animal ib exposure days 2,6,10 and 15 for measurement of the blood ethanol levels. The rats were then scored according to a set of behaviours : 1=no signs of intoxication, 2=slightly intoxicated(slight motor impairment), 3=moderately intoxicated(obvious motor impairment but able to walk), 4= highly intoxicated (dragging abdomen, loss of righting reflex) 5= extremely intoxicated(loss of righting reflex and loss of eye blink reflex)(Davidson et al 2014). An operant set-shiftng procedure was then put into place. For this procedure several phases were developed.
The first phase is termed the training phase, which involved the rat learning to press a levor, which determined their side preferance. The next phase is the visual-cue discrimmination phase, the rat learned to respond to visual cue where the correct lever was indicated by a stimulus light located directly above it (Davidson et al 2014) . The last phase was named the response discrimination phase in which the rat was required to shift its discrimmination strategy from the previously learned visual-cue rule to an egocentric spatial location rule, where the correct response was independent of the visual-cue light (Davidson et al 2014).
Through the course of this experiment electrophysiological recordings were noted. These recordings were obtained from the animal on the last day of alcohol exposure. They involved the rapid decapitation of the animal and placement of brain into a sucrose based dissection fluid. Recordings were taken from FS interneurons and spikes occured at highly variable frequencies, so the current spikes were then increased until a final spike was obtained. Following this another experimentation called the western blotting was undertaken. It involved the expression of NMDA receptor subunits in the PRL mPFC of control and CIE exposed rats were analysed (Davidson et al 2014). Receptor autoradiography was examined. D2 and D4 receptor density and D2 receptor-stimulate GTP gamma-s binding was determined in the mPFC of control and CIE exposed rats using in vitro receptor autoradiaography. The D2 receptor density was determined using D2 receptor anatagonist called raclopride. Non-specific binding was discovered by presence of butaclamol, another D2 anatagonist.
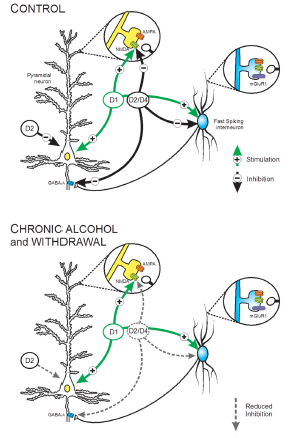
Figure 1 A schematic summary depicting the effects of CIE exposure on the intrinsic and synaptic actions of DA receptors on pyramidal neurons and FS interneurons in Layer 5 of the medial PFC. Top, Under control conditions, activation of D1 receptors enhanced the intrinsic firing of both cell type. Heather Trantham-Davidson,The Journal of Neuroscience, 34(10):3706 –3718.2014
The total binding was then determined by incubation for 1 hour in a buffer solution. The D2 receptor stimulated G-protein was also discovered using autoradiography. The measurements of total binding were taken form the mPFC of both hemispheres from 4 serial sections and averaged together into a single measurement for each subject. Specific and D2 recpetor stimulated binding were determined by subtracting nonspecific and basal binding,respectively, from total binding in adjacent sections (Davidson et al 2014). The concluding experiment involved immunochemistry, this involved the D2 receptor immunoreactivity was determined in the mPFC of conrol. Free floating sections containing the mPFC were processed for D2 receptor labelling uisng a standard avidin-biotin complex (Davidson et al 2014).
The final results of these experimentations proved the hypothesis to be correct. The effects of cyclic ethanol exposure and withdrawal on behavioural and also on the electrophysiologival properties of PFC in an experimental rat of chronic alcohol eposure by vapour inhalation were investigated. The resukts of the set-shifting experiment observed in the first week,following alcohol exposure, illustrated deficits in behavioural flexibilty. CIE exposure destroyed both D2 and D4 receptors, which were responsible for the modulation of evoked firing and synaptic transmission without altering either the D1 receptor or mGluR1 modulation of neurotransmission .
