Biological Clock
Contents
The Biological Clock is an endogenous mechanism responsible for regulating various physiological and behavioural rhythms in mammals. Mammals are warm-blooded vertebrates that belong to the class of the Mammalia. They can be characterized as eukaryotes observed through their endocrine and phenological features. Also, mammals give birth to live young, who are then fed milk produced by the maternal mammary glands. This class includes species like primates, carnivores, rodents and ungulates. Rhythmic changes occurring due to the biological clock happen in all mammals. These changes are genetically programmed and can be observed yearly, monthly and daily. Daily cyclical rhythms are also called Circadian Rhythms (from the Latin circa dies, “about a day”). Period lengths less than 24 hours are called ultradian.
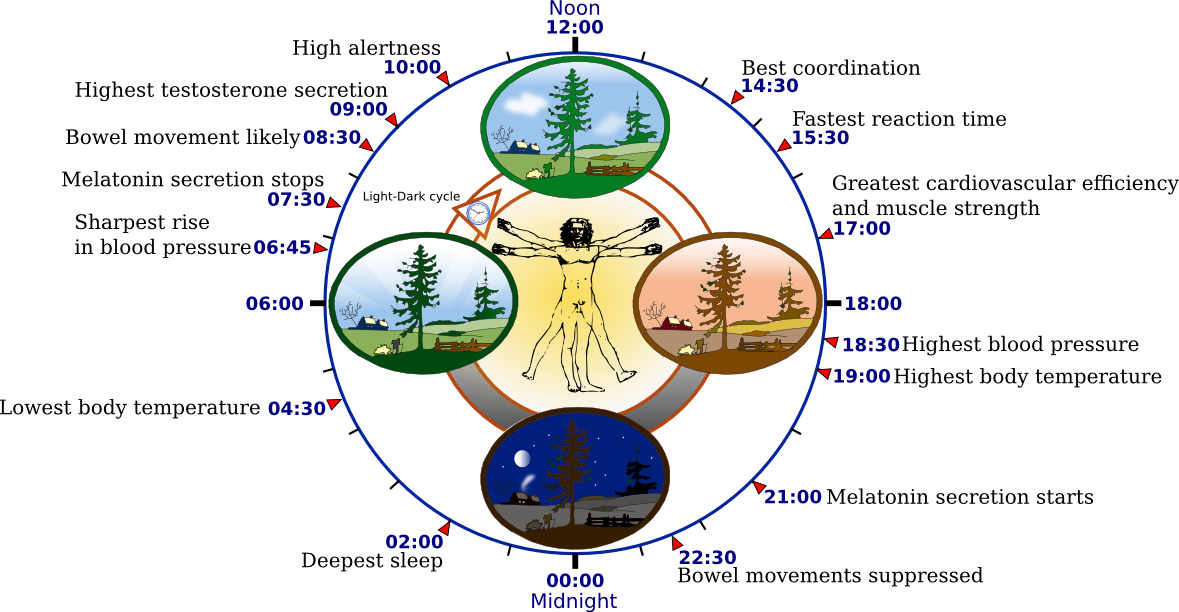
Fig. 1: Biological Clock of Humans
Process
The regulators responsible for the generation and synchronization of these circadian rhythms reside in the supra-chiasmatic nucleus (SCN) of the anterior of the Hypothalamus. It is organized in a hierarchy of oscillators.
Hypothalamus
Hypothalamus is the highest cerebral integrator of the autonomic functions. It coordinates neural and hormonal regulative activity. The morphologicalcharacteristics of the hypothalamus are as follows; it is divided into two areas, the Magnocellular area and Parvocellular area that consist of large and small cells andreach the posterior and anterior lobes of the pituitary gland respectively, thus,releasing their hormones.
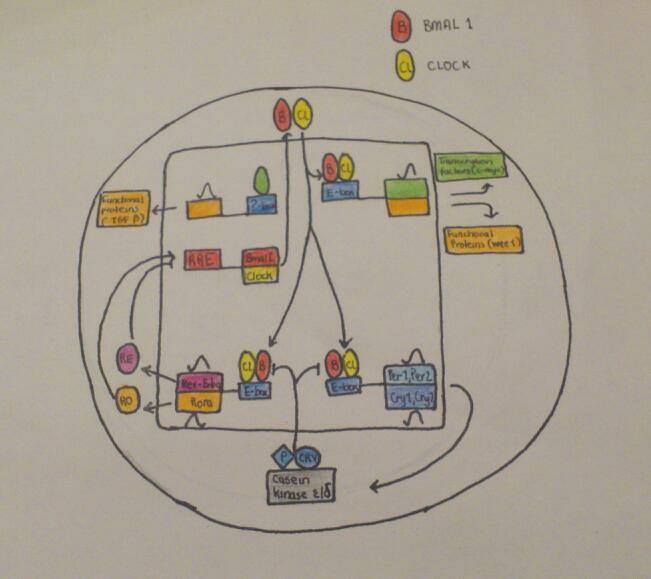
The SCN is responsible for coordinating independent peripheral oscillators so that acoherent rhythm is synchronized at the organismal level. The Clock mechanism consists ofa network of transcriptional – translational feedback loops that have a continuous cyclic 24hours expression pattern of core clock components. These are expressed as genes whose protein products are necessary for the generation and regulation of circadian rhythms within individual cells throughout the organism (Takahashi & Ko, 2006).
