Functional differences between current COVID19 Vaccines from a physiological perspective
Contents
Abstract
The following essay aims to give an understandable overview of the different physiological processes that take place in the human body after the introduction of the different SARS-CoV-2 vaccines that are currently in development or already in use. (2021)
Introduction
“Severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) induces the coronavirus disease 2019 (COVID-19), which leads to pneumonia and can be fatal. The first case of COVID-19 was recorded in December 2019 in Wuhan, Hubei Province, China, which subsequently spread to many other countries. This eventually led to the declaration of a worldwide pandemic by the World Health Organization on March 11 2020. Researchers in Wuhan published the nucleic acid sequence of the SARS-CoV-2 in mid-January, which gave international researchers the ability to consider possible vaccine solutions (Silveira et al, 2021)
Physiological symptoms and immune response to SARS-CoV-2
Since SARS-CoV-2 is an airborne pathogen the immune system within the respiratory tract and lung has to protect the body via a mucosal protective barrier which is constantly active. This protective surface consists of many layers of epithelium, which can catch pathogens that will be recognized via the mucosal pattern recognition receptors and can then be eliminated from the body by coughing. (Chung et al, 2020) In case of infection SARS-CoV-2 will induce symptoms in not only the lung, causing an acute respiratory distress syndrome, but also other organs like the heart, liver, brain, kidney and pharynx. (Jeyanathan et al, 2020) For most patients COVID-19 will only be manifested via little or no symptoms. In case no symptoms are detectable we talk about “asymptomatic” patients. Other courses of the disease would be fever, chills, fatigue, coughing, loss of appetite, loss of smell (anosia) and taste. During a severe course of the disease the following symptoms of blood clotting, renal damage and cardiovascular collapse can also be seen. (Tregoning et al, 2020)
SARS-CoV-2 induces a strong adaptive immune response in most individuals. This way T memory cells as well as B cells will be stimulated. About 40 to 60% of today's human population, which was not infected with SARS-CoV-2, have detectable SARS-CoV-2 reactive CD4+ T cells in their body due to prior exposure to other viruses of the Coronaviridae family. But that does not necessarily mean full protection against the outbreak of COVID-19 in 40-60% of the population. (Ura et al, 2021) In addition to the immune response of T cells, immunoglobulins are produced by B cells, mainly against nucleocapsid and spike proteins (N and S proteins). These will only appear around the 10th day after infection by the virus and will lead to Ig antibody production which will be seroconverted (https://en.wikipedia.org/wiki/Seroconversion) about three weeks after infection. (Silveira et al, 2021)
Structure of SARS-CoV-2
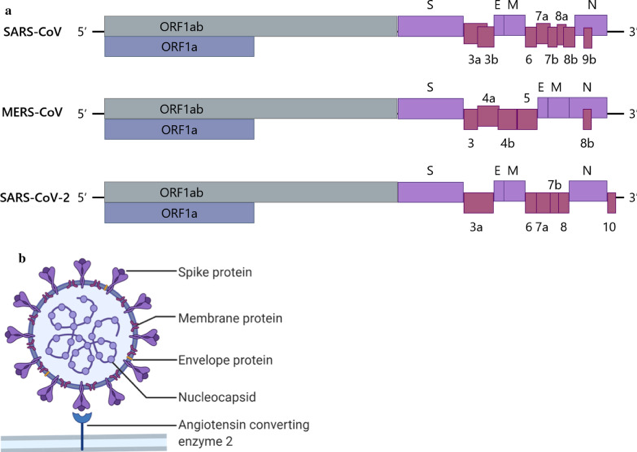
The coronavirus is an enveloped virus containing envelope proteins (E), membrane glycoproteins (M) Hemagglutinin-esterase dimer proteins (HE), nucleocapsid proteins (N) (assisting in virus budding) and spike proteins (S). (Chung et al, 2020) SARS-CoV-2 is adsorbed to the host cell by binding to the host cell's ACE2 receptor using its spike proteins (S). The fusion of the S proteins with the endosomal and viral membrane as well as the proteolytic cleavage of S proteins lead to the release of viral RNA into the cytosol of the host cell. Therefore, most vaccines against SARS-CoV-2 target the receptor-binding domain of the S proteins. (Ura et al, 2021)
