Viral triggers for Alzheimer’s disease
Contents
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease and the most common cause of dementia. It manifests as a progressive deterioration of cognitive functioning through memory loss, behavioral changes and language impairment. The progression of the disease is due to the continuous loss of neurons as a result of β-amyloid plaques and neurofibrillary tangles, which will be discussed in detail. Alzheimer's disease is estimated to affect 115.4 million people worldwide by 2050, of which the highest prevalence is in ages >60 (Prince et al, 2013).
In this essay, we are going to address the relations between Alzheimer’s disease and viruses. There is found a link between multiple herpesviruses. We will take a closer look at herpesvirus type 1, herpesvirus type 4 and herpesvirus type 6.
Physiology
Dementia is a description of a range of symptoms such as poor memory, difficulty obtaining new information, poor spatial skills and language impairment - which causes great difficulty in leading an independent life for the affected patient. Dementia itself is caused by damage or loss of brain cells, which can result from a variety of diseases - of which Alzheimer's disease is the most common cause of dementia.
Alzheimer’s disease is considered a neurodegenerative disease, responsible for causing the destruction of neurons in the brain, especially in the cortex. Ultimately, neuronal loss leads to the symptoms characteristic of dementia. In the final stages of the disease, significant brain atrophy can be observed as a result of neuronal loss.
Although the etiology of Alzheimer's disease is unknown, plaques and tangles are frequently mentioned as important players in its progression. The amyloid precursor protein (APP) is found in the cell membrane of neurons and is involved in their self-growth and healing after injury, according to most studies (O’brien et al, 2011). Over time, APP degrades and is eventually broken by the enzymes alpha and gamma secretase, resulting in a soluble peptide. When gamma secretase combines with another enzyme called beta secretase, a monomer called amyloid-beta is formed; this excess fragment is non-soluble. Beta-amyloid plaques are formed when many amyloid beta monomers join together. This accumulation of amyloid beta appears to begin in the neurons of the human (Seeman et al, 2011). These plaques can enter neural synapses and block neuron-to-neuron communication. Beta-amyloid plaques are considered to initiate an immune response, causing inflammation which may damage surrounding neurons. Secondly, plaques are capable of depositing around blood vessels in the brain (amyloid angiopathy). The occurrence of amyloid angiopathy results in the weakening of the blood vessel walls, ultimately increasing the risk of hemorrhage or rupture and blood loss.
The second major role in the cause of Alzheimer’s disease is neurofibrillary tangles, located inside the neuron, as opposed to the beta-amyloid plaques which are located extracellularly. The cytoskeleton of neurons is responsible for the structural build and consists of microtubules, intermediate filaments and actin filaments. These structures transport nutrients and chemicals across the cell’s length. A protein named tau prevents the breakage of this transport.
Neurofibrillary tangles and extracellular amyloid plaques (illustrated in figure 1) have been observed in AD brain autopsies, specifically in the hippocampus and other subcortical areas which are crucial for cognitive function.
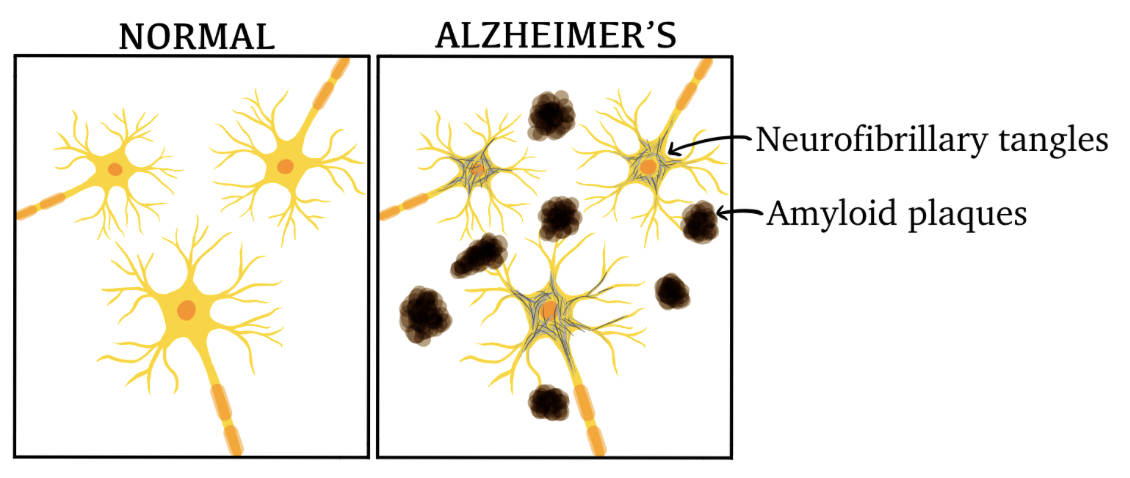
Figure 1: Illustration of neurons in a healthy brain versus a brain affected by AD.
