Dreaming animals
Student essay written by Aimonetti Emma, Balayn Perrine and Dorléans Marie
Contents
- Dreaming animals
Introduction
Any pet owner has already seen his animal moving during his sleep : the end of their legs frantically stirs and the animal often emits little squeaks. The explanation that traditionally comes to mind is that they are dreaming. It has been proven that dreams are most likely to occur during the Rapid-Eye-Movement or REM-sleep. This particular sleep phase, originating from the brain stem, presents characteristic behavioral manifestations that are rapid eye movements and a partial or total atonia of the muscles. Such a sleep state has been observed in warm-blooded vertebrates (mammals and birds). Issues with definitions of sleep and REM make it hard to draw conclusions about the dreaming of cold-blooded vertebrates and invertebrates. Dreams in animals seem to have a role in neurogenesis, learning and memorization.
Methods to study animals sleep and dreams
The standard method used to study sleep is called polysomnography . It uses electrode patches placed on specific parts of the animal's head and body to record electrical activity on a polygraph (resulting in readable data that looks like scribbled lines).
It records simultaneously the electrical activity of the brain, the eye movements, the muscles tone, the heart activity and the respiration of the animal. The electroencephalogram (EEG) is a measurement of bioelectric brain activity, by the mean of electrodes placed on the scalp. When looking at EEG levels, sleep researchers can clearly see distinctions between the different stages of sleep, as well as when the initial sleep onset actually occurs. The electro-oculography (or EOG) is the standard method used to measure eye movements during sleep. The front of the eye (the cornea) is electrically positive compared to the back (the retina). Therefore, when the eye moves the change in voltage can be recorded by electrodes placed on the sleeper’s face and recorded on the polygraph. The muscle activity is measured by a method called the electromyogram (or EMG). Muscles emit electrical potentials when they move, and that electricity is also detected by electrodes and recorded on the polygraph. EMG electrodes are usually placed over the animal’s chin muscles. Similarly, the electrocardiogram (ECG) records from the body surface and registers the differences in electrical potential generated by the heart.
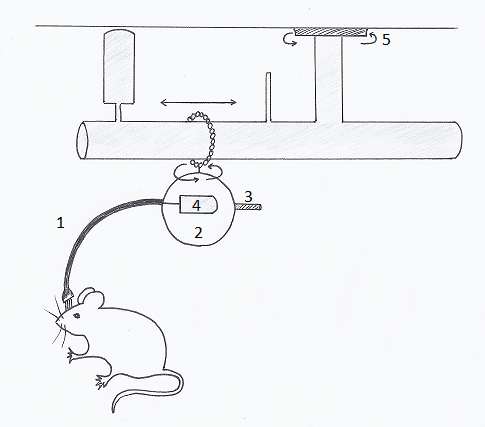
These systems involve either tethered systems that are restrictive and heavy on the animal or wireless systems using transponders that are large relative to the animal and surgically invasive for implantation, thus natural behavior/activity might be altered. But a study (Zielinski and al, 2013) using a novel telemetric system to measure polysomnography biopotentials in freely moving mice (Fig 1) showed good results in analysis of sleep architecture.
|
The mouse head cap containing EEG/EMG electrodes is connected to a telemetry transponder (4) encapsulated in a protective covering (2) by short cables contained in a lightweight protective sheath (1). This system is counterbalanced (3), rotates on an O-ring that swivels 360 degrees and slides horizontally between two contained ends, and possesses an additional swivel allowing the maximal range of movement within the mouse housing (5).
When do dreams happen during sleep?
Two states of sleep in mammals
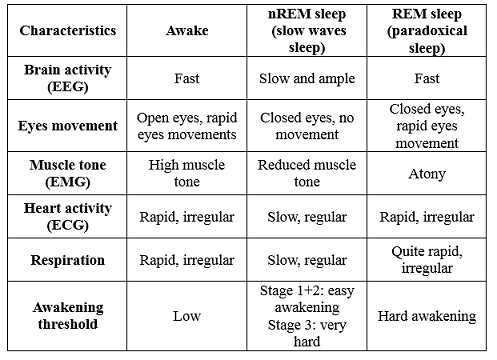
This type of studies - made since 1953 on humans (Aserinski, Kleitman and Dement, USA) and since 1959 on cats (Jouvet, France) – have proved that there is two distinct stages of sleep: the slow-wave sleep or nREM sleep (non Rapid Eyes Movement) and the paradoxical sleep or REM sleep, defined by their electrophysiological characteristics (Table 1).
|
These phases alternate cyclically in a highly structured pattern throughout the night. Those cycles last about 90 min in human and 28 min in cats. Jouvet (1994) has proved the existence of a duty ratio, calculated by dividing the average duration of sleep cycles by the average duration of REM sleep episodes. It is close to 4 in the vast majority of species (except rabbits and monkeys).