The positive elements of the primary feedback loop are the CLOCK and BMAL1. These belong to the transcription – factor family, (basic helix-loop helix) bHLH -Pas(Period-Arnt-Single-minded). The CLOCK and BMAL1 heterodimerize and initiate transcription of specific target genes. The negative feedback, however, is achieved by the translocation of these heterodimers back to the nucleus, thus suppressing their own transcription. In other regulatory loops, CLOCK:BMAL1 heterodimers activate transcription of retinoic acid (Takahashi & Ko, 2006).
The autoregulatory feedback loops are controlled by post – translational modifications,such as phosphorylation and ubiquitination. These processes affect the stability and nuclear translocation of the core clock proteins. Casein kinase 1 epsilon and casein kinase1 delta are critical factors that regulate the core circadian protein turnover in mammals.
|
Fig. 2: Circadian cycle Process
Regulation of Biological clock
Light is the most important stimulus for resetting the biological clock. The Light-Dark cyclemirrors the intensive changes in the environment like the alterations in temperature,availability of food and predation. (Halle & Stenseth, 2000). Animals are divided into two major groups. The first group consists of the diurnal animals,which can be more active and carry out their daily routine during the day time, whereasthe second group comprises of nocturnal animals which are primarily active at night.However, both groups have in common the hormone rhythm of Melatonin release fromthe pineal gland. Its role is the transduction of light/dark information into hormonal secretions in a rhythmic pattern. Secretion of melatonin depends on light exposure.Decreased illumination (darkness) acts positively while strong illumination (daylight) acts negatively on melatonin production. Melatonin in certain species influences reproduction, torpor and changes in pelage (Bartness & Goldman, 1989).
Glutamate (Glu) is the neurotransmitter that mediates light stimulation. Together with itsprecursor which is a dipeptide, they are localized in retinal fibers innervating thesuprachiasmatic nucleus. Glu is released after stimulation of the optic nerve. One of itsmost important antagonists is NMDA (N-methyl-D-aspartic acid) subtype of Glu receptor.It blocks light phase shifts. Its activation leads to calcium ions influx which can activate NOsynthase in order to produce Nitrogen monoxide. NO is a gaseous neurotransmitter andcan activate Guanylate cyclase or Adenosine diphosphate – ribosyl transferase. NMDAsubtype of postsynaptic Glu receptor has long lasting effects due to Gluneurotransmission. NO has binding affinity to heme-moieties. Hemoglobin acts as a NO-eliminator by competing other targets of NO like guanylate cyclase. The transductionof light signals in SCN requires the formation of NO, which can also permeate cellmembranes and glial cells. Regarding glial cells the possibility that neurons and glia canparticipate in light regulation is raised. Increased NO concentration may lead to therelease of neurotransmitters at synapses of SCN. These transmitters are: Substance Pwhich is localized in retinal fibers and Somatostatin which is endogenous to SCN neuronsand elicits phase shifts similar to those caused by Glu (Ding et al, 1994).
Experiments/Researches manifesting the function of Biological clock
The criteria that need to be taken into consideration for evaluating a circadian rhythm are: 1) the controlled laboratory conditions should be kept constant and; 2) The cycle should have a free running rhythm with a continuously changing period length.
One experiment shows the activation of biological clock pathway using a sliced rat brain kept in vitro for 3 days. Firstly, 10 mM of Glu were added to the SCN of the rat and then the neuronal activity was measured. The result observed is that Glu depending on the phase of the circadian cycle, at which it was administered, caused strong phase shifts:
• When applied at early night, neuronal circadian rhythm was delayed by 3 hours. By this the peak activity remained delayed for the next two days.
• Administration at late night, the opposite was observed, because Glu application proceeded the circadian rhythm by 3.5 hours.
• Glu application at midday did not change circadian rhythm. This proves that effects of Glu on phases of SCN happens only nocturnally.
For a better estimation of Glu clock sensitivity, SCN was treated at much more points across circadian cycles (Ding et al, 1994).
Reproductive effects:
Changes in reproductive competency are influenced by the environmental change in the time of sunrise and sunset. These changes go alongside the endogenous biological clock of the mammal. Together, they both control the daily secretion of the pineal hormone, Melatonin (Reiter, 1980). The daily rhythm of melatonin production, in addition with other circadian neural and hormonal signal, regulates the onset of seasonal fertility, puberty and parturition. The CLOCKΔ19 mutation has significant but subtle effects on reproductive function, which reduces fertility and fecundity without manifesting an obvious infertility.