Viral triggers for Alzheimer's disease
HSV-1
Herpes simplex virus (HSV) is categorized into HSV type 1 (HSV-1) and HSV type 2 (HSV-2). HSV-1 is primarily transmitted via oral secretions and wounds, of which the majority of acquired infections occur during childhood. An estimated 3.7 billion people under the age of 50 have HSV-1 (as of 2016), representing 67% of the world's population under the age of 50 (World Health Organization, 2022).
As a neurotropic virus, HSV-1 resides within neuronal cell bodies where it remains latent. During latency, the trigeminal ganglion is the main reservoir. In patients diagnosed with clinical AD before death, researchers found HSV-1 DNA located in the trigeminal ganglion in 90% of 147 autopsies (Devanand, 2018).
Periodic reactivation of the virus is due to immunosuppression, mainly triggered by stress, inflammation and illness. Research suggests that repetition of "mild" HSV-1 brain infections might cause neuronal damage similar to that seen in neurodegenerative diseases like Alzheimer's disease (AD) through amyloid aggregation.
T.J. Ball proposed that cerebral Herpes Simplex Virus Type-1 (HSV-1) infection has a role in Alzheimer's disease pathogenesis. Ball based this on the ability of HSV-1 to move from the peripheral trigeminal ganglia to the brain regions most affected by Alzheimer's disease. Following that, postmortem studies of small patient groups discovered not yet manifested HSV-1 in brain samples from healthy elderly controls and Alzheimer's disease patients.
There is also a link between Alzheimer's disease patients who possess both HSV-1 infection and the apolipoprotein E type 4 allele. ApoE is a component of lipoproteins in the cerebrospinal fluid (CSF) and interstitial fluid (ISF) of the brain parenchyma in the central nervous system. As a major lipid transporter, apolipoprotein E is of great importance in the regulation of various important signaling pathways, including the development, repair and maintenance of the central nervous system (Husain et al, 2021).
In 2017, a total of 31 clinicians and researchers expressed concern about the disregard for AD treatment (Itzhaki et al, 2017). Three decades of research and approximately 100 studies on the effects of HSV-1 led researchers to identify HSV-1 as the most probable culprit of AD progression. Based on their research, a trial of antiviral treatment has been proposed to potentially halt or stop the disease progression in AD patients. Antiviral agents foscarnet, acyclovir and penciclovir were tested in a cell culture and resulted in decreased HSV-1 particles, in addition to a decline in Aβ and phosphorylated tau accumulation (Devanand, 2018).
HHV-6
Nearing a 100% seroprevalence, human herpesvirus 6 (HHV-6) is highly prevalent among the world population, of which two variant groups are recognized: A and B. The virus replicates in the salivary glands and is shed in the saliva after initial infection, which is the known route of transmission for variant B strains. HHV-6 remains latent in lymphocytes and monocytes and lingers at low levels in cells and tissues (Campadelli-Fiume, 1999).
There is also found human herpesvirus type 6 (HHV-6) in the cortex samples of the brain. In samples taken from elderly and AD brains, there are significantly higher levels of HHV-6 (also HHV-7) in the brains affected by AD (Santpere et al, 2020). The majority of HHV-6 infected people do not show any clinical signs, although HSV-6 is a known cause of encephalitis in immunocompromised patients, displaying similar symptoms as dementia.
