Itt írjon a(z) ElectricOrgansFish-ról/ről
Electric Organs of Fish:
Introduction:
History:
abcThe functioning of electric organs has only in the past 60 years become more well understood. In the late 18th century it was generally understood that electric fish such as Electrophorus electricus (Fig.5) used electric discharges originating from specific electrogenic tissue while some fish were suspected to produce electric discharges that, at the time, couldn’t be detected (Markham, 2013). In the 19th century, Charles Darwin, during his investigation of the electric eel noted his suspicions of the convergent evolution of electric organ (Darwin, 1869). At this point in time, not much was known about how these organs had evolved independently, and indeed, a great number of mysteries surrounding the electric organ evolution are yet to be uncovered. It wasn’t until 1951 when Lissmann did the first comprehensive overview of electric organ function in a variety of fishes and layed out the foundation for future research on the subject (Lissmann, 1951).
Overview and Evolution:
The ability to use biologically produced electric currents for the purposes of location of objects, orientation to the environment, communication and hunting is called electrogenesis. While many water-restricted vertebrates can recognize the presence of electric fields not all of these organisms can produce them (Kramer, 1996). In order to be able to produce electric currents, the presence of an electric organ is required, and while the functioning of such an organ is very complex, surprisingly, it has evolved at least six times independently (Gallant et al., 2014). This has occurred twice in the case of the rays, and among teleosts, four times (Kramer, 1996).
In electric fishes, the electric sense dominates over any other, being the preferred means of obtaining information from the outside world. While eyesight is still used to learn about the environment, it is generally poorly developed (Dangelmayer et al., 2016; Lissmann, 1951). This is to be expected, most electrosensitive fish come from freshwater, an environment that generally lacks clarity, as such the ability to find objects by non-visual means would prove to be more useful in such a niche.
Electric fish are generally classified as being weakly or strongly electric depending on the strength of their discharges (Kramer, 1996). A lot of progress has been made with regards to the anatomical, neural and hormonal aspects and their respective implications on the fish’s general behaviour in both cases.
Microstructure
Electric Organs consist of electrocyte cells with an efferent innervation to brain ganglia or nuclei. The respective influx of Sodium ions followed by the subsequent efflux of potassium ions through their respective channels on both sides of the electrocyte are responsible for producing an action potential(AP) which causes an electric organ discharge (EOD) On the other hand electroreception occurs through afferent innervation of the electroreceptors in the skin. Both these systems work together to help the fish produce and detect electric signals on its own.
Development of Electrocytes:
Electrocytes are derived from muscle cells (myocytes). During development, the myocytes fuse and transform. Their membranes now contain a great number of voltage-gated ion channels and ion transporters instead of muscle proteins (Stoddard, 2015).
Channels and Innervation of Electrocytes:
The electrocytes are not innervated on one side, instead a large number of papillae are present to increase the surface area. On the other side they are innervated by spinal nerves(Kramer, 2009).The electric organ is enveloped by connective tissue, with each electroctye being innervated by a spinal electromotor neuron via a nicotinic cholinergic synapse(Bennett et al, 1967; Kramer, 1996). The neural command signal must reach each cell together, as the excitation of one electrocyte prevents its neighboring cell from firing, which is why each electrocyte is innervated by a separate motor nerve(Bennett, 1971)
Myocytes and electrocytes contain actin, myosin, desmin, and Na+ channels in varying quantities(Ayres Sá et al, 2001) To modify electric activity, Na+ channels act along with other channels(eg. K+) (Zakon et al, 2006)
NaV1.4, is a Na+ channel gene. The genes in gymnotiformes are NaV1.4a and NaV1.4b. NaV1.4a is expressed only in the electrocytes with no variation of the levels. NaV1.4b has a long (NaV1.4bL) and a short (NaV1.4bS) variant. It is expressed in muscles and the electrocytes.(Markham, 2013)
The Kv1 gene is a voltage dependant K+ channel, its has 3 expressions in Gymnotiformes- Kv1.1a, Kv1.2a, and Kv1.2b. In fish with high EOD frequencies, Kv1.1a and Kv1.2a are in high levels whereas in fish with low EOD frequencies, Kv1.1a and Kv1.2a are in low levels with Kv1.2b as the main subunit. (Stoddard et al, 2006)
Arrangement of electrocytes and its relation to function:
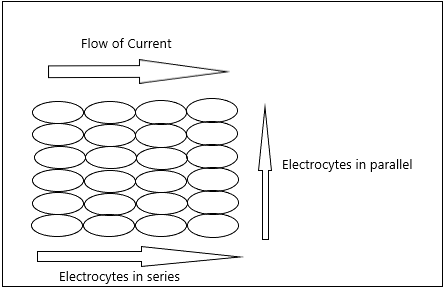
Electric organs have groups of electrocytes arrayed in series, they can be arranged in parallel to increase current(Fig 1). The skin is polarized due to flow of sodium ions into the electrocytes in the electric organ, if the flux of sodium ions is in the headward direction, the head of the fish is positively polarized because these fluxes sum through the organ and vice versa(Stoddard, 2015).
