Immunostimulation by Beta Glucan in Aquaculture
Aquaculture represents one of the fastest growing food producing sectors in the world (Winward, 2013). In an attempt to increase productivity, fish are farmed intensively in narrow spaces under high densities. The overcrowding tends to make the fish susceptible to various infecious diseases. The immune system is an important defensive mechanism against invading organisms; impaired immune functions will decrease resistance to infectious diseases. Immunostimulants are considered as an effective tool for enhancing immune status of cultured organisms, and hence hinder the development of pathological diseases. Beta-glucan is one of the most favourable immunostimulants currently used in aquaculture but has also shown promising effects in humans as well (Chan et al, 2009).
Contents
Immune System of Fish and Crustceans
Fish immune systems are very similar to that of mammals, in that they have an adaptive and innate immunity and utilise several organs, cells and chemicals in order to remove pathogens and pathogenic substances from the body. Since fish are free living from an early stage of life, they must protect themselves against a large number of factors working against them. This leads to a more developed innate immune system (Pilar Alvarez-Pellitero 2008, 14). The figure below shows the general response of a fish to a pathogen.
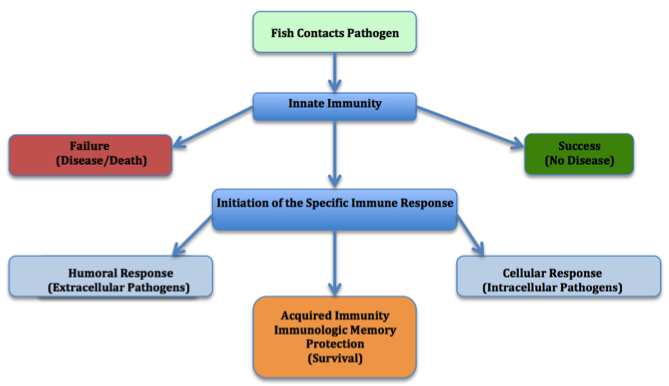
Fig 1. Schematic representation of a fish after encountering a pathogen.
Invertebrates, however, are not equipped with cells that are analogous to antibody producing lymphocytes in fish and warm-blooded animals. Thus, they are highly dependent on their innate immune system. Furthermore, they lack the ability to produce memory cells (Raa et al, 2000).
Immune organs
The immune organs of fish have certain differences to that of mammals (Uribe et al, 2011).
In cartilaginous fish:
1. The Epigonal Organs, a special tissue found surrounding the gonads, which serves a similar function to that of bone marrow in mammals
2. The Leydig organs, situated around the oesophageal wall
3. The Spiral Valve in the intestines, a modification of the ileum
4. The Thymus
5. The Spleen
In bony fish
1. The Kidney
2. The Thymus
3. The Spleen
4. Scattered Mucosa Associated Lymphoid Tissues (MALT)
In crustaceans, the lymphoid organ is found exclusively in penaeid prawns. Other crustaceans such as crabs, lobsters and crayfish do not possess lymphoid organs (Rusaini et al. 2010).
Immune cells
Fish have lymphocytes which are analogous to T-cells, B-cells, cytotoxic cells, macrophages and polymorphonuclear leukocytes. The cells of elasmobranchs (like Sharks) and teleost fish (like Salmon), also express Major Histocompatibility Complexes (MHC) and T-cell receptors on their cell surfaces, which are absent in jawless fish and invertebrates (Uribe et al, 2011). Dendritic Cells (DCs)have also been found in tissues that is part of the first line of defense (Tort et al, 2003). They use Toll-like Receptors (TLR), which can recognise microorganisms and activate the innate immune systemby activating lymphocytes as an Antigen Presenting Cell (APC) and thus promote inflammation.
Shrimp have white blood cells (WBC) with the same biological properties and functions as the macrophages, granulocytes and natural killer cells (NK) in vertebrates (Raa et al, 2000). These cells can be found in the blood as well as in tissues around the digestive organ. Crustaceans have three different types of circulating hemocytes;
1. Hyaline hemocytes - phagocytic activity
2. Semi-granular hemocytes - contain small granules (resemble the granulocytes)
3. Granular hemocytes - filled with large granules - stores the pro-phenol oxidase enzyme
In its active form the phenoloxidase (PO), catalyses the oxidation of phenols, which due to their high reactivity, will kill microbes.
Non-specific Immunity
Non-specific immunity refers to non-specific line of defence of the immune system. In fish (and crustaceans) this is considered very important due to the limitations of the adaptive immune system, their poikilothermic nature, limited repertoire of antibodies and slow proliferation, maturation and memory of their lymphocytes (Uribe et al, 2011). The complement system is one of the central non-specific immune responses in humans as well as fish.