Furthermore, HHV-6 type A is a neurotropic virus that enters the CNS via olfactory pathways. HHV-6A has the ability to dysregulate the autophagy of damaged cell components, as well as activate endoplasmic reticulum stress which can lead to incorrect folding of proteins, resulting in declined protein function and cell death. This is relevant as studies show that neurodegenerative diseases frequently occur together with autophagy dysregulation.
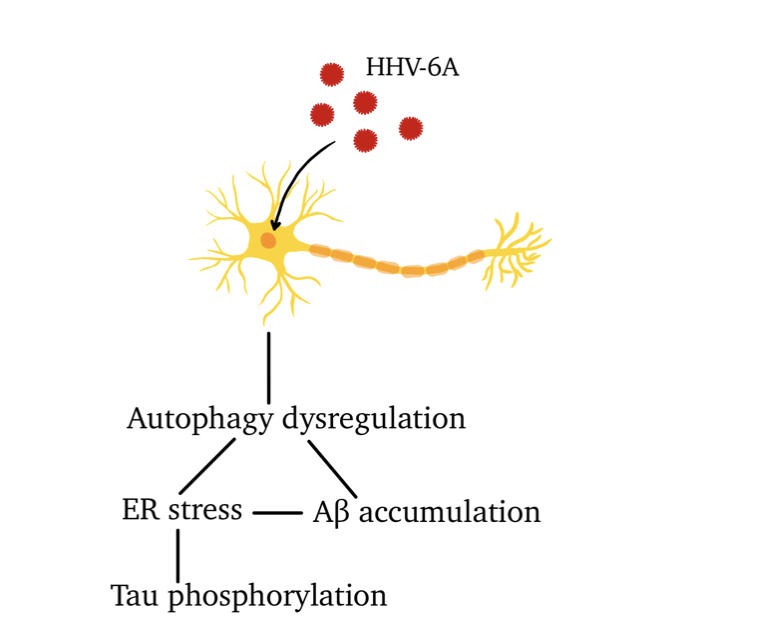
Figure 3: effects of HHV-6A on neurons.
Relevance
Amyloid beta-peptide (Aβ) depositions are important in the pathogenesis of Alzheimer’s disease as they have an antimicrobial effect by entrapping viruses in amyloid deposits (Eimer et al, 2018). The Aβ fibrillization and deposits are, as previously stated, the fundamental basis of neuronal loss in AD patients. Antimicrobial activities linked to Aβ are mediated by fibrilization pathways, which explains how an HHV-6 or HSV-1 infection may cause beta-amyloid depositions in the brain as the Aβ peptides bind to surface glycoproteins of the viruses. Ultimately, herpesvirus infections can dramatically increase the beta-amyloid depositions in the brain.
EBV
Herpesvirus type-4 (HSV-4) known as Epstein-Barr virus (EBV) is detectable in 90-95% of the population. EBV is a dsDNA virus found as both a latent infection and in lytic replication states. Through the infected B-lymphocytes, EBV can infect neurons both directly and indirectly, contributing as an effector of the occurrence of Alzheimer’s disease.
Huang et al investigated the connection between different herpesviruses and Alzheimer’s disease. To investigate if there is an association between four different herpesviruses, the researchers applied the two-sample Mendelian randomization analysis. The result of this analysis was a significant association between Alzheimer’s and mononucleosis from the Epstein-Barr virus (EBV). Another study saw a connection between the increased amount of EBV-specific T-cells in the brain of an AD patient (Gate et al, 2020).
Discussion
Alzheimer’s is the leading neurological disease that causes dementia. Consequently, extensive research on Alzheimer’s and the causes of the disease has been conducted. Despite some known rare hereditary forms of Alzheimer’s, there are no concluded causes of the disease, although several triggers have been identified. The most common triggers of AD are age and genetic factors. The reasoning for age as a trigger is not known, but there are several hypotheses as to why age is a trigger; the impairment of the cholinergic function is a critical for risk AD, the failure of energy metabolism in brain cells, or that the key element is a change in amyloid β-protein synthesis and processing.
Conclusion
For over three decades, researchers have investigated the viral hypothesis of Alzheimer’s disease and concluded with considerable scientific and epidemiological evidence in its connection to viruses. Due to the lack of efficient prevention and treatment of AD, an antiviral trial could potentially improve AD treatment, although HSV-1 is unlikely to be the sole cause of AD. Alzheimer's disease is a diagnosis based on exclusions, and therefore causes greater difficulty in both treatment and, naturally, the scientific background of how AD develops.