|
Fig.1 Arrangement of electrocytes in series and parallel |
Electric Organ Discharges(EODs):
The pulse discharge in strong electric fish is monopolar, while in weak electric fish it is bipolar (Kramer, 1996). The pulse-type EODs are short and separated by periods of electric silence, there can be between 1 and 6 phases. Wave type EODs are continuous, there is no electric silence and have between 1 and 4 phases. The frequency of wave type discharges is greater than in pulse type as can be seen in Table 1 (Albert and Crampton, 2005)
Table 1 |
Pulse type |
Wave type |
Mormyrid |
1-150Hz |
250-300Hz |
Gymnotiformes |
|1-120Hz |
20-2200Hz |
Electroreceptors:
There are 2 types of electroreceptors: ampullary and tubulary. Ampullary electroreceptor cells are used for detecting electric fields. Teleosts have only microvilli, while non teleosts can have a kinocilium as well as microvilli (Szabo, 1974). Tubular electroreceptors are used to detect Electric Organ Discharges(EODs), they consist of epithelial cells to create a capacitance in series with the receptor cells (Tricas and Carlson, 2012).
Function:
Electric fish are divided into two major groups based on the strength of their EOD. Strong electric fish’s EOD falls between 10 to 600V and is typically used for predatory and defensive purposes (Albert and Crampton, 2005). Weak electric fish discharge is less than 1V, these include fish from Gymnotiformes order (South African knifefish), Mormyridae & Gymnarchus species from the Osteoglossiformes order (Stoddard, 2010).
