Itt írjon a(z) BloodGlucoseBone-ról/ről
The effect of blood glucose on bone health
Glucose is the foundation of energy for most organisms on the planet. It makes sense that glucose will have an effect on normal bone development because it is the most essential energy biomolecule for organisms capable of respiration. As we know, bones have a powerful role in blood formation. Hence, bones will have a large capillary network for nutrients and products to leave/enter. Glucose’s main mode of transport is indeed the blood so the bones will have direct contact with fresh blood with glucose present. Our results concluded that diseases, such as diabetes mellitus, can cause fluctuation in blood glucose levels and hence effect bone health. Type 1 diabetes highlighted a result of lower bone mineral density (BMD) leading to a higher risk of osteoporosis. Whereas, type 2 diabetes causes increased BMD. However, the increased calcification is irregular and similarly to type 1, bone fractures were also a considerably high risk. This study revealed evidence to prove blood glucose has a role in bone metabolism.
Contents
- Introduction
The skeleton is often seen as an inert entity. However, old bone fragments are broken down and replaced with new skeleton tissue continuously. In fact, the skeleton replaces itself within ten years. New studies reveal a close connection between bone cells and blood glucose levels. This assignment aims to investigate bone health and how the effects of differing blood glucose levels can change the make-up of bones in the body.
- Normal Bone Development
During the early stages of embryonic development, the embryo’s skeleton consists of hyaline cartilage and a fibrous membrane. By the seventh week of embryonic life, the process of bone development, ossification (osteogenesis) starts. There are two different osteogenic pathways, intramembranous ossification and endochondral ossification. Bone is the same, regardless of what pathway produces it.
a.Cartilage Templates
Bone is a replaceable tissue, it uses a model tissue to lay down its mineral matrix. For skeletal development the most common template is cartilage. During foetal development a structure is laid down that determines where the bones will eventually form. This structure is a semi-solid matrix which is flexible and produced by chondroblasts, it contains water, collagen fibres, hyaluronic acid and chondroitin sulphate. Cartilage is avascular, therefore it does not have blood vessels that can supply nutrients and remove any waste product. These functions are carried out through diffusion through the matrix. This is why damaged cartilage does not repair itself as easily as other tissues. Through foetal and childhood growth and development, bone will form on this cartilaginous matrix. When the foetus is born the majority of the cartilage will be replaced with bone. The additional cartilage will be replaced with bone throughout childhood development and some remnants of cartilage will remain. The two stages of bone development through foetal development is Intramembranous ossification and Endochondral ossification. The four general categories of bones are long bones, short bones, flat bones, and irregular bones. Long bones include the clavicles, humeri, radii, ulnae, metacarpals, femurs, tibiae, fibulae, metatarsals, and phalanges. Short bones include the carpal and tarsal bones, patellae, and sesamoid bones. Flat bones include the skull, mandible, scapulae, sternum, and ribs. Irregular bones include the vertebrae, sacrum, coccyx, and hyoid bone. Flat bones form by membranous bone formation, whereas long bones are formed by a combination of endochondral and membranous bone formation. (Clarke, B., 2008.) Bone undergoes longitudinal and radial growth, modelling, and remodelling during life. Longitudinal and radial growth during growth and development occurs during childhood and adolescence. Longitudinal growth occurs at the growth plates, where cartilage proliferates in the epiphyseal and metaphyseal areas of long bones, before subsequently undergoing mineralization to form primary new bone. (Clarke, B., 2008.)
b.Endochondral ossification
Endochondral ossification is the type of bone ossification where bone tissue is created directly over the cartilage.

- fig.1 Endochondral ossification (Barnes-Svarney, et al.2016.)