Dreams and REM sleep
At the end of the nineteenth century, a scientist first made the assumption that rapid eye phenomena were due to feelings and representations of dreams (Manaseina, 1899). Then, studies found that by waking his sleeping human patients during REM-sleep and interviewing them, they remember their dreams much clearly. So scientists made the hypothesis that most dreams occur during REM sleep (Dement et al, 1957).
However, the forced awakening of patients during nREM sleep gives equivocal results. Memorizing dreams is not demonstrated but in 8 to 30% of the cases, elementary mental activity defined as "dream" was found. (Cavallero et al, 1990)
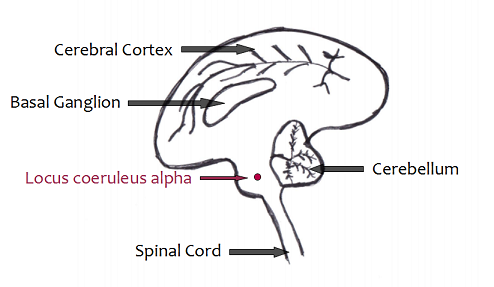
In animals, experiences on cats have proved that the destruction of a specific area, the locus coeruleus alpha (Fig 2), prevents muscle atonia normally observed during REM sleep (Jones, 1977). In fact, the cortical synchronization, rapid eye movements and the activity of the pons bear witness of REM sleep in these cats. However, animals simultaneously present motor behaviors characteristic of the species: predatory attack (round back, erection of hairs), fear, grooming, exploration. All of these behaviors are never referred to an element of the environment and the animal is largely insensitive to any external stimulus. The animals seem to "live" the dreams they have during REM-sleep.
|
Pontic origin of the REM-sleep
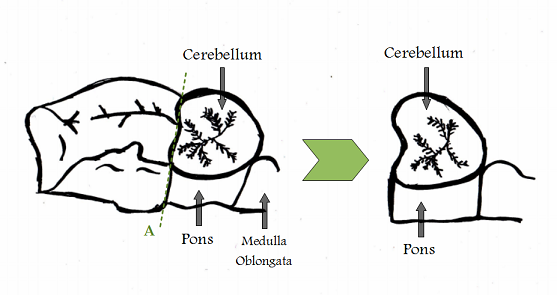
Michel Jouvet (1961), French researcher, found the cerebral origin of the REM-sleep by experimenting on “pontic cats”. It means cats whose brain stems (cerebral trunk) are connected to electrodes.
During the experiences, the part of the brain located forward to the pons is removed (Fig 3). The cats can still survive for several months if the following conditions are ensured artificially: thermal balance, nutritional and hydromineral supply. Symptoms of REM-sleep (rapid eye movements and periods of atonia) are observed in relationship with a ponto-geniculo occipital activity (PGO), proving that REM activity does arise from the pons. (Convers, 2005)
|
The REM basic mechanisms are responsible for two complementary functions. First, they involve an endogenous excitatory system of the brain through the PGO activity. This excitation is transmitted via neurons of the pontine reticular formation to sensory systems (mostly visual, explaining the rapid eye movement) and motor systems (pyramidal neurons of the motor area). These descending signals are conveyed to the spinal cord to trigger actions and behaviors. Secondly, to prevent the motor activity (atonia), another mechanism must arise: it consists on a powerful descending inhibition on motor neurons of the spinal cord. Thus, the dreamer is "paralyzed" and cannot move. (Jouvet, Conference 1996)
Which animals dream?