As a summary these results and observations led to the proof that chronic alcohol exposure leading to ethanol induced deficits in executive function were responsible for the distruption of D2/D4 recpetor modulation of the neural networks found in the PFC, these underline cognition. The vast amount of results from this investigation also portrayed that the correct functioning of the PFC has a neccessary role in the excretion of inhibitory control over impulsing behaviours characterising drug and alcohol addiction. Abstinent alcoholics who exhibit deficits in executive function have a greater probabolity of relapse and therapuetic enhancement of cognitive functioning in the anstinent alcoholic may reduce the incidence of relapse (Davidson et al 2014). As a concluding point these studies showed that the impact of chronic alcohol exposure on cognition are confounded by potential that the cognitive deficits may represent a pre-existing condition and are therefore not the result of chronic alcohol consumption (Davidson et al 2014)This explains why rodent experiments are very helpful in the investiagtion of cellular and molecular properties, but primarily for control of the confounding factors such as pre-exsiting cognitive status.
The Affect of alcohol on the membranes of Neurons
Chronic also exposure was also believed to alter the behavioural and synaptic plasticity of the rodent PFC. During the course of this experiment acute brain slices were taken from a rodent, after testing it was discovered that CIE exposure led to an increase in the NMDA/AMPA current ratio. This ratio was present 1 week after the last episode of ethanol exposure. Along with the continous increase in synaptic NMDA currents another experimental technique called western blot analysis of insoluble PSD containing membrane fraction was undertaken. This proved that increase in NR1 and NR2B subunits occured, but no change in GluR1 subunits in these same tissues was seen immediately after the termination of the last alcohol exposure. However, if these NR1 and NR2B were measured 1 week after the last alcohol exposure no increase was observed. The next step was to perform a structural level analysis.
This reveleaved that dendritic spikes illustrated a selective increase in the denisty of mature spines that persisted even after withrawal of ethanol for a week. CIE exposure and withdrawal was also associated with abberennt expression of NMDAR-mediated STDP (Sven Kroener et al 2012). The final conculsion for approvement of this hypothesis was the observance of behavioural changes. This was carried out using a mPFC-dependent task. This task showed that CIE exposure was associated with deficits in behavioural felxibilty that persisited up to week1 after the last period of alcohol exposure (Sven Kroener et al 2012). The summary of these final results proved the fact that chronic ethanol exposure induces changed in PFC plasticity. This may contibutary facotr to the loss of appropraite attention or sontrol over behaviour.
Foetal Exposure
As discussed previously the dangers of foetal exposure to alcohol is a widely known fact, the true damage ethanol exposure can cause to the foetus is not as well conveyed.
Many studies have been carried out to determine the precise effect that the ethanol has on the brain of the foetus.(Guerri et al., 2009). Showed that the cerebral cortex is one of the brains regions most effected by in utro alcohol exposure in humans or Foetal alcohol spectrum disorder (FASD). Many of the studies in this field have focused on the exposing ethanol during the first postnatal week of the test subject ie. Rats. (Dobbing and Sands 1979). Showed that early postnatal exposure to ethanol reproduces several of foetal alcohol effects in humans. Due to this fact it allows us to study such thing as the electrophysiological features of the pyramidal neurons in the somatosensory cortex . (Geranato et al 2012) showed this by using simultaneously recordings from the stoma and apical dendrite they can demonstrate that dendrites of layer 5 neurons are less excitable in rats exposed to alcohol compared with controls.
The rats were exposed to the ethanol (95%v/v) via a pump which conveyed the ethanol at 2.5 ml/min for 3 hours day. Once they rats were exposed for a set period of time they selected layer 5 and layer 2/3 pyramidal neurons. They tested the Subthreshold and the Suprathreshold of the layers. (Geranato et al 2012 )showed that as to L2/L3 neurons there was no significant difference in the Subthreshold and the Suprathreshold properties between the Control And Ethanol exposed rats. Unlike the L5 neurons where Alcohol exposure lead to a change in excitability between the control and ethanol exposed rats. Dendritic calcium channels have also been shown to affect the firing properties of L5 ( Larkum and Zhu 2002) . (Geranato et al 2012) showed that Direct dendritic patch recordings indicated that an impairment of dendritic calcium singling is responsible for the permanent down regulation of dendritic electrogenesis in L5 pyramidal neurons. Their experiment showed that early alcohol exposure has a dramatic effect on output of pyramidal neurons in response to the distal dendritic input.