Bone develops by replacing hyaline cartilage. This does not become bone. Instead, the cartilage serves as a template to be entirely replaced by new bone (see fig.1) Endochondral ossification takes a much longer time than intramembranous ossification. Long bones and bones at the base of the skull form throughout this endochondral ossification. Development of endochondral bones initiates shortly after the formation of the limb bud with the condensation of loose mesenchyme, marked by expression of type II collagen. Condensing mesenchyme forms an anlage for the endochondral skeleton and can either branch or segment to form individual skeletal elements. Differentiation of condensing mesenchyme gives rise to a proliferating population of centrally localized type II collagen-expressing chondrocytes and more peripherally localized type I collagen-expressing perichondrial cells. At this stage, chondrocytes begin to elaborate a specialized extracellular matrix containing type II collagen. Midway between the ends of this elongated cartilaginous template, chondrocytes exit the cell cycle, hypertrophy, and begin to synthesize type X collagen in place of type II collagen. (Ornitz, D.M. and Marie, P.J., 2002) To synthesize bone, a centre of ossification forms in the mid-hypertrophic zone by neovascularization of the initially avascular cartilaginous template. The secretion and mineralization of a type I collagen-containing extracellular matrix is mediated by osteoblasts that are associated with the newly developed vasculature. As bones grow, this centre of ossification propagates toward the two epiphyseal plates. (Ornitz, D.M. and Marie, P.J., 2002)
- Effects of glucose and normal bone formation
Glucose has a huge effect on normal bone development, whether it be high or low level of glucose each have different negative effects on bone health. You can’t mention both high and low levels of glucose without mentioning the most important glucose related disease diabetes mellitus, but that will be spoken about later on. Glucose can directly affect bone mineral density (BMD) and calcification of bones. In a study bone calcification was investigated whether glucose affects osteoblastic calcium deposition. MC3T3-E1 ( osteoblast precursor cells obtained from the calvaria of mice) cells were incubated in media containing a normal (5.5 mmol/L)[control], a high glucose concentration (15 mmol/L) or mannitol (15 mmol/L). Osteoblast activity was measured by alkaline phosphatase (bone specific alkaline phosphatase isoenzyme is elevated as a result of increased osteoblastic activity). The bone nodules were examined daily. During matrix maturation and formation cultures undergo a phase of increased calcium deposition. Results show that the size of the nodules was larger by up to four times in the high concentration of glucose but the shape was very irregular and distorted. Despite being much larger, the size of the calcified area relative to the size of the nodule was the same across the samples by percentage of mass that was calcified. However in the presence of glucose, the daily calcium uptake was sizably inhibited compared to the control and mannitol, see Graph 1. Therefore larger nodules were created but with less calcification. (E. Balint et al. 2001)
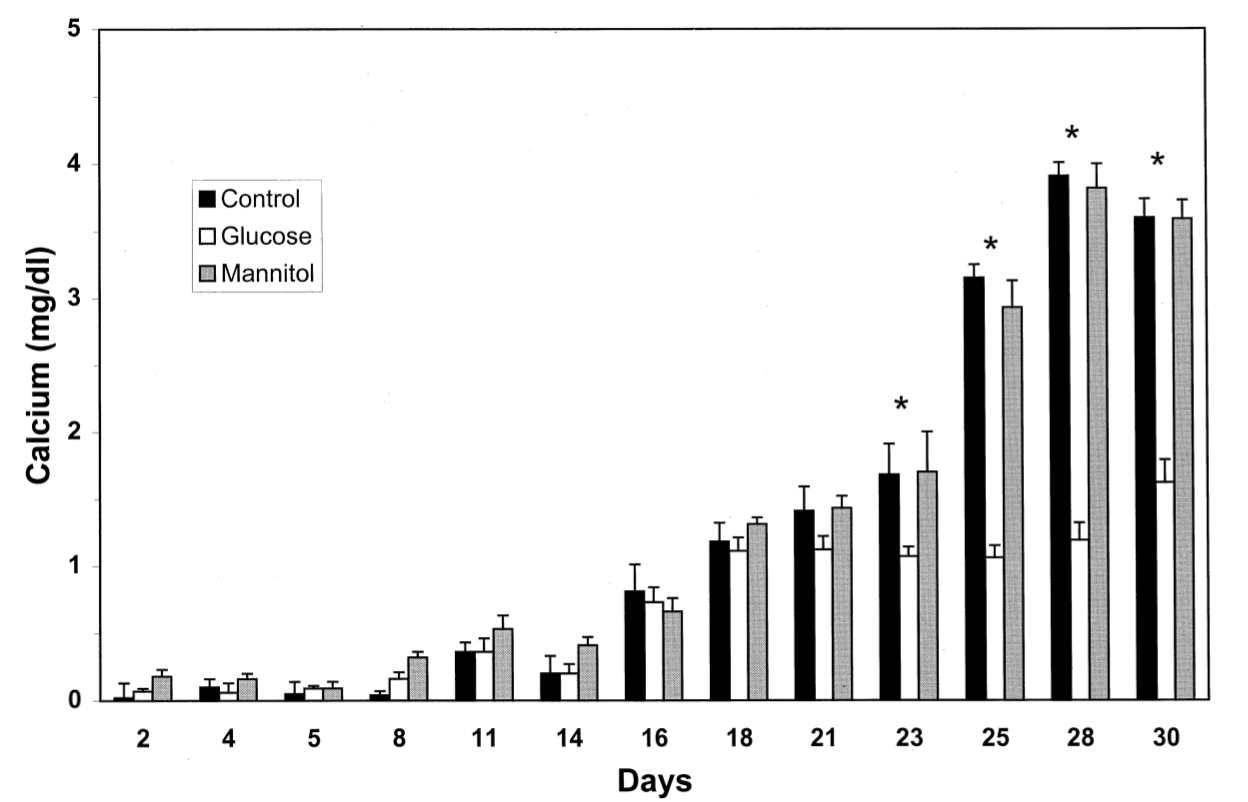
Graph 1. illustration of daily calcium uptake (E. Balint et al. 2001)
Regarding bone mineral density another study was carried out on patients with hip fractures that had type II diabetes mellitus which is characterised with levels of high blood glucose levels. The aim was to see what influence would type II diabetes have on BMD. In order to investigate this primary human osteoblast like cells were stimulated by using a high glucose concentration with advanced glycation end products to simulate the diabetic patient. A factor that has to be mentioned is that BMD can be influenced in other ways that are linked to high glucose i.e. type II diabetes is linked with obesity which is linked to high BMD, some of the patients in this study were quite old and had signs of osteoporosis (low BMD). In light of these the results showed that the femoral neck samples treated with the high glucose concentration of Dulbecco’s modified eagle medium (DMEM) of 4.5 mM glucose containing 20 % heat-inactivated foetal bovine serum, came out with a lower BMD, which in turn leads to increased risk of bone fractures. In addition increased levels of advanced glycation end products alter the mineralisation and repair of micro damaged bone tissue through the formation of crosslinks in collagen. As the organism ages the level of advanced glycation end products rises and it is fair to say that they are an essential factor in the decrease of bone quality in type II diabetes mellitus. (Miranda et al. 2016)