Weak Electric Fish:
Active Electrolocation:
Active electrolocation is the ability of weak electric fish to recognize distortions in there own EOD for navigational purposes by differentiating between the electrical properties of objects (which cause characteristic distortions of the EOD) from those of the neighbouring water. The recepted signal is translated to an electrical image on it is skin depicting information on the size, location and material of the object (Caputi et al, 1998 ; Babineau 2006). This ability is of great importance for nocturnal electric fish species (Emde, 2007)
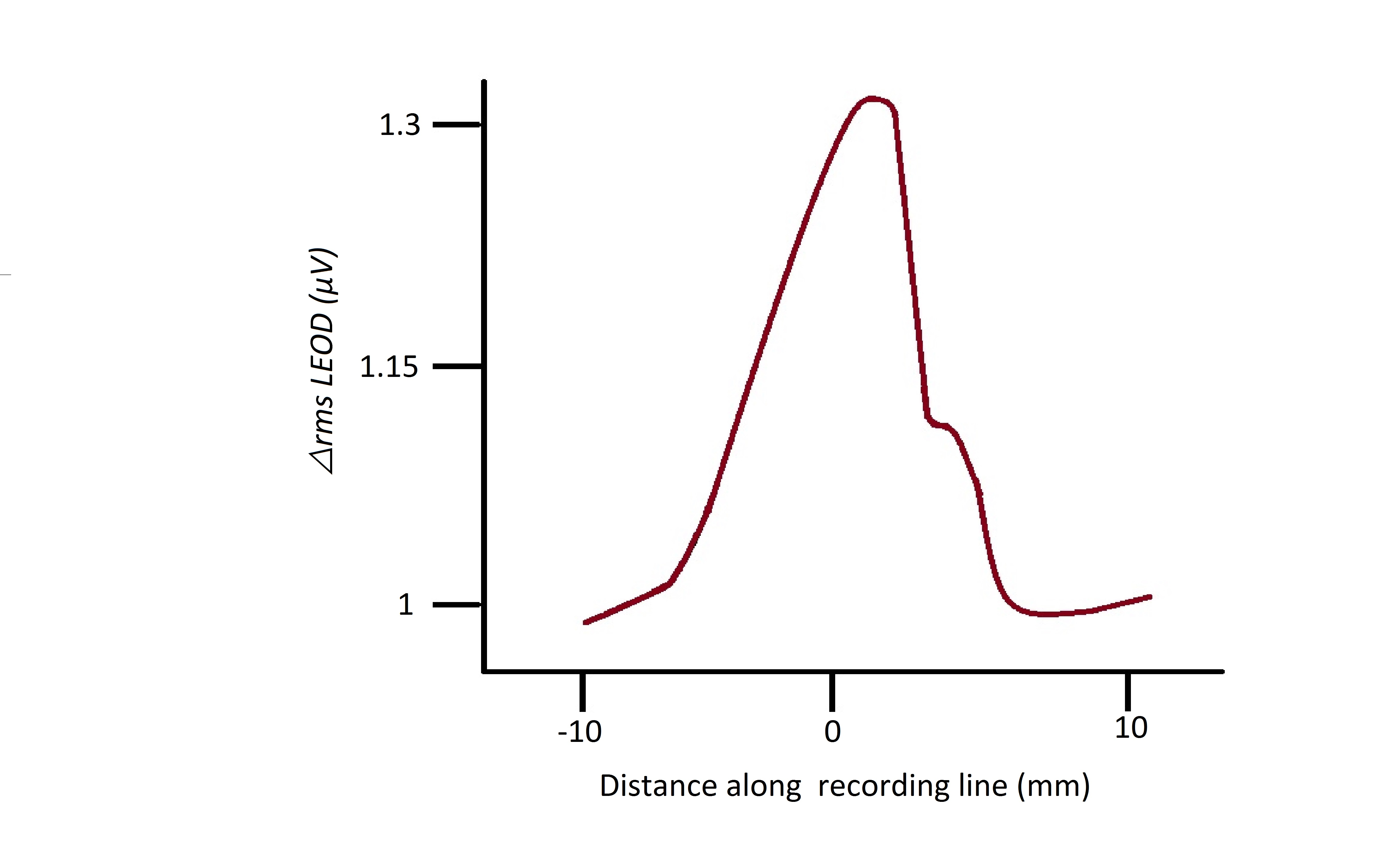
An object’s distance from the fish is determined by the area of skin stimulated during electroreception. Several electrical properties modify the amplitude of the EOD, as such recognition of the object's properties usually relies on more than one reading than just change in amplitude. Distance is an exception, it is calculated with soley amplitude(Gottwald et al, 2017). The electroreceptors closest to the object translating the EOD, register an increase amplitude, these electroreceptors would be surrounded with other electroreceptors of decreased amplitude (Caputi et al, 1998).The mexican hat profile (fig. 2) characterizes this mechanism. von der Emde showed that object distance is independent of size, shape and material of the object (Emde, 2004).There is no focusing mechanism for electric images, as such objects can be effectively recognized at maximum of 4 cm distance from the fish (Emde, 2010).