If we admit that REM sleep is accompanied by dreams, all animals that have REM sleep, should also have dreams. Similarly, the absence of REM-sleep in the other animals would preclude the existence of dreams (Tafti, 1996). However, dreams use stored elements, both in the short-term and long term memory. Even if the brain structures necessary for memory, exist in many of the animals we know very little about their memory capacity. Nevertheless, we must admit that the vast majority of animals (except maybe insects and fish) probably has traces stored, necessary for the realization of dreams.
A lot of studies have been carried out on over a hundred of species of mammals, the most studied ones, but also on birds, reptiles, fishes and even insects.
Problem in the definition of sleep states
The problem for defining sleep in general or the phases of nREM and REM sleep, is that standardized physiological criteria applicable for all species don't exist. The sleep state can be defined by the absence or reduction of activity and response to external stimuli, a specific body posture and an easily reversible state. The latter is crucial in order not to confuse that state with coma, hibernation or hypothermia. The behavioural signs of REM sleep are a partial or total atonia (especially in the neck), rapid eyes movements and sudden movements of the extremities.
Sleep states in warm blooded vertebrates
Mammals
A crucial point in evolution is the divergence between the phyla of live-bearing mammals (marsupials and placental) and egg-laying mammals (monotremes). Oviparous mammals, echidna and platypus, were first thought not to have REM. But, Siegel had them re-tested with newer technology and it appeared that they presented a kind of REM as well (Siegel et al, 1996). The echidna presents a less pronounced brain wave pattern, but had these tiny REM-like bursts more often. It seemed that the animal was having micro-dreaming throughout the night. The platypus showed many REM indicators too, except that there wasn't the low voltage REM characteristic of most mammals.
In all land viviparous mammals studied, REM-sleep exists. It is very easy to recognize by its classic criteria: activation of the EEG, suppression of muscle tone, rapid eye movements.
Modifications are implemented in marine mammals (Moukhametov, 1990), the largest of these being the need to rise to the surface to breathe. In dolphins and in some other cetaceans, sleep is unilateral. While one cerebral hemisphere shows typical signs of REM sleep, the other hemisphere is awake. As breathing is voluntary in these animals, this feature allows them to simultaneously perform two vital functions: sleep and breathe. The dolphin, which never stops to swim even while sleeping, do not seem to have REM sleep. This is an exception because other marine mammals studied, such as seals and sea lions, have a REM sleep comparable to terrestrial mammals. The possible lack of REM sleep in the cetacean is one of the biggest puzzles of the phylogeny of sleep.
Birds
A dozen bird species was studied with polygraph methods (Klein and al, 1964). There are notable EEG differences between the awake state (rapid activity and low voltage) and sleep (slow activity high voltage). During the behavioral sleep, there is indisputable evidence of the periodic appearance of REM sleep. It is characterized, as in mammals, by activation of the EEG by rapid eye movements and a decrease, sometimes not total, of the muscle tone. Complete atony of the neck, one of the mammals own characteristics, is rarely seen in birds, except the goose. These episodes are very brief (10 to 20 seconds) but are repeated often during the night. There are significant differences in the organization of sleep in different species. During migration, the albatross or swifts can fly nonstop for long periods. It is possible that these birds have a unihemispheric sleep, like dolphins.
Activity and inactivity states in cold-blooded vertebrates
Fishes
Many studies (Bauchot 1984, Karmanova 1979, Tobler 1992), mainly behavioral, has proved the existence of sleep-like in many fish species. Some sink into the sand to spend the night. Other changes color at night. Some parrotfish can secrete a mucous envelope in which they remain motionless all night. During these states rest, the fish react more slowly to sensory stimuli. In tench (Tinca Tinca), were recorded simultaneously the electrical activity of the brain, the activity of gills and different muscles, respiratory rate, heart rate and finally reactivity to sensorial stimulation (Hartse, 1994). There are no significant differences between the rapid and low voltage electrical activity of the brain during awake state (at night) or during rest (day). (Zhdanova, 2006, for similar results in the zebra fish). Moreover, no periods of eye movement accompanied by autonomic changes has been observed during resting period. There is therefore no evidence to suggest that periodic state, similar to REM sleep, is present in fish.