To grasp how each vaccine type functions, it helps to understand the genome structure of the three main coronaviruses: SARS-CoV-1, MERS-CoV and SARS-CoV-2. MERS-CoV stands for Middle East Respiratory Syndrome coronavirus and is another highly pathogenic virus. The coronavirus discussed in this paper focuses primarily on SARS-CoV-2. For some vaccine platforms, the development began with treating SARS-CoV-1 before being applied to SARS-CoV-2. The 3’-terminus of the coronavirus genome is what encodes the four major structural proteins seen in the figure below as well as genus-specific accessory proteins that vary between the 3 types. The order being S, E, M and N proteins. (Li et al, 2020)
|
Figure 1 Demonstrating the genome and virion of CoV’s, coronaviruses. (Li et al, 2020)
The aim of vaccines and how they work
The development of a well-functioning, safe and cheap vaccine is of utmost importance since it is widely assumed that life will not return to a pre-pandemic state without it. (Jeyanathan et al, 2020) However there are still a few things that are unknown at this point. As of the beginning of 2021 it is unknown whether the protection against COVID-19 is long-lasting since several cases of patients being repeatedly infected with SARS-CoV-2 have been reported. A long lasting immunity induced by a vaccine would be the ultimate goal in vaccine development, however considering our current knowledge, an annual vaccination would be more likely like in the case of influenza. (Silveira et al, 2021) Additionally, natural immunity might be different to vaccine induced immunity since viruses form specific strategies remain in the host unnoticed and therefore evade the immune system. (Jeyanathan et al, 2020)
The goal would be to vaccinate 70% of the population successfully so that herd immunity would be assured. Other non-vaccination treatment options, such as antiviral drugs for COVID-19, have also been considered and in some cases applied. However, those drugs would only mitigate symptoms and would slow down the progress of the disease. (Chung et al, 2020)
Under normal circumstances the development and the issuing of a licensure for commercial use of a vaccine would take 10 to 15 years. During this emergency situation however, the timeline for producing a vaccine was considerably reduced to 1 to 2 years. (Jeyanathan et al, 2020) Even though a new vaccine is highly desired at the moment, all safety and efficacy measures need to be considered to produce an adequate vaccine which should also have scalable production. (Silveira et al, 2021)
Vaccines against SARS-CoV-2 inducing adaptive immune response might reduce severe clinical symptoms by facilitating adaptive immune activation. We know today that not only the induction of antibody response (humoral immunity) by a vaccine is important. T memory cell mediated immunity (CD4+ and CD8+ T cells) plays a crucial role in the prevention of severe COVID-19 symptoms and therefore the full activation of adaptive immunity. (Jeyanathan et al, 2020) Vaccine induced very strong innate immunity is non-desired. In contrast a moderate innate immune reaction will increase the adaptive immune response and it therefore aimed for. (Silveira et al, 2021)
Vaccine platforms
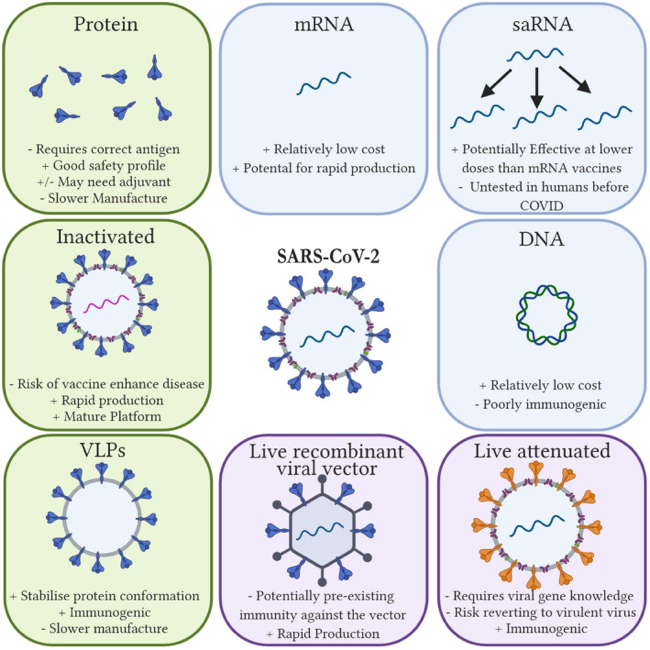
Generally, we differentiate between 6 different categories of vector platforms consisting of inactive virus vaccines, protein subunit vaccines, nucleic acid vaccines (including mRNA and DNA), virus like particles (VLP), live attenuated vaccines and viral vector vaccines. (Jeyanathan et al, 2020) (see figure 2) Three of which will be discussed in this paper.