Luteinizing Hormone (LH) surge: During maturation of a follicle, there is an increase of Gonadotropin Releasing Hormone (GnRH) secretion stimulation, resulting in the surge release of LH from the pituitary and subsequent ovulation of mature oocytes. Demonstration of this process was carried out by administrating phenobarbital in the afternoon of prooestrus in both hamsters and rats. The LH surge was delayed by 24 hours, since the following afternoon there was a normal LH surge in animals treated with the drug (Everett & Sawyer, 1950)
Damage of SCN:
Lesions of the SCN cause the loss of gating response to elevated oestrogen. This results in the inhibition of ovulation in rats (Mosko & Moore, 1979) , but not in sheep (Scott et al, 1995), indicating that different mechanisms may be present in different species.
The SCN also acts on the GnRH-positive neurons in the hypothalamus, which comprises of oestrogen receptors as well. This can serve as a pathway for the steroid to influence the timing of ovulation.
Oocyte and early embryo:
It is possible that the developing embryo is exposed to maternal rhythmic signals during its progress through the oviduct. It has been recently shown that the rat oviduct expresses clock and clock-controlled genes rhythmically over 24 hours. Among the genes shown to be rhythmically expressed was plasminogen activator inhibitor-1 (Pai-1), which has been associated with the protection of the developing embryo during its transport along the oviduct. It is likely that a systematic evaluation of gene expression profiles within the oviduct would uncover additional rhythmically secreted proteins that promote healthy and timely embryo development. The importance of this rhythmicity for the developing embryo is not known, but when an embryo is cultured in vitro, there are no rhythmic changes in its environment (Boden & Kennaway, 2006)
Ovary:
In rats, there is an accessory feedback loop, which establishes cellular rhythmicity. It is expressed as DEC1 (sharp2) and it’s stimulated temporarily by Chorionic Gonadotropin (eCG and HCG) in both theca and granulose cells. DEC1 acts as a repressor, altering the expression of genes in the ovary such as the follicle stimulating hormone receptor, prostaglandin endoperoxidase synthase 2 and the E-box-dependent genes in a gonadotropin –dependent manner. Speculation suggests that there may be circadian gating of cellular processes at the ovarian level as well as within the hypothalamus at the time of ovulation (Boden & Kennaway, 2006).
In conclusion, there is growing evidence that circadian rhythmicity and reproduction are interconnected. The circadian timing system influences a wide range of physiological systems via hormonal and neural routes and the disruption of clock gene expression can lead to a spectrum of reproductive (Miller et al, 2004) and metabolic changes in animal models (Takahashi et al, 2001). An exception may be the Bmal1 gene, which appears to be crucial and when the function of this gene is disrupted, there are severe alterations to reproductive function.
Photoperiodic Regulation of multiple traits:
Body Weight:
Changes in body weight and fat content are common tactics for coping with winter energy stores. Gonadal hormones influence body weight. However, seasonal changes in gonadal hormone secretion account for only a part of the photoperiodic modulation of this trait. Seasonally regulated changes in prolactin secretion may contribute to changes in body weight in Siberian Hamsters (Niklowitz and Hoffmann, 1988) Pinealectomy is one method to investigate this concept, however more research is required. Studies show that pinealectomy completely blocked photoperiod-dependent changes in body weight in Siberian hamsters (Vitale et al, 1985)
Pelage:
The endocrine mechanism mediating reproductive activity and seasonal molt are separable, at least interspecifically, when undergoing winter molts. The molt of winter pelage depends on a decrease in circulating prolactin concentration (Goldman B. D and Nelson R., 1993) and is largely independent of gonadal hormones. The role of photoperiod – mediated changes in prolactin secretion in regulation of seasonal changes in pelage has been studied in Siberian hamsters and mink and in these species the pelage rhythm is pineal dependent. In male meadow voles, pinealectomy eliminated short-day induced changes in guard hair length, but no in under hair growth (Takakashi et al, 2001)
Advantages of Biological clock usage
Therapeutic drugs and biological clock connection:
Stress is produced in the organism by the effect of drug administration. Many observations have shown that the biological clock is significantly involved in subsiding the side effects of therapeutic drugs; therefore these effects can be better tolerated at specific times. Furthermore, regarding anticancer therapy, tumor cells have increased sensitivity at certain times of day, thus permitting a more beneficial action of the drug (Antoch & Kondratov, 2013).
Circadian clock in Chondrocytes:
An experiment was made, based on cartilage tissue taken from a young adult and an aged mouse that were treated with PER2::luc. After experimental examination, intrinsic molecular circadian clock existence was found that could affect the expression of genes at certain times. These results revealed an autonomous circadian clock in chondrocytes that could be implicated in key aspects of cartilage biology and pathology. Subsequently, circadian malfunction may account for increased susceptibility to joint damage or disease. (Gossan et al, 2013)
References:
2) Bartness T.J., and Goldman B.D., 1989 Mammalian pineal melatonin: a clock for all seasons. Experimentia 45: pp.939-945
8) Halle S. and Stenseth N.C. 2000 Activity patterns in small mammals: an ecological approach. Springer – verdag berlin Heidelberg
Pictures