Mild HSV-1 causes similar neural damage to the brain as AD does. There is also found an association between the HSV-1 and the apolipoprotein E type 4 allele.An increased amount of HSV-6 was found in the frontal cortex of several AD patients, and there was also found HSV-7 in these samples. Another herpesvirus, EBV, which can cause mononucleosis is found in association with AD through multiple EBV-specific T-cells in an AD brain biopsy.
According to different studies, there are multiple triggers of Alzheimer’s disease, of which herpesviruses are considered a trigger of Alzheimer’s, but as one of many potential triggers. This indicates that patients can be affected by Alzheimer’s even if they have never contracted any form of herpesvirus.
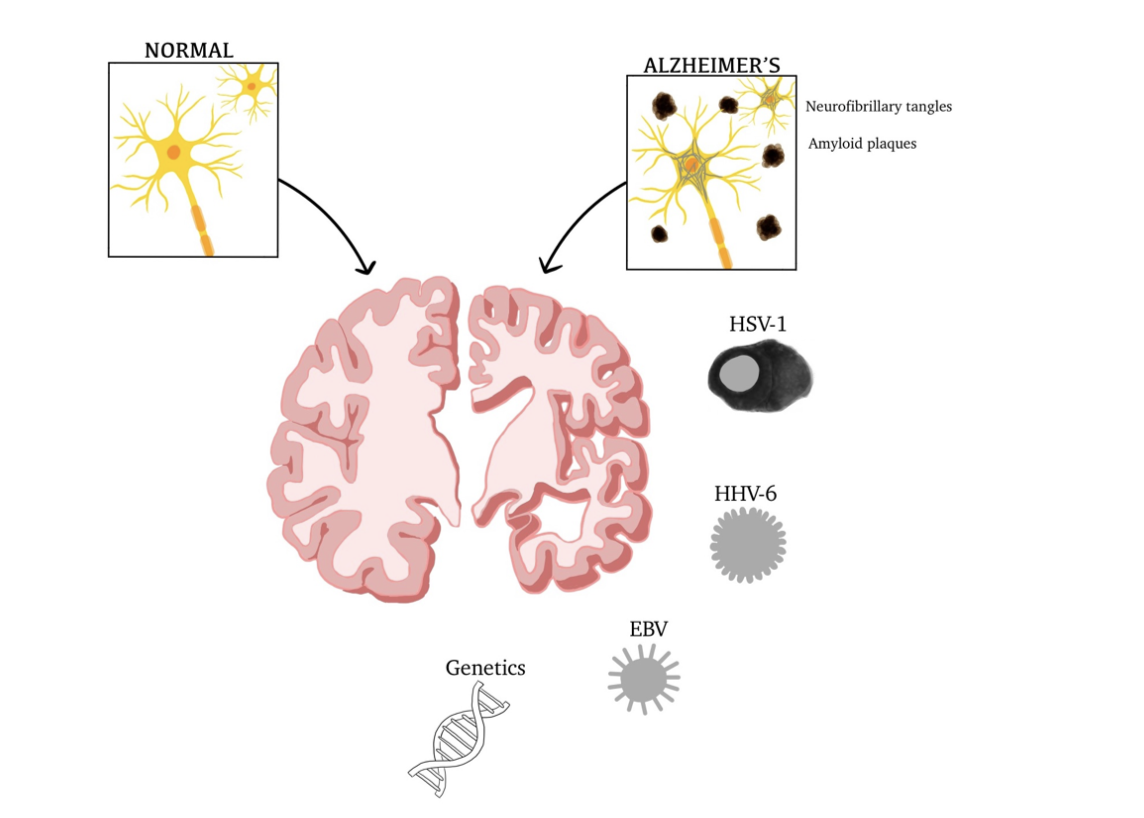
Figure 2: Factors contributing to AD, physiological and anatomical differences.