Amphibians
Amphibians are probably the most poorly studied taxa among tetrapods with sleep–wake data existing for only a handful of species (0.14% of all known species). Despite conflicting results from some studies, the amphibian species studied to date display behavioural characteristics of sleep (Libourel et al, 2015). The majority of studies did not report complete atonia or eye movements during the resting state suggesting the absence of an active sleep state in amphibians. However, one did report an ‘activation phase’ with an EEG similar to that observed during the awake state, motor automatisms, and a phasic transitory heart rate increase in R. temporaria. (Karmanova et al, 1979)
Reptiles
Three studies on turtles reported the presence of a mammalian active sleep-like state, and one the presence of mammalian quiet sleep-like state. Turtle is among the few reptiles believed to have REM sleep with rapid eye movement and suppression of muscle tone of the neck. In crocodiles, in contrast to other reptilian species, the EEG amplitude and frequency during sleep-like states appears to change, similar to what is observed during mammalian quiet sleep. Only one study reported eye movements and motor automatisms during behavioural sleep not associated with muscle atonia. All studies agree on the presence of sleep-like states in squamates, but the diversity of findings prevents clear conclusions regarding the electrophysiological nature of sleep in these animals. None of the studies on squamates performed to date have examined all of the electrophysiological and physiological traits that allow the characterization of active sleep in mammals. As a conclusion, if reptiles do have REM, it must be quite different from mammalian REM. Reptiles don’t have the brain development of mammals and don't show the extreme EEG differences that mammals do between wake and sleep. Further, reptiles don't seem to have atonia during sleep. This means that the three measurements, brain activity, input/output gating and neuromodulation are all going to be quite different, if they exist at all (Libourel et al, 2015).
Sleep-like state in invertebrates
Sleep research in invertebrates started with bees, cockroaches and scorpions (Kaiser, 1983; Tobler, 1983). More recently, Drosophila (Hendricks, 2000), and the roundworm C. elegans were discovered as promising models for sleep research (Raizen, 2008). In the beginning, the field was rather preoccupied with proving the existence of sleep in invertebrate species. The research strongly contributed to the implementation of general criteria for the definition of sleep. As no typical mammalian EEG signal is present in these species, the identification of sleep-like states in invertebrates relies primarily on behavioral signs like inactivity and the presence of a specific body posture, an increased threshold to arousing stimulation, as well as the demonstration of a rebound in the sleep-like state that occurs as a consequence of experimental sleep deprivation (Vorster et al, 2015). In fact, invertebrates studied so far like honey bees and flies, with the exception of crayfish, seem to lack synchronous neuronal activity during sleep (Mendoza-Angeles et al., 2007). Moreover, sleep intensity seems to vary as seen in cock-roaches, bees and flies suggesting the existence of different sleep stages possibly serving different functions (van Alphen et al., 2013). But no proper REM sleep as in mammals has been observed.
Factors influencing the amount and rhythmicity of REM-sleep
Studies have highlighted some correlations regarding the amount of REM sleep in mammals and birds:
¤ The average duration of REM sleep episodes increases with the increase in body and brain weight (Parmeggiani, 2011).
¤ The more an animal is immature at birth, the more he is likely to have big amount of REM sleep. The amount of REM sleep then decreases with ontogenesis (Jouvet-Mounier, 1968).
¤ The safety of the refuge and the availability of food positively influences the amount of REM sleep. Ruminants, which need considerable amounts of grass to meet their food needs, sleep and have very little REM sleep when grazing, but sleep more and have a greater amount of REM sleep if they are to the barn with unlimited access to food. (Allison et al, 1976).
¤ There is a conjecture that REM changes with the predatory/prey conditions of an environment. Predatory animals show more REM than herd and prey animals (Allison et al, 1976).
¤ Low temperatures usually decrease the amount of REM sleep (Haskell et al, 1981).
¤ Sleeping postures that need a minimal muscle tone are less compatible with REM sleep.
REM sleep is not continuous during sleep: it appears periodically and its rhythmic recurrence structures sleep cycles. Periodicity is unique for each animal species: it occurs roughly every 4 minutes of sleep in mice, every 12 minutes in squirrels, every 27 minutes in cats, every 60 minutes in the horse, every 90 minutes in humans and every 100 minutes in the elephant.