The EOD’s peak-to-peak amplitude drop in an electroreceptor compared to that of the electroreceptors around it also gives an indication of the object’s conductivity. The electrical resistance of the object is characterized by it’s impedance, a high impedance object (non-conductor) will translate into a lower EOD peak-to-peak amplitude and vice versa. recording at local electroreceptors. Since non-conductors will have a lower change in rmsLEOD (root mean square of local self generated field) their mexican hat profile character is horizontally inverted compared to a conductor’s profile as seen in (Fig 2) (Emde, 1999) .
|
Fig. 2 Mexican-Hat profile Local EOD (LEOD) change at receptors is calculated as change in spatial patterns’ root mean square (△rmsLEOD)(Caputi et al, 2011). |
Capacitance detection is used to discriminate between animate and inanimate objects, both Mormyridae and Gymnotiformes are capable of this however the mechanism is not yet fully understood in Gymnotiformes. In Mormyridae, capacitance of an object (the ability to store electrical charge) is measured via the EOD amplitude & the EOD waveform and timing. The presence of membrane electrical gradients in living animals can be detected as capacitance values(Emde, 1999).
Jamming Avoidance Response:
Two weakly electric fish in each other’s vicinity with similar EOD frequencies will alter their EOD frequency, the fish with the lower EOD will lower it is EOD and vice-versa (Watanabe and Takeda,1963). The resultant alteration is termed J.A.R. (Jamming Avoidance Response), it is due to the interaction of the 2 electric fields producing ‘beat’ patterns with a frequency called ‘beat rate’. The beat rate frequency is identical to the difference between the frequency of the 2 electrical fields, depending on the range of this frequency the electrosensory ability of the fish may weaken. Eigenmannia viscerens (ghost knifefish)(Fig 3) will exhibit J.A.R. behaviour if the beat rate frequency is in the range of 3-8Hz(Tan et al, 2005). A beat rate frequency of 4Hz elicits J.A.R. behaviour in Gymnarchus niloticus (African knifefish) (Heiligenberg, 1975).
|
Fig. 3 Eigenmannia virescens |
Electrocommunication:
Weak electric fish are known to communicate via discharging a modulated EOD targeted for reception by another fish. Communication related to EOD modulation is deciphered into courtship and agnostic information (Ho et al, 2010). The EOD of a particular species is typically uniform and characteristic when not being used for communication. Modulations of the EOD are categorized into chirps and gradual frequency rises (GFRs) on the basis of change in frequency over a period of time. Chirps are large frequency changes (50-600Hz) over a short duration (10-1000ms) while GFRs are characterized by a frequency change of <100Hz over a longer duration (10ms-60s)(Turner et al, 2007).
Fish belonging to the Mormyridae family send information pertaining to their species’s sex and size with their characteristic, invariable EOD. They are able to modify their inter-discharge-interval (IDI) to vary information sent, this has been shown in recordings of Mormyrus rume discharge during different behavioural periods shown in Table 2. It is apparent that IDI dynamics can be used to determine behavioural state governed by the current activity the fish is partaking in. Determination of these behavioural states are not solely done by researchers but as is shown by synchronization behaviour, certain IDI patterns instigated an echo response between one another(Emde et al, 2012). IDIs may also be relevant in species discrimination, this has been shown inPetrocephalus bovei and Campylomormyrus rhynchophorus (Baier and Kramer, 2007).