References
Ball MJ (1982): Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved?. The Canadian Journal of Neurological Sciences 9: (3) 303-306
Ball MJ (1986): Herpesvirus in the hippocampus as a cause of Alzheimer's disease. Archives of Neurology 43: (4) 313
Breijyeh Z, Karaman R (2020): Comprehensive Review on Alzheimer's Disease: Causes and Treatment. Molecules 25: (24) 5789
Campadelli-Fiume G, Mirandola P, Menotti L (1999): Human Herpesvirus 6: An Emerging Pathogen. Emerging Infectious Diseases 5: (3) 353-366
Devanand DP (2018): Viral Hypothesis and Antiviral Treatment in Alzheimer's Disease. Current neurology and neuroscience reports 18: (9) 55
Dunys J, Valverde A, Checler F (2018): Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?. The Canadian Journal of Neurological Sciences 9: (3) 303-306
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE, Moir RD (2018): Alzheimer's Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 11;99: (1) 56-63
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los Santos MB, McBride A, Pluvinage J, Elahi F, Tam GK, Kim Y, Greicius M, Wagner AD, Aigner L, Galasko DR, Davis MM, Wyss-Coray T (2020): Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 577: (7790) 399–404
Gouras GK, Olsson TT, Hansson O (2015): β-Amyloid peptides and amyloid plaques in Alzheimer's disease. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics 12: (1) 3-11
Huang SY, Yang YX, Kuo K, Li HQ, Shen XN, Chen SD, Cui M, Tan L, Dong Q, Yu JT (2021): Herpesvirus infections and Alzheimer's disease: a Mendelian randomization study. Alzheimer's Research & Therapy 13: (1) 158
Husain MA, Laurent B, Plourde M (2021): APOE and Alzheimer's Disease: From Lipid Transport to Physiopathology and Therapeutics. Frontiers in Neuroscience 15: 630502
Itzhaki RF (2018): Corroboration of a Major Role for Herpes Simplex Virus Type 1 in Alzheimer’s Disease. Frontiers in Aging Neuroscience 10: 324
Itzhaki RF, Lathe R, Balin BJ, Ball MJ, Bearer EL, Braak H, Bullido MJ, Carter C, Clerici M, Cosby SL, Del Tredici K, Field H, Fulop T, Grassi C, Griffin WS, Haas J, Hudson AP, Kamer AR, Kell DB, Licastro F, Letenneur L, Lövheim H, Mancuso R, Miklossy J, Otth C, Palamara AT, Perry G, Preston C, Pretorius E, Strandberg T, Tabet N, Taylor-Robinson SD, Whittum-Hudson JA (2017): Microbes and Alzheimer's Disease. Journal of Alzheimer’s Disease: JAD 51: (4) 979-984
Marcocci ME, Napoletani G, Protto V, Kolesova O, Piacentini R, Li Puma DD, Lomonte P, Grassi C, Palamara AT, De Chiara G (2020): Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol 28: (10) 808-820
Moreira PI, Carvalho Cristina, Zhu X, Smith MA, Perry G (2010): Mitochondrial dysfunction is a trigger of Alzheimer's disease pathophysiology. Biochimica et biophysica acta 1802: (1) 2-10
O'Brien RJ, Wong PC (2011): Amyloid precursor protein processing and Alzheimer's disease. Annual Review of Neuroscience 34: 185-204
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013): The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s & Dementia: The Journal of the Alzheimer's Association 9: (1) 63–75
Romeo MA, Montani MSG, Gaeta A, D’Orazi G, Faggioni A, Cirone A (2020): HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer's disease 1866: (3) 165647
Santpere G, Telford M, Andrés-Benito P, Navarro A, Ferrer I (2020): The Presence of Human Herpesvirus 6 in the Brain in Health and Disease. Biomolecules 10: (11) 1520
Seeman P, Seeman S (2011): Alzheimer's disease: β-amyloid plaque formation in human brain. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics 12: (1) 3-11
Other referenced materials
Figure 1: based on https://www.researchgate.net/figure/Major-pathological-hallmarks-of-AD-are-amyloid-plaques-and-neurofibrillary-tangles-B_fig3_337715716
Figure 2: based on https://ars.els-cdn.com/content/image/1-s2.0-S0925443919303771-ga1_lrg.jpg
Figure 3: based on https://www.alamy.com/alzheimers-disease-brain-comparison-image353184441.html