Alcohol effect on Neural Enzymes monoamine oxidase
Monoamine oxidases (MOA-A) and Monoamine oxidases B (MOA-B) regulate neurochemistry by degrading monoamine neurotransmitters (serotonin, dopamine and norepinephrine). Changes in MAO levels have drastic effects on the brain and behaviour by lowering or raising neurotransmitter levels and producing toxic reactive oxygen species. These fluctuations are caused by the oxidising of amines from both endo and exogenous sources.
As outlined in the introduction MAO is impacted by alcoholism. It can also bring about depressive disorders but the focus of my attention is on MAO and alcoholism as our essay is about the effect of alcohol on the neurons. Alcoholism is a psychiatric condition that causes approximately one third of alcoholics to suffer from neuropsychological difficulties(Ducci F et al. 2007). Ethanol has been shown to reduce prefrontal cortex volume(Paul CA et al. 2008). Abuse of alcohol also lowers densities of neuronal and glial cells in brain(Miguel-Hidalgo JJ et al. 2006). Glyceraldehyde -3-phosphate dehydrogenase is a protein referred to in the research of MOA. It was found through studies that GADPH was elevated in in the prefrontal cortex in the brains of human alcoholics(Alexander-Kaufman K et al. 2006), it also increased the brains of rats were exposed to ethanol(Ou XM et al. 2010).As an experiment human brain cell lines were treated with ethanol to establish the relationship between GADPH and the KLF11-MAO-B cascade. The results show that MAO-B mRNA increased by 4 fold and GAPDH by 3.5 fold in the nucleus with a 1.8 fold increase overall post ethanol treatments(Ou XM et al. 2010). The results indicate that GAPDH can increase MAO-B via KLF11 in the presence of ethanol(Ou XM et al. 2010). This results in further damage in neuronal cells exposed to ethanol due to increased MAO-B mediated oxidative stress. It was also discovered from the experiment that a new MAO-B inhibitor could decrease ethanol- induced cell death by preventing nuclear translocation of GAPDH in cell cultures in vitro(Ou XM et al. 2009).
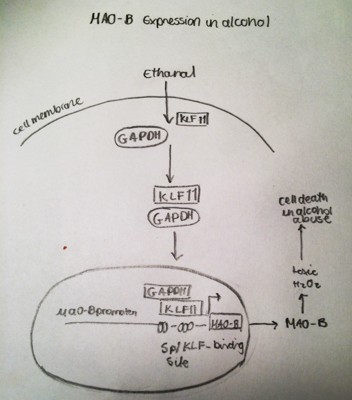
Figure 2. A representative structure of the transcriptional regulation of MAO-A gene expression in alcohol-related disorders. Duncan J1 2012 Jun;6(3):112-22.
Studies examining the Prefrontal Cortex of post-mortem subjects with alcohol dependence showed a large rise in protein levels of GAPDH and MAO-B compared to normal subjects. Ethanol preferring rats showed a two fold increase in GAPDH and a 1.7 in MAO-B. Rats chronically treated with ethanol not only displayed an increase in GADP and MAO-B but also a 1.6-fold increase of KLF11, a 1.37-fold increase in MAO-B catalytic activity and a 1.8 fold increase in active caspase 3, an apoptotic protein in the PFC compared to control rats. On the other hand the anti-apoptotic, Bcl-2was decreased by 41% in rats exposed to ethanol compared to control rats(Ou XM et al. 2011). The decrease in apoptotic Bcl-2 and increase in apoptotic caspase 3 in the PFC of rat brains post ethanol exposure simply that ethanol induces apoptosis that may be mediated by an increase in the KLF11-MAO-B cell death cascade(Ou XM et al. 2011). The GAPDH-KLF11-MAO-B cascade is a novel way to explain ethanol –induced neuronal death due to chronic alcoholism.