Table 2 |
Resting behaviour |
Aggressive behaviour |
Foraging behaviour |
Inter-discharge interval |
180-250ms |
8-80ms |
16-50ms |
Average discharge frequency |
4.8Hz |
13.9Hz |
28.3Hz |
A separate study carried out on Mormyrus rume proboscirostris found that there was a direct correlation between the hierarchy and the peak-to-peak EOD amplitude, weight and size of the fish (Worm et al, 2017). In Eigenmannia virescens(fig 3) and Apteronotus leptorhynchus (Gymnotiformes) hierarchy level has also been linked to EOD amplitude, frequency and waveform (Hagedorn and Heiligenberg, 1985). pH also plays a role in the frequency of the EOD (Squire and Moller, 1982)
Sexual dimorphism also plays a role in the modulation of EOD, in this regard a great degree of species difference can be noticed. Androgens play a role in the development of sexual dimorphism which are accredited to morphological changes such as thickening of the skin, which in turn alters the electrocyte function. Male Sternopygus macrurus (Fig 4) and Apteronotus albifrons discharge at a lower frequency and longer waveform (retaining the species characteristic wave) (Hopkins, 1988) . Male Apteronotus leptorhynchus and Apteronotus rostratus discharge at higher frequencies than their female counterparts (Ho et al, 2010) . Eigenmannia virescens(fig 3) and Apteronotus leptorhynchus courtship involves the male continuously discharging marginal higher frequency chirps, in an attempt to stimulate spawning by the female (Hagedorn and Heiligenberg, 1985). Sternopygus macrurus(Fig 4) EOD’s modulation related to courtship has been observed to follow a pattern of a rise in frequency followed by a decrease to resting discharge frequency. The resting discharge frequency is different in males and females, the oscillations in frequency play a role in attracting and recognising potential mates (Hopkins, 1974).
|
Fig. 4 Sternopygus macrurus |
Strong Electric Fish
Strong electric fish are capable of discharging within 10-600V (Albert and Crampton, 2005), this high voltage EOD is used to stun during predatory or defensive behaviour. EODs of these fish last 0.03s, they are fired in rapid successions of pulses termed trains. A shock is a succession of trains. The electromotive force of the EODs is species specific and varies with the condition of the fish (Tee-Van, 1948). Strong electric fish include species belonging to Torpediniformes, Paradoxoglans, Malapretus, Electrophorus and stargazer family. The order of Torpediniformes (Electric rays) is further subdivided into the families Torpedinidae, Hypnidae and Narcinidae(Nacinidae and Narkidae subfamilies).
Electric rays:
Hypnos monopterygius (Coffin ray) is the only member of the family Hypnidae, commonly thought of as a subfamily for Torpedinidae. Torpedo marmorata(Marbled torpedo ray) can produce an EOD of up to 200V with a frequency of up to 200Hz (Fishbase.org Torpedo Marmorata summary page). P.Belbenoit (1985) described 2 predatory behaviours in this species, jumping and creeping responses. Along with this 2 defensive behaviours were also described. All of these behaviours use EOD sets to stun and immobilize their target. The sequence of events during predatory jumping behaviour are the jumping response followed by an engulfing response and suction responses. During the jump response an immediate tail stroke lifts the ray, subsequent gliding. The first EOD pulses are discharged during the jump response. The aim of the fish is to entrap it is prey beneath it's disc, when accomplished the movement of the prey underneath the discs stimulates the engulfing response. The engulfing response can be split into 2 phases, the first phase involves stunning the prey with EODs while phase 2 involves bringing the prey closer to the mouth with a tailstroke. Torpedo nobiliana (Atlantic torpedo ray) can produce EODs within the range of 50-60V (Grundfest, 1960), however up to 220V ad 600HZ have been recorded (Sharktrust, n.d.). Hypnos monopterygius has been observed to deliver up to 50 successive shocks in 10 mins. Voltage and power of EOD progressively decline with increasing fatigue. Close relation to torpedos, Hypnidae & Torpedos have no labial cartilage connecting two jaws, other electric rays do. (Tee-Van, 1948).