|
Figure 2 Showing the different vaccine platforms as well as advantages (+) and disadvantages (-). These include protein, inactivated, VLP, viral vector, live attenuated, and nucleic acid vaccines. (Tregoning et al, 2020)
Inactivated virus vaccine
Function
Unlike other vaccine types, inactivated virus vaccines uses the purified whole SARS-CoV-2 components. (Ura et al, 2021) They are created using a traditional method of manufacturing with well-established methods and infrastructure. It is therefore a very hands-on approach to a pandemic situation as it can be rapidly generated in large quantities. (Jeyanathan et al, 2020)
To produce the vaccine type, one needs to completely inactivate or kill the pathogen. They will induce protective antibodies against epitopes on hemagglutinin glycoprotein seen on the surface of the virus when it is injected into the host. (Rawat et al, 2020) The method used to inactivate the virus particles is vital to ensure an effective and safe vaccine. They can be inactivated by heat, chemicals or radiation. (Chung et al 2020) Specifically, formalin with added carbonyl groups, beta-propiolactone, UV or gamma radiation are used. The mode applied during the inactivation process can influence the quality of antibodies as well as the polarization of T cell response to the vaccine. (Tregoning et al, 2020) Due to the inactivation, they are deemed safer than live-attenuated viruses. They are incapable of replication as the RNA is destroyed and it expresses viral epitopes that can induce antibody responses. (Chung et al, 2020) However, inactivated vaccines may not be sufficiently immunogenic and in some cases can lead to an enhanced disease pathology. (Brisse et al, 2020) There is also the concern of vaccine-induced immunopathology seen in vaccines for SARS-CoV-1. (Tregoning et al, 2020)
T helper cell (THcell) phenotype of vaccine-induced T cells of CD4+ Th cells show TH1 or TH2 cell responses, which are relevant to how they mediate protection and depend on the adjuvant in use. TH1 are seen in less severe cases of SARS-CoV-2, whilst TH2 responses are seen with enhancement of lung disease following infection in hosts parenterally vaccinated with the inactivated viral vaccines. (Jeyanathan et al, 2020) This was detected in the double inactivation through UV light and formalin of SARS-CoV-1. It showed an enhancement of the eosinophilic response from the vaccine, which elicited a proinflammatory pulmonary response. Conclusively failing to provide complete protection. (Tregoning et al, 2020) They are also poor inducers of cytotoxic CD8+ T cells, which as discussed earlier, is vital for adaptive immunity. (Jeyanathan et al, 2020)
Delivery
Similarly to the mRNA and DNA vaccines, inactivated virus vaccines require repeated vaccination and an adjuvant such as alum or CpG to be effective. Alum enhances TH2 cell responses, which prevents them from being used for respiratory mucosal delivery due to being potentially involved with “ADE”, antibody-dependent enhancement of disease, which can potentially be worsened in hosts of an older age. It is delivered intramuscularly, which does provide some mucosal immunity most likely due to the transport of systemic antibodies to the lungs. (Jeyanathan et al, 2020)
There are currently seven early clinical trials testing inactivated SARS-CoV-2 vaccines including PiCoVacc developed by “Sinovac”. It provides protection against SARS-CoV-2 with antibodies to S protein and nucleocapsid, and reduced viral titres in rhesus macaques. (Jeyanathan et al, 2020) This particular candidate grows the virus In Vero cells and inactivates them with beta-propiolactone, and no virus was detected in the pharynx or lungs. (Tregoning et al, 2020) Based on the safety and efficacy data from the large studies conducted on the COVID-19 vaccines, of candidates in Phase III as of August 2020, 50% of them are inactivated vaccines. (Tregoning et al, 2020)
Vector vaccine
The viral vector type of vaccine was developed about 40 years ago. A vector responsible for expressing the Hepatitis B surface antigen was tested with great success in a chimpanzee animal model. This vaccination platform is regularly applied all around the world in everyday veterinary clinics. (Brisse et al, 2020)
We can differentiate between “replication-competent” and “replication-defective” viral vectors. (Ura et al, 2021) The following part will be limited to “replication-defective” types of viral vector vaccines as only those were approved by the WHO in human use to combat the COVID-19 situation. “Self- replicating” viral vectors are currently only being tested in animal models because the possibility for them to have an uncontrollable exponential effect is still too high. (Brisse et al, 2020)
Function
Viral vector vaccines give us the possibility to supply the patients with a strong dose of DNA encoding the antigenic spike glycoprotein of the virus causing the disease.The antigen encoding information is transported in a non-pathogenic way by a DNA carrying virus that has been genetically modified to include the sequence coding for the surface antigens of the ssRNA SARS-2 virus. (Brisse et al, 2020) In order to transport the genetic information a human adenovirus and a chimpanzee adenovirus were chosen to transport the genetic information. The human adenovirus type 5 (-Ad05) and a type 26 (-Ad26) as well as the chimpanzee adenovirus (ChAd) are able to induce strong cellular and humoral immune responses. Normally the human adenoviruses only cause the “common cold”. (Ura et al, 2021) Therefore it is safer to use the chimpanzee derivative that has not been seen in humans before.This is done in order to avoid any kind of unwanted reaction. (Chung et al, 2020) To make sure that all the aforementioned viruses are replication defective the viral gene region E1A and E1B were modified. (Ura et al, 2021) Inactivation of the replication site is done by the deletion of the E1 and the deletion of the E3 site allows the insertion of a gene sequence up to 8Kb. The size of gene sequences that can be transported this way is much more significant than most other types of vaccines and is thus a large benefit. (Chung et al, 2020) After the genetic modification we talk about the “Ad26CoV-2-S” and the “ChAdOx-1n CoV-19” (Ura et al, 2021) The Ad5-nCoV encodes the full length of the “S protein” of SARS-2 whereas most mRNA only encode for specific subunits. (Chung et al, 2020)
Delivery
As with most vaccines the vector virus type is most often injected intramuscularly which often causes a strong cell mediated type of immune response.