The complement system
The complement system, when activated, through either the classical, the alternative or the MBLectin pathway, anaphylactic factors C3a and C5a will be produced. These factors will in turn induce the release vasoactive amines, like 5- hydroxytryptamine (5-HT), from thrombocytes and eosinophilic granular cells. The 5-HT will induce a local vasodilation and extravasation of neutrophils and monocytes into the infected site. The C5a component has chemotactic activity for fish phagocytes and will thus accumulate at the site of infection. LPS will further stimulate fish leukocytes to induce secrete interleukin-1 (IL-1). (Galindo-Villegas and Hosokawa, 2004)
Prophenoloxidase System (ProPO)
Prophenoloxidase activation system, in invertebrates, involves a serine proteinase cascade with similarities to blood clotting system and complement system of vertebrates (Meena et al, 2013, López et al. 2003).). When components such as lipopolysaccharide (LPS) or beta-1, 3-glucan are recognized by pattern recognition proteins (PRP), serene proteases will be activated, which then triggers activation of the serine proteinase cascade, leading to activation of the prophenoloxidase -activating proteinase (PAP). PAP then converts inactive proPO to active PO, which in turn can produce toxic compounds to micro organisms by oxidising phenol to melanin.
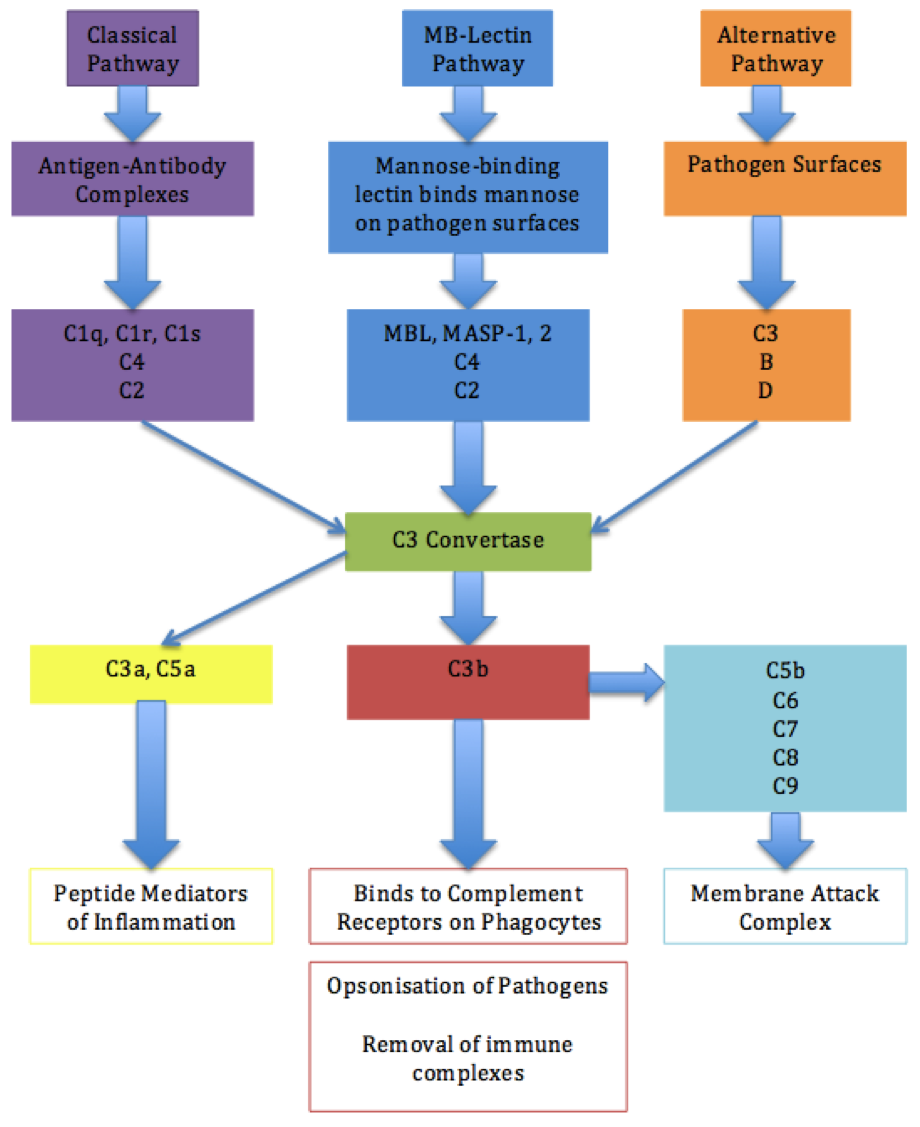
Fig 2. The complement system
Respiratory Burst Activity
Respiratory Burst Activity (RBA) is a highly efficient, non-specific cellular defense mechanism in fish (Haugland et al. 2012). The phagocytes increase their oxygen consumption to produce superoxide ions, peroxides or radicals using a special oxidase enzyme, which is made of 5 parts; 1 membrane bound component and 4 cytosolic parts. The phagocyte then uses these oxide ions, in the form of phagosomes, to destabilise harmful pathogens by degrading their internalized particles. (Dahlgren and Karlsson 1999).