Electric eel:
The Electrophorus electricus (Electric eel)(Fig 5) belongs to the order of Gymnotiformes, it is the only strong electric fish in this group. It possesses 3 electric organs (the main organ, Hunter’s organ and Sach’s organ) which are capable of producing 2 types of EOD, a high voltage one and a low voltage one. The main organ and ⅔ of Hunters organ are responsible for the high voltage discharge which can reach up to 600V (+100V per 30 cm body length). Sach’s organ along with ⅓ of Hunters organ produces low voltage discharge of 1-10V (Albert and Crampton 2005). Low Voltage EOD is used for active electrolocation and electrocommunication. A continuous low voltage discharge is produced with varying frequency during rest (1Hz) and during swimming (10Hz). Low voltage EOD activity is higher during the night. Electric eels use their strong electric discharge to hunt by immobilizing prey, this is done by stimulating the prey's efferent neurons, subsequently eliciting muscle contraction in turn evokes tetanic contractions of the prey’s muscle(Catania, 2015a). K. Catania (2014) recorded high frequency (400 Hz) high voltage EOD pulses just 10-15ms before the eel strikes, he noted it took 3-4ms for the prey to be immobilized.
|
Fig. 5 Electrophorus electricus |
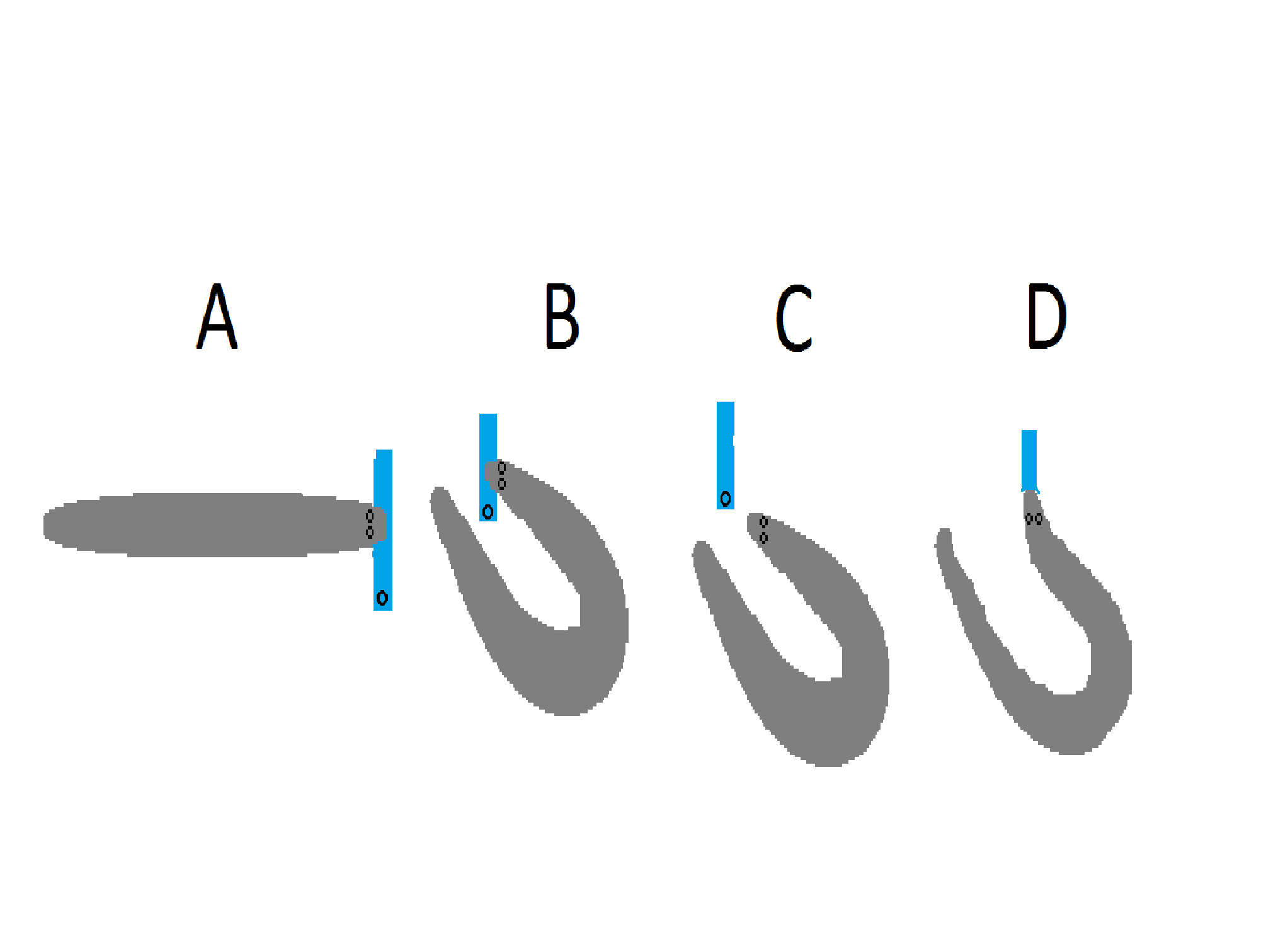
Curling behaviour further strengthens the shock by intensifying the electric field, this is done by placing the tail (negative pole) behind the prey, since the head is the positive pole the electrical field is greatly intensified between these 2 extremities. Curling behaviour is used when dealing with large prey (which are more resistant) and when repositioning of captured fish (in mouth) for swallowing is necessary. Repositioning starts with the electric eel capturing its prey with a high voltage discharge and an initial strike, the prey is captured lengthwise, as such it cannot be swallowed (This can be seen in Fig. 6A). The eel orients its head and tail into curling position and the fish is released simultaneously with high voltage discharges as can be seen in Fig. 6B&C. A new opportunity for the eel to capture the fish presents itself (Fig. 6D). It can be commonly observed in young eels and in medium sized eels but rarely in large eels, since the high voltage discharge increases by 100V for 30cm body length it seems logical that juvenile and medium sized eels would struggle more when hunting hence profiting greatly from this mechanism (Catania, 2015a). It has also been shown that the high voltage discharge is also used to locate prey during striking, modification of strike direction (Catania, 2015b).