After the injection the viral vector will be transported throughout the body and reach the system circulation and most importantly the lymphatic system. During the transport it will be presented to many different cell types and the viral vector will use the “normal” virus approach to enter into the cell via adhesion to the virus specific receptors. Once this step is complete the intracellular transport of the nucleic acid into the nucleus of the host’s cell will take place. This will induce the target gene expression after the internuclear transcription and intracellular protein translation. (Chung et al, 2020) This step completed the spike antigen protein will be secrete and be used for cross-presentation by macrophages, immature dendritic cells and which via the aid of immature B-cells will produce plasma cells that finally lead to the protective effect of specific antibody production. These immunity inducing steps are very comparable to the mode or action of the mRNA vaccine. (Chung et al, 2020)
Even though DNA vaccines could be injected by gene gun with the aid of gold nanoparticles that could increase potency and efficiency the method of administration for a vector vaccine is the classical injection. (Brisse et al, 2020)
Both the human and the chimpanzee adenovirus viral vectors have their benefits and their downsides when it comes to delivery and transport. A notable advantage over other vaccine types is that both have the ability to transport large amounts of genetic information into the cell without the use of adjuvants while still causing a strong immunogenic response. Adjuvants can be left out because the vector itself will take over the role of the adjuvant and “activate” the immune response cascade as the presentation to the APC’s is efficient enough this way. (Brisse et al, 2020)
The delivery of the antigen coding DNA to the nucleus of the host’s cells is definitely not risk free as the possibility of DNA reversion is still significant which poses a huge risk in case a replication competent virus was originally used. (Brisse et al, 2020) Another noteworthy risk is the pre-existing percentage of the herd immunity to the human adenovirus. This means that the immune system of patients, that were previously in contact with variations of the virus used as the vector, will recognize the vector as a foreign substance that needs to be eliminated. The nuclease enzyme will kick in and degrade the genetic information before it physically reaches the cell where it can have its wanted effects. (Chung et al, 2020)
An additional risk of this type of delivery is the introduced DNA has to get into the nucleus of the host cells which could cause intense cancer formation in case it would integrate into the oncogenic or regulatory sequence of the nuclear DNA. (Chung et al, 2020)
Nucleic acid vaccines
There are two major types of nucleic acid vaccines which have been used for developing vaccines against COVID-19 namely mRNA and recombinant plasmid DNA vaccines. DNA vaccines have been used and investigated for many years whereas mRNA vaccines are a rather new field of research. (Jeyanathan et al, 2020)
DNA vaccine
Plasmid DNA vaccines are similar to mRNA vaccines not only since both of those vaccines use nucleic acid as their main vaccine platform, but also since both vaccine types are easy to produce and comparably safe to use. (Jeyanathan et al, 2020) There are various reasons why DNA vaccines are considered to be less successful than other vaccines. (Liu, 2019) DNA vaccines are less immunogenic than many other vaccines which means that multiple doses are needed to induce full immunity against a certain virus. Additionally DNA vaccines need adjuvants to take full effect. (Jeyanathan et al, 2020) Concerns have also been raised that DNA vaccines could be integrated into the host genome which could lead to mutations and cancer. An advantage of DNA vaccines is the relatively low production cost and stability. (Rawat et al, 2021) DNA vaccines can be produced in large scale using Bacteria. (Silveira et al, 2021)