As other physiological parameters such as the duration of the cardiac, respiratory or sexual cycles, the periodicity of the REM sleep is inversely proportional the basal metabolic rate of the animals (exceptions are ruminants). Thus the mouse, which has a basal metabolic rate 25 times greater than the elephant (that is to say, it consumes twenty five times more oxygen per gram of body weight per hour), has REM sleep 25 times more often the elephant. If the dream mouse more frequently, she dreams shorter than the elephant.
Phylogeny of sleep states
By now, it is proven that birds and two of the three groups of mammals - placental and marsupial - do have REM sleep. A theory proposed that this phase of « dreaming » would be an evolutionary advance. Indeed, the purpose of it would be to keep the brain warm and sprightly, and avoid to fall deeply in state of cerebral inactivity. (Siegel, 2005)
The common ancestor of birds and mammals are reptiles, so we would expect that instead of an independent development of REM sleep in those classes, there exists a common reptilian ancestor with REM. Nevertheless, the presence of REM is hard to determine in reptiles. But if Siegel is right, there is a form of REM-sleep in reptiles and it would go along with the theory of a common ancestor. (Siegel, 1999)
Besides, among mammals, the amount of REM does not seem to be related neither to the degree of evolution nor to the size of the brain. Primates with higher intelligence, have less REM than rats. Furthermore, although the amount of REM-sleep per species seems to be stable, it can vary significantly within an Order. This observation makes us conclude that it is not directly correlated with the evolution of that Order. (Wilkerson, 2003)
Scientists asked themselves about the third group of mammals: monotremes, represented by echidnea and platypus, most ancient group of living mammals. In both cases, we observe a special pattern: it seems that monotremes have a single sleep state that combines elements of nREM and REM sleep. This suggests that these states were first blended and became segregated afterwards during evolution. REM-sleep would have evolved after the three branches of mammals split (150-200 million years ago), but while placental and marsupials were still together. Indeed, the hypothesis of an independent evolution within the groups after they were separated seems less likely. (Siegel et al, 1998)
To understand the phylogeny of REM-sleep in the class of birds, a group of researchers studied the sleep of ostriches as they are members of the most basal group of living birds. They found a unique cerebral pattern in those animals when they were sleeping. Sequences of REM sleep presented characteristics similar to those observed in other birds and mammals engaged in REM sleep. However, ostriches' forebrain activity varied between REM-like activation and SWS-like slow waves, just like we observed in the monotremes. This discovery tends to confirm the way that sleep evolved. The evolution of sleep in mammals and birds seems to own a common scheme: SWS and REM sleep arising from a single heterogeneous state and then becoming segregated into two distinct states later on. What's more, the forebrain activation during REM sleep appears to be an evolutionarily new feature that would be involved in performing new sleep functions not found in more basal animals. (Lesku et al, 2011)
Dreams content in animals
Animals can present gestures during their sleep, which looks like the reflexion of their «dreams» : running, barking, etc. In 2001, researchers at the Massachusetts Institute of Technology (Louie et al, 2001) performed experiences on rats to try to understand better what the cerebral activity during REM-sleep consists of. They monitored the animals ‘hippocampus while they were either learning to run through a maze for a food reward or sleeping. In about half of the REM sleep episodes, they found the same unique patterns of neuron firing that were recorded during the task. The correlation was so close that researchers were even able to reconstruct where it would be in the maze, and whether it was dreaming of running or standing still. First of all, this tends to confirm that animals do have complex dreams as humans do. Secondly, even if we still ignore if there would be other contents of their dreams, it is now proven that some of them concern pas events they experienced and stored into their memory. Furthermore, this is also interesting regarding the role of REM sleep.
Roles of dreaming for animals
The experience described above makes us believe that REM-sleep would be a phase in which rats could re-process and memorise the information collected during awakeness.
Interestingly, researchers of MIT also discovered that such a replay occurred during non-REM sleep or the so-called slow-wave sleep. Though, this replay is very different from the one above: unlike the REM one where it lasts the real time of the event, the NREM one is experienced at higher speed. What's more, this replay appeared right after the event, whereas the “off -line” practice sessions in REM sleep could still be found in the brain patterns after 24 hours. Thus, the slow-wave sleep replay appears more as an initial storage process, while the REM sleep replay would consist in recalling and analysing older memories (Halber, 2003).