|
Fig 6. Curling behaviour of Electrical eel 4 Stages of curling behaviour. A= capture fish requiring reposition for swallowing. B= Tail and head orient close to each other in preparation of high voltage discharge. C= Simultaneous release and immobilization of prey via high voltage discharge. D= Reposition & swallowing successful (Catania, 2015a) |
Regulation:
Regulation of electric organs consists of a variety of mechanisms that act on both the short term and the long term. Changes in the short term (milliseconds) are under neural control, those in the long term (days to weeks) are under steroid hormone control (mainly androgens and oestrogens), while changes in an intermediate length of time (minutes to hours) are down to control by melanocortin peptides (Stoddard et al., 2006).
Neural Regulation of the EOD:
The neural aspects of regulation are mainly under the control of the medullary command nucleus (MCN), a cluster of between 50 to 100 pacemaker neurons (Stoddard et al., 2006). This nucleus is on the receiving end of projections coming from two other major nuclei, those being the precommand nucleus of the mesencephalon (PCN) as well as the dorsal posterior nucleus of the thalamus (DPN); however it is the medullary command nucleus that initiates the electric organ discharge itself (Carlson, 2003). From the aforementioned nuclei, the PCN has been shown to be excitatory in nature to the MCN (von der Emde et al., 2000) while the DPN is yet to be described.
The neurons of the MCN are extensively synaptically interconnected to each other and to relay cells forming the medullary relay nucleus (MRN) (Elekes & Szabo, 1981). Such close synaptic relationships between neurons allows for the synchronous firing of action potentials (APs) (Stoddard et al., 2006). The neurons of the MRN project to the spinal cord and synapse with modified spinal neurons in the form of electromotor neurons present here (Bennett et al., 1967). These electromotor neurons then go on to innervate the electrocytes via a nicotinic cholinergic synapse and therefore transmit the signal for an EOD to occur (Bennett et al., 1967).
The MRN also initiates a secondary regulatory signal. This serves a dual purpose: it acts as a reference for further EOD discharge while also having an inhibitory effect on the PCN. This means that the MCN is mainly concerned with the timing of the EOD (Carlson, 2003).