Conclusion
It is clear that excessive alcohol has major impacts on neurons.. It is clear from experiments and carried out by the authors of this paper that alcohol’s primary product ethanol can have a major influence on the neurons of the fetus, the membrane of neurons, Dopamine and on MAO producing by-products which have major impacts on the neurons. However in final conclusion some of the papers that were reviewed stated that more research in this area is warranted over the coming years.
Bibliography
1.Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I.(2006) Differential protein expression in the prefrontal white matter of human alcoholics: A proteomics study. Mol Psychiatry. ;11:56-65.
2.Ducci F, Enoch MA, Funt S, Virkkunen M, Albaugh B, Goldman D.(2007) Increased anxiety and other similarities in temperament of alcoholics with and without antisocial personality disorder across three diverse populations. Alcohol. ;41:3-12.
3.Granato Alberto,1 Lucy M. Palmer,2 Andrea De Giorgio,1 Daniela Tavian,1 and Matthew E. Larkum2 (2012) Early Exposure to Alcohol Leads to Permanent Impairment Of Dendritic Excitability in Neocortical Pyramidal Neuron. http://www.jneurosci.org/content/32/4/1377.full.pdf+html
4.Guerri C, Bazinet A, Riley EP (2009) Foetal alcohol spectrum disorders andalterations in brain and behaviour. Alcohol Alcohol 44:108 –114.
5.Heather Trantham-Davidson,1 Elizabeth J. Burnett,1 Justin T. Gass,1 Marcelo F. Lopez,Patrick J. Mulholland,1Samuel W. Centanni,1 Stan B. Floresco,2 and L. JudsonChandler1 (2014 ) Chronic Alcohol Disrupts Dopamine Receptor Activity andthe Cognitive Function of the Medial Prefrontal Cortex • 34(10):3706 –3718 http://www.jneurosci.org/content/34/10/3706.full.pdf+html
6.Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G.(2006) Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 30:1845-1855.
7.Ou XM, Lu D, Johnson C, Chen K, Youdim MB, Rajkowska G, Shih JC.(2009) Glyceraldehyde-3-phosphate dehydrogenase-monoamine oxidase B-mediated cell death-induced by ethanol is prevented by rasagiline and 1-R-aminoindan. Neurotox Res. 16:148-159.
8.Ou XM, Johnson C, Lu D, Johnson S, Paul IA, Austin MC, Iyo AH, Miguel-Hidalgo JJ, Luo J, Bell RL, Grunewald M, Wang J, Sittman DB. (2011) Ethanol increases TIEG2-MAOB cell death cascade in the prefrontal cortex of ethanolpreferring rats. Neurotox Res.19:511-518.
9.Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, urjus GJ, Dieter L, Chen K, Lu D,Johnson C, Youdim MB, Austin MC, Luo J, Sawa A, May W, Shih JC.(2010) A novel role for glyceraldehyde-3-phosphate dehydrogenase andmonoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatry. 67:855-863
10. Paul CA, Au R, Fredman L, Massaro JM, Seshadri S,Decarli C, Wolf PA.(2008) Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. ;65:1363-1367.
11.Sven Kroener Patrick J. Mulholland,Natasha N. New, Justin T. Gass, Howard C.Becker, L. Judson Chandler (2012) Chronic Alcohol Exposure Alters Behavioral and Synaptic Plasticity of the Rodent Prefrontal Cortex http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0037541
Figures
1.Figure1-Heather Trantham-Davidson,1 Elizabeth J. Burnett,1 Justin T. Gass,1 Marcelo F. Lopez,Patrick J. Mulholland,1Samuel W. Centanni,1 Stan B. Floresco,2 and L. JudsonChandler1 (2014 ) Chronic Alcohol Disrupts Dopamine Receptor Activity andthe Cognitive Function of the Medial Prefrontal Cortex • 34(10):3706 –3718 http://www.jneurosci.org/content/34/10/3706.full.pdf+html
2.Figure2-Jeremy Duncan, Shakevia Johnson, Xiao-Ming Ou* Monoamine oxidases in major depressive disorder and alcoholism Drug Discoveries & Therapeutics. 2012; 6(3):112-122.