In levels of high glucose certain transcription factors ( e.g.RunX2) have decreased expression which is responsible for the osteoblast differentiation. (Thrailkill et al. 2005). The transcription factor RunX2 is regarded as the master regulator of osteoblast differentiation, in its absence there are no osteoblasts anywhere in the skeleton. On the contrary if there is too much activity of RunX2 then it can lead to early closure of the sutures in the developing skull. There is a factor called smurf1 which inhibits osteoblast differentiation because it can target RunX2 for degradation. The mice that were used to prove this were discovered to be hypoglycaemic and hypoinsulinemic. (Shimazu et al. 2016). RunX2 levels in an organism with high glucose is expressed much lower than that of an organism of with normal glucose levels further proving that glucose has an effect on osteoblast differentiation and also we can draw the conclusion that smurf1 has both an effect on glucose levels and bone formation. (Miranda et al. 2016)
- Diabetes and the effects on bone health
The most common disease affecting carbohydrate metabolism in the body is diabetes mellitus. Studies carried out by the World Health Organisation estimate in 2014, 9% of adults (older than 18 years old) had diabetes (Sundararaghavan, V. et al 2017)
Diabetes is a condition that affects the hormone insulin and leads to high levels of glucose in the body, known as hyperglycaemia. There are two forms of diabetes mellitus. Type 1 is referred to as insulin dependant diabetes mellitus (IDDM) and stems from the inability of the pancreas to produce insulin. Occurrence is often in the young or in those after suffering from a pancreatic disease e.g. insulinoma. Type 2 is known as non-insulin dependant diabetes mellitus and is characterized by insulin resistance, where cells are unable to properly utilise the insulin produced. With progression, the pancreas encounters difficulty producing insulin to the point where none is produced at all. The condition is most prominent in obese or old patients (Gardner, D. G., Shoback, D. and Greenspan, F. S., 2007)
a. Effects of Type 1 diabetes on bone metabolism
Harmful advanced glycation end products (AGE'S) accumulate in diabetic patients due to the inability to metabolise e.g. use of the GLUT -4 transporter for glycogen synthesis. Instead, the glucose reacts with lipids or proteins without the use of proteins. Research has shown that the build up of AGE's causes apoptosis of mesenchymal stem cells (death of cells) preventing the differentiation of osteoblasts, cartilage and adipocytes (Yamagashi et al 2005). Furthermore, high glucose levels inhibit synthesis of the protein osteocalcin from osteoblasts. Osteocalcin plays a major role in the body. Firstly, it’s pro-osteoblastic meaning it's implicated in bone mineralisation and calcium ion homeostasis. Secondly, it stimulates beta cells in the pancreas to release insulin and adipocytes to secrete adiponectin causing fat cells to be more sensitive to circulating insulin (Sundararaghavan, V. et al 2017). Studies have also highlighted that type 1 diabetes affects the composition of bone by decreasing the bone mineral density (BMD). Changes in the mesenchymal cells found in bone marrow of type 1 patients favoured adipogenesis (Kurra, S. and Siris, E., 2011). This is further emphasised in a meta-analysis hypothesising that reduced expression of insulin like growth factor (IGF-1) resulted in decreased bone growth. The same analysis also stated ' a 6.9 relative risk of hip fracture, when the expected relative risk of fracture was only 1.4 based on BMD'. This data reveals that increased risk of fracture cannot be based solely on lower BMD, but other affects of type 1 diabetes must be taken into account such as ophthalmic, nephropathy and neurological associated difficulties (Vestergaard, P., 2007).