Specific immunity
Although the specific immune system of fish is less developed (absent in crustaceans), it is similar in most ways to that of mammals, in that it utilises immunoglobulin, it has immunological memory of certain antigens and it uses humoral cytokines as a means of defence (Raa et al, 2000). The specific immune system can be divided into humoral and cellular immune reactions.
Environmental factors affecting the immune system
Given that fish are poikilothermic animals, that is, their body temperature generally varies around the environment they are in, the lower the temperature the less efficient the immune system is. Since fish are aquatic animals, their surroundings also affect them in certain ways; for example, the amount of suspended material present in the water may increase the hematocrit level due to the blocking of the gills, this would cause lysozyme activity and IgM concentrations to increase as a response to the presence of pathogens in the particles (Uribe et al, 2011).
Immunostimulants
Immunostimulants are biological and synthetic chemicals which have a documented ability to enhance or modify non specific or specific immune system activity by interacting with the cells of the system in both animals and humans (Raa et al, 2000).
Immune-stimulants may provide particular benefits when used in order to:
a) Reduce mortality due to pathogens
b) Enhance disease resistance of farmed fish and shrimp
c) Enhance the efficacy of anti-microbial substances
d) Enhance the resistance to parasites
e) Enhance the efficacy of vaccines
These are chemicals such as nucleotides, probiotics, which allow the animal to improve its intestinal microbial balance, and glucans, which are used the most in aquaculture in various chemical forms.
Beta Glucan
Structure and Sources
There are two types of glucans based on their molecular structure, alpha and beta. The complex nature of the beta-glucans enhances their ability to stimulate immune responses in an organism. Beta-glucans are naturally occurring polysaccharides consisting of glucose molecules interconnected by β-glycosidic bonds and is commonly found in yeast and mushrooms. Other sources include some species of seaweed, oat and barley (Meena et al, 2013). Glucans derived from different sources have different structures but the most common ones are the β-1,3-glucans, with side chains of β-1,6 or 1,4-type.
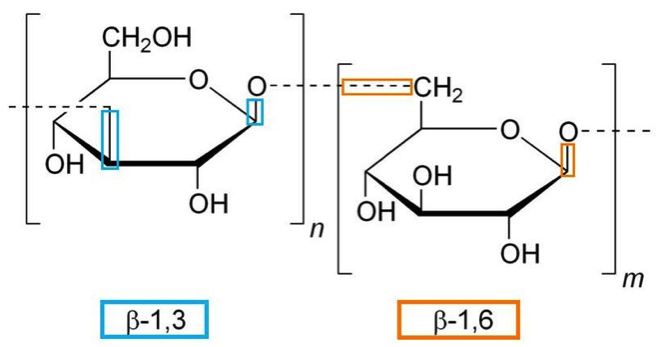
Fig 3. Typical structure of a 1,3/1,6-beta-glucan molecule
Not all beta-glucans have been shown to exhibit the immune-enhancing properties. The larger, insoluble glucans have shown more favourale stimulating ability than their smaller, soluble counterparts. Today beta-glucans are used more and more widely, both by humans through the health-food industry, and as a supplement in agriculture and aquaculture (Meena et al, 2013).
Receptors
Beta-glucans bind to specific recepts on macrophages, neutrophils and natural killer cells, and thus modulate the innate immunity of the host cell. Beta-glucans receptors are opsonin-independent receptors which can activate the alternative complement activation pathway. There is a difference between the innate and adaptive immunity reeceptors. Innate immunity is the first line of defence, where genetically predetermined receptors (PRR) recognise biomolecules. Adaptive immunity on the other hand recuire receptors that have encountered the antigen before. Several different recpetor types have been identified (Meena et al, 2013) incuding:
1. Scavenger Receptors - bind to many polyanionic receptors
2. Complement Receptors (CR) – Expressed on neutrophils, monocytes and NK cells
3. Lactosylceramide (LacCer) – Found in plasma membranes, will induce inflammation
4. Dectin-1 (D1R) Receptors – The main beta-glucan receptor
5. TLR– Will activate the aquired immune response
Stimulation Mechanism
When the beta-glucan receptors are activated they stimulate the macrophage, although its exact mechanism is not yet clear. It has been documented that orally administrered beta-glucans were taken up by macrophages via the D1R or TLR and transported to the spleen, lymph nodes and bone marrow in humans (Hong et al, 2004). There, the macrophages degrade the large, insoluble glucans into smaller, soluble fragments which are eventually taken up by the complement receptor (CR3) of various, circulating granuxlocytes,monocytesor macrophages. These activated granulocytes showed enhanced phagocytic activity, including killing inactivated complement 3bopsonised tumour cells.