Trying to understand the role of “dreaming”, or at least REM sleep, is complicated. One method that is often used by scientists is the sleep deprivation. Two studies focused on the effect of REM sleep deprivation on the development of the hippocampus. The first researches (Guzman-Martin et al, 2008) concern a special part of the hippocampus, the dental gyrus, which is composed of many progenitor cells able to differentiate into neurons. As a consequence of a decrease in REM sleep, the percentage of those proliferating cells was strikingly reduced.
In a second study (Ravassard et al, 2009), researchers observed a reduction in transcription of several neurotransmitters and a significant decrease in synaptic transmission in the dorsal hippocampus. Furthermore, the latter, according to a study (Fanselow et al, 2010), appears to be specifically involved in memory function whereas the ventral pole would modulate emotional and affective processes.
Thus, it seems to be that REM-sleep plays a role in both neurogenesis and functioning of the hippocampus. The fact that it concerns this particular cerebral area is interesting. It shows one more time the involvement of REM sleep in the processes of learning and memorisation.
References
Articles
1. Allison, T., & Cicchetti, D. V. (1976). Sleep in mammals: ecological and constitutional correlates. Science, 194(4266), 732-734.
2.Bauchot, R. (1984). La phylogénie du sommeil chez les vertébrés. L'Année biologique, 23(4), 367-392.
3. Cavallero, C., Foulkes, D., Hollifield, M., & Terry, R. (1990). Memory sources of REM and NREM dreams. Sleep: Journal of Sleep Research & Sleep Medicine.
4. Dement, W., & Kleitman, N. (1957). The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. Journal of experimental psychology, 53(5), 339.
5. Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron, 65(1), 7-19.
6. Guzman-Martin, R., Suntsova, N., Bashir, T., Nienhuis, R., Szymusiak, R., & McGinty , D. (2008) Rapid Eye Movement Sleep Deprivation Contributes to Reduction of Neurogenesis in the Hippocampal Dentate Gyrus of the Adult Rat. Journal of Sleep 31(2): 167-175
7. Hartse, K. M. (1994). Sleep in insects and nonmammalian vertebrates. Principles and Practice of Sleep Medicine, 2nd ed. Philadelphia: WB Saunders, 95-104.
8. Haskell, E. H., Palca, J. W., Walker, J. M., Berger, R. J., & Heller, H. C. (1981). The effects of high and low ambient temperatures on human sleep stages. Electroencephalography and clinical neurophysiology, 51(5), 494-501.
9. Hendricks, J. C., Finn, S. M., Panckeri, K. A., Chavkin, J., Williams, J. A., Sehgal, A., & Pack, A. I. (2000). Rest in Drosophila is a sleep-like state. Neuron, 25(1), 129-138.
10. Jouvet M. (1994). Phylogénèse des états du sommeil. Acta psychiatrica Belgica 94(4-6):256-67.
11. Jouvet-Mounier, D. (1968) Ontogenése des états de vigilance chez quelques mammifères. Imprimerie des Beaux-Arts. unreferenced page on google scholar.
12. Kaiser, W., & Steiner-Kaiser, J. (1983). Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect.
13. Karmanova, I. G., & Lazarev, S. G. (1979). Stages of sleep evolution (facts and hypotheses). Waking and sleeping, 3(2), 137-147.
14. Klein, M., Jouvet, M., & Michel, F. (1964). Etude polygraphique du sommeil chez les oiseaux. Comptes-rendus des seances de la societe de biologie et de ses filiales, 158(1), 99.
15. Lesku, J. A., Meyer, L. C., Fuller, A., Maloney, S. K., Dell'Omo, G., Vyssotski, A. L., & Rattenborg, N. C. (2011). Ostriches sleep like platypuses. PLoS One,6(8), e23203.
16. Libourel, P. A., & Herrel, A. (2015). Sleep in amphibians and reptiles: a review and a preliminary analysis of evolutionary patterns. Biological Reviews. doi: 10.1111/brv.12197, page non referenced on Pub Med.
17. Louie, K., & Wilson, M. A. (2001). Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron, 29(1), 145-156.
18. Manaseina, M. M. (1899). Sleep: Its Physiology, Pathology, Hygiene, and Psychology. publisher not identified.
19. Mendoza-Angeles, K., Cabrera, A., Hernández-Falcón, J., & Ramón, F. (2007). Slow waves during sleep in crayfish: A time–frequency analysis. Journal of neuroscience methods, 162(1), 264-271.