Generally DNA vaccines are non-replicating and non-infectious. (Rawat et al, 2021)
Function
DNA vaccines induce both adaptive and innate immune response. (Silveira et al, 2021) The most crucial part of promoting adaptive immunity is the triggering of innate immune response. Innate immune response will be stimulated by the DNA vaccine in the cytosol, before entering the nucleus, causing inflammation. (Tregoning et al, 2020) The inflammation can be explained by the intense release of cytokines and chemokines stimulated by the vaccine (inhibiting viral replication) as well as interleukins produced by dendritic cells. (Silveira et al, 2021)
DNA vaccines consist of bacteria derived plasmids containing a mammalian expression cassette. The expression cassette consists of a Kosak sequence as well as a promoter region and a polyA tail (a 3’ polyadenylation tail). The main function of this expression cassette is the regulation of transgene (plasmid) (https://en.wikipedia.org/wiki/Transgene) expression. (Tregoning et al, 2020) The plasmid encodes the antigen that ought to be produced in the cell nucleus using the host’s cellular machinery for transcription. (Silveira et al, 2021) Therefore, DNA vaccines can only function by entering the cell nucleus of the host cell where antigen expression takes place. (Tregoning et al, 2020)
Subsequently a major histocompatibility complex (MHC) will be activated since the newly produced antigens will be presented to T cells by antigen presenting cells (APCs) or myocytes.(Silveira et al, 2021) This way an adaptive immune response will be induced. (Tregoning et al, 2020) An example for APCs would be dendritic cells (DCs) which use the MHC-II pathway and present the antigens to CD8+ as well as CD4+ T memory cells. Interleukins and TNF-alpha (tumor necrosis factor alpha) which were released by the DCs will also activate CD8+ T cells which will then activate CD4+ T cells. Myocytes are non-APC cells which use MHC-I for antigen presentation activating mainly CD8+ T cells. (Silveira et al, 2021)
B cells are also activated by vaccine proteins via MHC-II and therefore start the production of antibodies (mostly IgG) as an active humoral immune response. Therefore a large amount of B cells as well as memory T cells will be stimulated by DNA vaccines. (Silveira et al, 2021)
Delivery
Usually DNA vaccines will be applied via an intramuscular or intradermal injection. This is used to maximize the delivery of the vaccine to the APCs. Intravenous and intraperitoneal delivery methods have been investigated in case of animals but they are not as common. Localized routes have proven to be the most effective in case of DNA as well as mRNA vaccines. (Brisse et al, 2020) Two newer forms of DNA vaccine delivery which have been developed to improve DNA vaccine stability and immunogenicity are the gene gun and electroporation delivery method. (Brisse et al, 2020) The electric current delivered by the electroporation increases the uptake of DNA vaccines. Biojectors which are so called “gene guns” can increase the humoral antibody response. (Tregoning et al, 2020) Other methods improving the immunogenicity of DNA as well as RNA vaccines would be the usage of nanoparticles which work like a carrier for the vaccine or the optimization of codons that are used to attach to the target host cells. (Brisse et al, 2020)
Nucleic acid vaccines like DNA vaccines cannot be administered via the respiratory mucosal route yet since they need to be delivered repeatedly and together with adjuvants. (Jeyanathan et al, 2020)
mRNA vaccine
The mRNA vaccines can be classified into two different groups. The “self-amplifying” and the “non-amplifying” vaccines. Even though the volume and the doses needed to gain a “safe immunity titer” via usage of self-amplifying mRNA vaccines is much lower, they are still not approved by the FDA for human use. These vaccines have a much higher capability and could easily spiral out of control. Therefore they are still in the early clinical trials. (Ura et al, 2021)