b. Effects of type 2 diabetes on bone metabolism
In contrast, evidence illustrates that type 2 diabetic patients display increased BMD when compared to those with type 1 or without diabetes mellitus when considering the femoral head, neck and spine (Ma et al 2012). As previously mentioned, type 2 diabetes is characterized by insulin resistance in cells. Therefore, insulin is still being produced by the pancreas causing hyperinsulinemia to occur in the body. Insulin can interact with the IGF-1 receptors present on the osteoblasts due to its similar structure. Hence, promoting osteoblast division and differentiation, see figure 2. (Sundararaghavan, V. et al 2017).
In conjunction, obesity and/or high BMI is linked to higher bone mineral density. Adipose tissue secretes adiopokines such as leptin which can both inhibit osteoclastogenesis (resulting in reduced bone reabsorption) and also stimulating osteoblastogenesis (inducing bone growth) (Holloway, W. R. et al 2002)
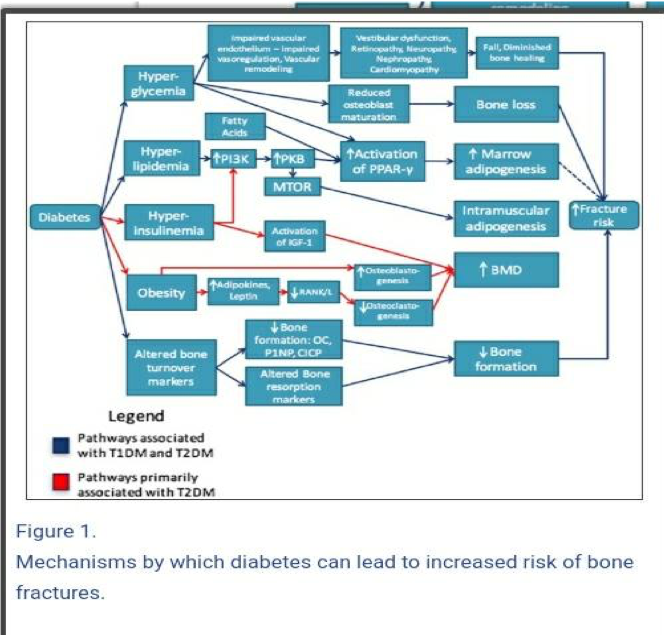
figure 2. Mechanism by which diabetes can lead to increased risk of bone fractures. (Sundararaghavan, V. et al 2017).
However, despite type 2 diabetics displaying higher BMD, they also showed lower bone turnover rate. Analysis concurs that bone markers osteocalcin and cross linked telopeptide were significantly lower among diabetics than non-diabetics (Starup-linde, J., et al 2014).Moreover, patients with type 2 diabetes showed high risk factor to fractures similar to those of type 1 diabetes, despite having higher BMD. Improper redistribution of bone mass occurs leading to increased trabecular bone density but with decreased intracortical bone as see in figure 3. Thus, the bending load is comprised (Leslie, W. D. et al 2012)
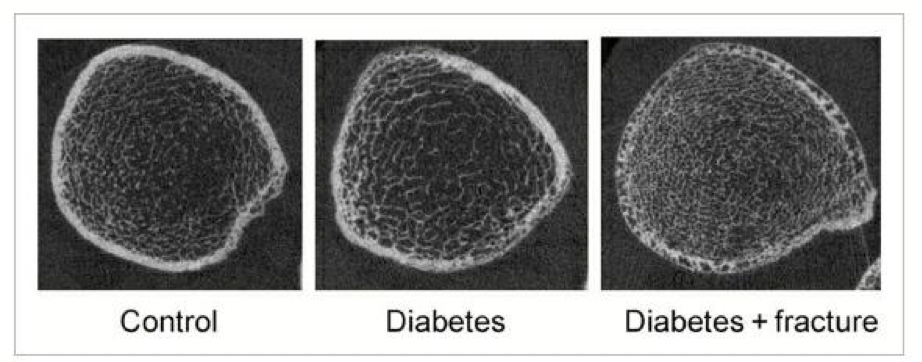
Figure 3: Cortical porosity in type 2 diabetes. Source: (Leslie, W. D. et al 2012)
Anti-diabetic medicine can also cause damage to bone. For example, thiazolodiones (TZD's) increase the body’s sensitivity to insulin. They promote adipocyte differentiation but greatly reduce osteoblast formation (Kurra, S. and Siris, E., 2011)
There are a range of carbohydrate metabolism disorders apart from diabetes mellitus. These can include insulinoma or pancreatitis, both affect insulin levels in the body similarly to diabetes. Another example is xylitol toxicosis, a condition that stimulates beta pancreatic cells causing hyperinsulinemia. Regardless of the condition, if the symptoms cause significant fluctuation in blood glucose levels, there will be a negative outcome in bone health.