19. Moukhametov, L. (1990). The Sleep Of Marine Mammals. Recherche, 21(217), 40-47.
21. Parmeggiani, P. L. (2011). Systemic homeostasis and poikilostasis in sleep. London: Imperial College Press.
22. Raizen, D. M., Zimmerman, J. E., Maycock, M. H., Ta, U. D., You, Y. J., Sundaram, M. V., & Pack, A. I. (2008). Lethargus is a Caenorhabditis elegans sleep-like state. Nature, 451(7178), 569-572.
23. Ravassard, P., Pachoud, B., Comte, J. C., Mejia-Perez, C., Scote-Blachon, C., Gay, N., ... & Salin, P. A. (2009). Para-doxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hip-pocampus. Sleep, 32(2), 227-240.
24. Siegel, J. M. (1999). The evolution of REM sleep. Boca Raton, FL: CRC Press. 87-100.
25. Siegel, J. M. (2005). Clues to the functions of mammalian sleep. Nature, 437(7063), 1264-1271.
26. Siegel, J. M., Manger, P. R., Nienhuis, R., Fahringer, H. M., & Pettigrew, J. D. (1996). The echidna Tachyglossus aculeatus combines REM and non-REM aspects in a single sleep state: implications for the evolution of sleep. The Journal of neuroscience, 16(10), 3500-3506.
27. Siegel, J. M., Manger, P. R., Nienhuis, R., Fahringer, H. M., & Pettigrew, J. D. (1998). Monotremes and the evolution of rapid eye movement sleep. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 353(1372), 1147-1157.
28. Tafti, M. (1996). Animal rêveries. Science et Avenir Hors-Série Le Rêve, 82-89
29. TOBLER, I., & NEUNER‐JEHLE, M. A. R. T. I. N. (1992). 24‐h variation of vigilance in the cockroach Blaberus giganteus. Journal of sleep research, 1(4), 231-239.
30. Tobler, I. (1983). Effect of forced locomotion on the rest-activity cycle of the cockroach. Behavioural brain research, 8(3), 351-360.
31 Van Alphen, B., Yap, M. H., Kirszenblat, L., Kottler, B., & van Swinderen, B. (2013). A dynamic deep sleep stage in Drosophila. The Journal of Neuroscience, 33(16), 6917-6927.
32. Vorster, A. P., & Born, J. (2015). Sleep and memory in mammals, birds and invertebrates. Neuroscience & Biobehavioral Reviews, 50, 103-119.
33. Zielinski, M. R., Gerashchenko, L., Karpova, S. A., & Gerashchenko, D. (2013). A novel telemetric system to measure polysomnographic biopotentials in freely moving animals. Journal of neuroscience methods, 216(2), 79-86.
34. Zhdanova, I. V. (2006). Sleep in zebrafish. Zebrafish, 3(2), 215-226.
Books
1. Jouvet, M. (2013). De la science et des rêves: mémoires d'un onirologue. Odile Jacob.
Websites
1. Convers, P., Jauzein, F. (2005) Structures impliquées dans la génèse du sommeil paradoxal.
2. Jouvet, M. (1996) Conference report. Le Sommeil, les rêves et l'éveil. 11 April 2016.
3. MIT News, Rats replay tasks during slow wave sleep. Massachussets Institute of Technology. 10 April 2016
4. Wilkerson, R. (2003 January). The Evolution of REM Dreaming: New Research Includes All Mammals. Electric Dreams.
Figures references
All the figures have been re-drawn by the students
Fig 1. : Original title: "Schematic of the components of the new system"
Realized by Zielinski and al (2013) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3679249/figure/F1/
Fig 2. : Original title: "Localisation du Locus Coeruleus alpha"
Realized by: Jauzein F. (2010) http://acces.ens-lyon.fr/acces/ressources/neurosciences/sommeil/DossierScientifSommeil/SommeilParadoxal/StructurGenesSommeilParadox
Fig 3. :0riginal title: "Localisation des sections réalisées pour obtenir un "chat pontique" (section en A)"