The following part will be limited to the “non-amplifying” type of mRNA vaccine.
Function
The mRNA is an antigen encoding nucleic sequence that is protected via a lipid nanoparticle.(Nanoparticle -https://en.wikipedia.org/wiki/Nanoparticle) mRNA will exert its effects in the cytoplasm and is therefore safer than its counterpart vaccine which needs to enter into the nucleus.The entry into the nucleus poses the risk of potential insertion mutation. (Jeyanathan et al, 2020) The nowadays available biotechnologies and bioinformatics make it possible to rapidly produce large amounts of vaccines with crucial accuracy. (Ura et al, 2021) As the sequence of SARS-CoV-2 was made public shortly after the outbreak of the pandemic it is possible to isolate the sequence coding for the surface spike protein also called (S) protein. (Chung et al, 2020) The mRNA is modified to increase the stability and translational ability by usage of UTR. “UTR” stands for “untranslated region” and these regions are added to the 3’ and the 5’ UTR which also includes a stop signal. The mRNA will then be translated by the ribosomes. From this point on the viral protein will enter into the proteasome which will split it into much smaller molecules. These molecules will be passed to the Golgi apparatus for maturation. The released MHC and newly synthetized spike proteins are transported to the cell membrane. Upon release from the cell the spike protein will cause cellular immune response cascades. These cytoplasmic steps are very similar to the DNA counterpart vaccine. The latter produces mRNA but the product has to evade the nucleus which is risky and could lead to insertional potential into the host cell’s genetic information, potentially leading to unwanted effects like tumors or cancer formation. (Liu, 2019)
The longer lasting more complete type of humoral and cellular immunity is sanctioned by the activation of the CD4+ (helper) T and CD8+T (killer) cells of the immune system that are. CD8+T cells will bind to the MHC1 and are responsible for the humoral memory and the destruction of the cells that present the SARS-CoV-2 spikes on their surface. (Ura et al, 2021) Helper cells will actively produce cytokines which are used for further intracellular signaling and inflammatory responses. (Jeyanathan et al, 2020) CD8+T and CD4+T are activated by the MHC1, MHC2 respectively. This is a necessary step for the powerful function of the mRNA vaccine. (Chung et al, 2020)
Delivery
As most conventional vaccines the mRNA type of vaccine is administered via a needle type of injection and needs 2 repeated doses. After subcutaneous or intramuscular injection the mRNA that is packaged into lipid nanoparticles will be taken up by endocytosis by antigen presenting cells (APC’s) such as dendritic or by B cells. (Jeyanathan et al, 2020) The nanoparticles are able to move and squeeze into the lymphatic system and into the systemic blood circulation. (Chung et al, 2020) In addition the RNA itself will act as an adjuvant when it is detected by the toll like receptors (Chung et al, 2020)
As the antigen coding mRNA will not pass the cellular membrane by itself. It needs to be packaged into a lipid nanoparticle in order to pass the membrane without damaging the mRNA. (Liu, 2019) This also allows the RNA to escape the host cell’s nucleosomes which would have severe consequences in the efficiency due to degradation. (Chung et al, 2020) The usage of nanoparticles (NP’s) is rather new in the domain of vaccines which allows a higher uptake rate of the mRNA to the APCs. They have very general characters that can easily be controlled like size, polarity and hydrophobicity. NPs are small capsules (between 1 and 100 nanometers) made of hydrogels and polymer matrices. Another advantage of the nanoparticles is that they allow a controlled delivery and protection during transport. In addition the mRNA is protected from degradation by macrophages. (Chung et al, 2020)
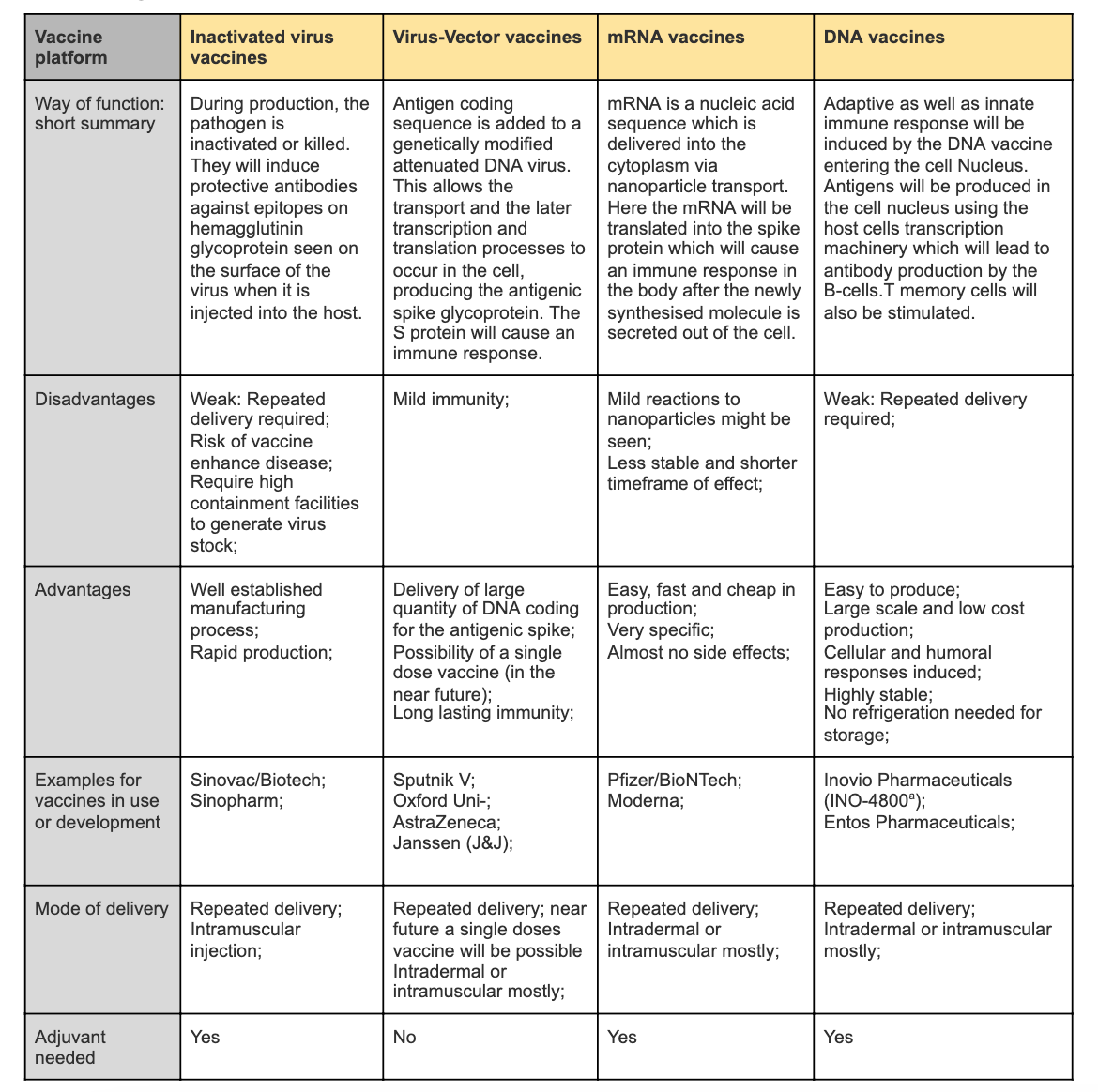
Summary Table
|
Figure 3 Simplified comparison of different vaccine types