- Conclusion
It is apparent that glucose has a profound effect on bone health. It affects not only bones directly but also the factors that influence ossification e.g.RunX2. It’s clear that organisms with glucose related diseases are at more susceptible to bone problems in particular fractures and brittle bones. Depending on the type of diabetes the organism can encounter various bone problems including low BMD, fractures and low mineralisation. The normal figure for blood glucose levels are as follows : Ruminants 3 mmol/L, monogastric animals 5 mmol/L, poultry 10 mmol/L. To combat these problems the organism can use insulin treatment to maintain normal glucose levels and help ensure no further bone damage.
- References
Balint, E., Szabo, P., Marshall, C.F. and Sprague, S.M., 2001. Glucose-induced inhibition of in vitro bone mineralization. Bone, 28(1), pp.21-28.
Clarke, B., 2008. Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology, 3(Supplement 3), pp.S131-S139.
Gardner, D.G., Shoback, D. and Greenspan, F.S., 2007. Greenspan's basic & clinical endocrinology. McGraw-Hill Medical,.
Holloway, W.R., Collier, F.M., Aitken, C.J., Myers, D.E., Hodge, J.M., Malakellis, M., Gough, T.J., Collier, G.R. and Nicholson, G.C., 2002. Leptin inhibits osteoclast generation. Journal of Bone and Mineral Research, 17(2), pp.200-209.
Kurra, S. and Siris, E., 2011. Diabetes and bone health: the relationship between diabetes and osteoporosis‐associated fractures. Diabetes/metabolism research and reviews, 27(5), pp.430-435.
Leslie, W.D., Rubin, M.R., Schwartz, A.V. and Kanis, J.A., 2012. Type 2 diabetes and bone. Journal of Bone and Mineral Research, 27(11), pp.2231-2237.
Ma, L., Oei, L., Jiang, L., Estrada, K., Chen, H., Wang, Z., Yu, Q., Zillikens, M.C., Gao, X. and Rivadeneira, F., 2012. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. European journal of epidemiology, 27(5), pp.319-332.
Miranda, C., Giner, M., Montoya, M.J., Vázquez, M.A., Miranda, M.J. and Pérez-Cano, R., 2016. Influence of high glucose and advanced glycation end-products (ages) levels in human osteoblast-like cells gene expression. BMC musculoskeletal disorders, 17(1), p.377.
Ornitz, D.M. and Marie, P.J., 2002. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & development, 16(12), pp.1446-1465.
Shimazu, J., Wei, J. and Karsenty, G., 2016. Smurf1 inhibits osteoblast differentiation, bone formation, and glucose homeostasis through serine 148. Cell reports, 15(1), pp.27-35.
Starup-Linde, J., Eriksen, S.A., Lykkeboe, S., Handberg, A. and Vestergaard, P., 2014. Biochemical markers of bone turnover in diabetes patients—a meta-analysis, and a methodological study on the effects of glucose on bone markers. Osteoporosis International, 25(6), pp.1697-1708.
Sundararaghavan, V., Mazur, M.M., Evans, B., Liu, J. and Ebraheim, N.A., 2017. Diabetes and bone health: latest evidence and clinical implications. Therapeutic advances in musculoskeletal disease, 9(3), pp.67-74.
Thrailkill, K.M., Liu, L., Wahl, E.C., Bunn, R.C., Perrien, D.S., Cockrell, G.E., Skinner, R.A., Hogue, W.R., Carver, A.A., Fowlkes, J.L. and Aronson, J., 2005. Bone formation is impaired in a model of type 1 diabetes. Diabetes, 54(10), pp.2875-2881.
Vestergaard, P., 2007. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporosis International, 18(4), pp.427-444.
Yamagishi, S., Nakamura, K. and Inoue, H., 2005. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Medical hypotheses, 65(6), pp.1013-1015.