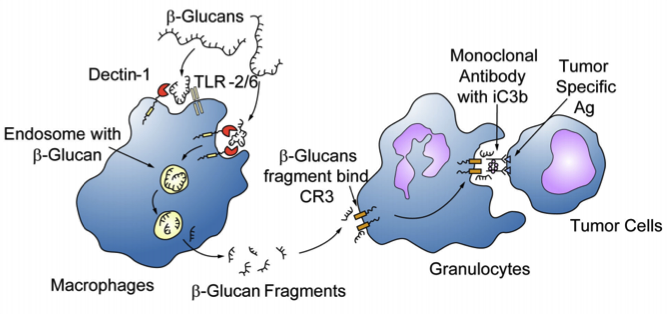
Fig 4. The uptake and subsequent actions of beta-glucans on immune cells
It has also been shown that beta-glucans can be directly bound and internalized by intestinal epithelial cells without the presence of D1R (Hong et al, 2004).
Beta Glucan as an immunostimulant
Beta-glucans are able to stimulate the specific as well as the non-specific immune system. By stimulating the non-specific immune responses, like macrophages, glucans also complement the activation of the specific immune system (Raa et al, 2000). Immune-stimulating glucans often have a structure similar to structures that exist in pathogenic microrganisms. It has been theorised that the bodies of bigger multicellular organisms percieve presence of these molecular structures as a danger sign, signalling that potentially harmful microrganisms are present, and that extra activity of the immune system is called for. (Vetvicka et al, 2007)
In fish
Beta-glucan is normally administered through the feed in the form of pellets although its injected form has also been tested (Meena et al, 2013. Hong et al, 2004. Siwicki et al, 2004. Vetvicka et al, 2007. Ringø et al, 2012 ). The time of administration plays a significant role in induction of resistance. Some of the observed effects of beta-glucan includes:
a) Stimulation of respiratory burst activity
b) Long and short term disease resistance
c) Elevation of complement factor activity in serum
d) Stimulation of superoxide anions
e) Enhanced efficacy of vaccines
f) Increase in survival rate and faster growth
g) Promotes lysosome activity of phagocytes (9-13)
In Shrimp
Beta-1,3/1,6-Glucans have proved to enhance the biological activity of shrimp hemocytes and to improve growth, survival rate and feed conversion efficacy under experimental conditions and in practical shrimp farming. Furthermore, it has also shown to increase the ProPO concentration in the blood of various shrimp species (Raa et al, 2000).
Negative Effects
It has been shown that continuous administration of beta-glucans to fish enabled their immune system to muster extra intense activity in response to experimentally induced stress, but that subsequent episodes of induced stress failed to elicit the same response. This was interpreted as a sign that beta-glucans might induce a state of constant activation of the immune system, which would finally result in exhaustion and unresponsiveness (López et al. 2003). If that is in fact the case, such a mechanism will have a strong influence on the recommended temporal pattern of administration of these compounds.
Conclusion
The drastic increase in human population has created an increasing demand for food. These demands need to be met through innovative approaches without compromising human health and environmental sustainability. Beta-glucan is a widely used and researched immunostimulant, in aquaculture, agriculture and human medicine alike. It is an easily obtainable and administered chemical, and furthermore, presents an environmental advantage in that it is biodegradable and can be used as a substitute to vaccines or antibiotics. However, there is still the risk of overdosage, and there is no guarantee commercially available products can be fully utilised without problems. Some future studies include; determining optimal doses, influence on the cellular and humoral defence mechanisms and protocol for feeding this substance to maximize protection, given the constraints of fish culture and economics (Siwicki et al, 2004).
References
Winward, F. ‘Coordinated efforts in aquaculture needed to meet global demand’, (2013)http://www.fao.org/news/story/en/item/202782/icode/
Figures
Fig 2. The complement system, adaption from (Garland Science. 2004)
Fig 3. Typical structure of a 1,3/1,6-beta-glucan molecule (Wikipedia)