Comparative Conclusion
mRNA has certain advantages over the other vaccines in terms of efficacy, issues of antivector immunity and safety, which contributes to why they were the first vaccines to be approved. For protein translation and post-translation modifications, the antigen-encoding mRNA complex with a carrier can be efficiently delivered in vivo into the cytoplasm of the host cell, for example lipid nanoparticles. They are non-infectious and synthesized by in vitro transcription, meaning that they are free of microbial molecules. (Jeyanathan et al, 2020) Comparing all the platforms is most applicable in their efficacy trials, however this is yet to be carried out for all variants. One must also be critical to compare directly as different ELISA and neutralization assay are used. (Tregoning et al, 2020)
The antigen used for nucleic acid vaccines and viral vector vaccines is the S protein, whilst inactivated virus vaccines use the whole virion. Inactivated virus vaccines that use the whole virus have the ability to include the coronavirus nucleocapsid, which is immunogenic. The antibodies against the SARS-CoV-1 N protein are abundant and live longer compared to the S protein in recovered patients. (Tregoning et al, 2020) However, using the whole virus may not be the best option as they include non-essential antigens that take up unnecessary space and decrease the yield of potent antigens. They also vary in their production as nucleic acid vaccines use genetically engineered RNA/DNA, respectively, to directly produce an antigen. Inactivated virus vaccines inactivate virus particles and viral-vector vaccines are genetically engineered with encoded target genes. (Chung et al, 2020)
As long as there is no respiratory mucosal vaccination available, the body is not able to create the much-needed trained immunity in the respiratory tract alveolar macrophages which is the first and key line of defense if we want to eradicate SARS-CoV-2. Not all vaccine platforms are suitable for respiratory mucosal delivery, however the most suitable platform would be viral-vector vaccines. (Jeyanathan et al, 2020)
The four vaccine platforms discussed demonstrate that determining the most effective vaccine based on their physiological processes alone, doesn’t determine which vaccine provides the best immune response for every country and every mutation of SARS-CoV-2. Due to the increase in rapid development of preclinical and current vaccine approaches, there has become more than one effective vaccine approach. (Chung et al, 2020) Inactivated and live attenuated vaccines are the traditional approaches, however research within other platforms such as nucleic acid and virus-vector vaccines offer innovative potential solutions to addressing existing challenges. They have advanced our application and understanding of vaccine immunology. (Brisse et al, 2020) The immune response, combination of immune responses and which antigen targets are crucial to determine efficacy, along with the consideration that vaccines alone may not be the best approach. As the world continues to develop vaccines within each platform, it becomes clear that researching the immunopathological basis of COVID-19 and its immune evasion mechanism can provide an essential insight into vaccine design strategies. (Rawat et al, 2020)
Glossar
Adaptive immunity
Adaptive immunity can be described as a specific but slow immune response. There are further subgroups of adaptive immunity: cellular response (memory T cells are mainly involved like CD4+ and CD8+ T cells) and humoral response (B cells mainly producing antibodies). (Silveira et al, 2021)
Adjuvants
Adjuvants are needed in the case of protein vaccines of low immunogenicity. (Tregoning et al, 2020) They are biochemical components that promote the functioning of a vaccine. This is achieved by stimulating innate immune cells and therefore eliciting an adaptive immune response. (Jeyanathan et al, 2020) In case of protein vaccines such adjuvants are administered which increase the production of cytokines and chemokines and thus increase antigen presentation and uptake which increases inflammasome activation. (Tregoning et al, 2020) Nucleic acid vaccines are often complemented by lipid nanoparticles, polyplexes and lysosomes. Especially in case of RNA vaccines those adjuvants are essential since they protect the vaccines from being degraded by extracellular RNAses. (Tregoning et al, 2020) Inactivated viral vaccines often use alum as an adjuvant which is why those vaccines cannot be used for respiratory mucosal delivery. (Jeyanathan et al, 2020)
Examples for vaccine platforms needing adjuvants are: inactivated viral vaccines, nucleic acid and protein vaccines. In vector vaccines the vector itself will take over the role of the adjuvant and “activate” the immune response cascade as the presentation to the APC’s which is why no adjuvants are needed. (Brisse et al, 2020)
Innate immunity
Innate immunity is a nonspecific and fast response of the immune system against invaders. (Silveira et al, 2021)
Written by: Schafzahl Lena Maria, Steckmest Anna Bettina, Workman Benjamin
References
Brisse M; Vrba SM; Kirk N; Liang Y; Ly H (2020): Emerging Concepts and Technologies in Vaccine Development. Frontiers in Immunology. 11: () 583077: DOI:10.3389/fimmu.2020.583077
Case BJ; Rothlauf PW; Chen RE; Kafai NM; Fox JM; Smith KB; Shrihari S; McCune BT; Harvey IB; Keeler SP; Bloyet LM; Zhao H; Ma M; Admas LJ; Winkler ES; Holtzman MJ; Fremont DH; Whelan SP.J.; Diamond MS (2020): Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host & Microbe. 28:(3) 465-474 DOI: 10.1016/j.chom.2020.07.018
Chung JY; Thone NM, Kwon YJ (2020): COVID-19 vaccines: The status and perspective in delivery points of view. Advanced Drug Delivery Reviews. 170: 1-25. DOI:10.1016/j.addr.2020.12.01
Chung YH; Beiss V; Fiering SN; Steinmetz NF (2020): COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS-NANO 14:(10) 12522-12537 DOI: 10.1021/acsnano.0c07197
Jeyanathan M; Afkhami S; Smaill F; Miller MS; Lichty BD; Xing Z (2020): Immunological considerations for COVID-19 vaccine strategies. Nature Reviews Immunology 20: (10) 615-632 DOI:10.1038/s41577-020-00434-6
Liu MA (2019): A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 7:(2) 37 DOI:10.3390/vaccines7020037
Li YD; Chi WY; Su JH; Ferrall L; Hung CF; Wu TC (2020): Coronavirus vaccine development: from SARS and MERS to COVID-19. Journal of Biomedical Science 27: 104 DOI: 10.1186/s12929-020-00695-2
Rawat K; Kumari P; Saha L (2021): COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. European Journal of Pharmacology 892:173751. DOI: 10.1016/j.ejphar.2020.173751
Silveira MM; Moreira GMSG; Mendonça M (2021): DNA vaccines against COVID-19: Perspectives and challenges. Life sciences 267: 118919. DOI: 10.1016/j.lfs.2020.118919
Tregoning JS; Brown ES; Cheeseman HM; Flight KE; Higham SL; Lemm NM; Pierce BF; Stirling DC; Wang Z; Pollock KM (2020): Vaccines for COVID-19. Clinical and experimental immunology 202: (2) 162–192 DOI:10.1111/cei.13517
Ura T; Yamashita A; Mizuki N; Okuda K ; Shimada M (2021): New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine 39:(2) 197-201 DOI:10.1016/j.vaccine.2020.11.054
Figures
Figure 1: Li YD; Chi WY; Su JH; Ferrall L; Hung CF; Wu TC (2020): Coronavirus vaccine development: from SARS and MERS to COVID-19. Journal of Biomedical Science 27: 104 DOI: 10.1186/s12929-020-00695-2 “Permission granted for use of image by author”
http://creativecommons.org/licenses/by/4.0/
http://creativecommons.org/publicdomain/zero/1.0/
Figure 2: Tregoning JS; Brown ES; Cheeseman HM; Flight KE; Higham SL; Lemm NM; Pierce BF; Stirling DC; Wang Z; Pollock KM (2020): Vaccines for COVID-19. Clinical and experimental immunology 202: (2) 162–192 DOI:10.1111/cei.13517 “Permission granted for use of image by author”
Figure 3: Simplified comparison of different vaccine types, information retrieved from: Brisse et al, 2020. Chung et al, 2020. Jeyanathan et al, 2020. Liu, 2019. Rawat et al, 2020. Silveira et al, 2021. Tregoning et al, 2020. Ura et al, 2021.