Steroid Hormone and Genetic Regulation of the EOD:
When considering long term changes in EODs the main regulators are steroid hormones i.e. androgens and oestrogens, and the types of ion channels being expressed. As a result, the characteristics of EODs are sexually dimorphic and therefore genetically determined (Markham, 2013).
EODs are sexually dimorphic in the sense that androgens generally reduce the frequency of the EOD and increase the duration of electrocyte APs, this being caused mainly by dihydrotestosterone (DHT). Therefore, when measured, the EODs of Sternopygus specimens were seen to be low frequency and having a longer AP duration if male, while the opposite was true in female specimens. Immature Sternopygus individuals had intermediate values for both parameters (Ferrari et al., 1995). In fact, constant treatment of Sternopygus with androgens resulted in a longer AP duration as well as lower frequency EODs (Mills & Zakon, 1991).
Steroids likely achieve this role directly via their regulation of expression of particular channel subunit types. The repolarizing K+ channels, are the primary regulatory target. There are three genes for such channels, but not all of them play a role in regulation. The Kv1.1a and Kv1.2a subunit genes’ expression is inversely proportional to the duration of the EOD i.e. treatment with androgens results in a decrease in their expression (Few & Zakon, 2007).
The Na+ channel is regulated in a similar fashion. In particular, the Nav1.4bL alpha subunit gene and a Beta-1 subunit gene are the main regulators. Such subunits are expressed less with increased EOD duration and after exposure to androgens (Markham, 2013). This is due to the fact that such a subunit speeds up the inactivation of the Na+ channel, if its expression decreases, then the AP lasts longer since the channel take longer to be inactivated (Liu et al., 2008). The way in which androgens interact with Na+ and K+ channels to regulate their components is likely to be the cause of the sexual dimorphism observed.
As can be expected, exposure to oestrogen had the opposite effect i.e. a feminization of EOD (higher frequency and shorter AP duration) (Dunlap et al., 1997)
Peptide Hormone Regulation of the EOD:
While steroid hormones are useful when it comes to changes that can afford to span weeks and months, social interactions need changes that occur in the relatively short term due to their ephemeral nature. Melanocortin peptides fulfill this role.
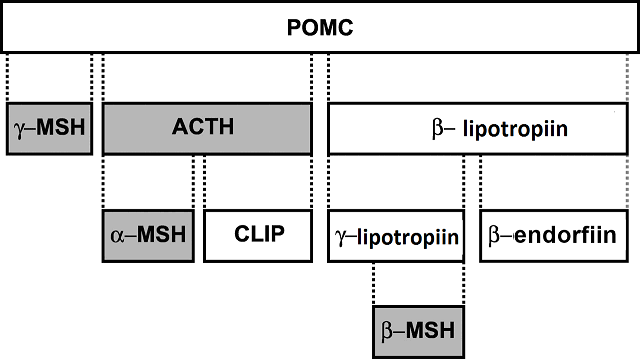
Two examples of such peptides are alpha melanocyte stimulating hormone (alpha-MSH) and adrenocorticotropic hormone (ACTH) - both hormones are produced from the same pro-hormone, proopiomelanocortin (POMC) as shown in (Fig 7). (Markham, 2013; Stoddard et al., 2006). These two hormones act in the same way, in that they bind to an extracellular receptor on the electrocyte with a G-protein part intracellularly and therefore activating an adenylyl cyclase - cAMP/PKA pathway. This pathway then modulates the waveform as well as the frequency of APs. This has the effect of masculinizing the EOD waveform (longer duration and lower frequency) (Markham & Stoddard, 2005). While it is known that the cAMP/PKA pathway is the mechanism by which changes in EOD waveform and duration are elicited, the phosphorylation targets of the PKA are unknown (Markham, 2013).
|
Fig.7; proopiomelanocortin being broken down into its components, most notably ACTH |
It is interesting to note, that such intermediate changes follow a circadian rhythm. Such circadian rhythms are important when it comes to socialization and courtship. Shortly after dark, the enhancement of peptide hormone-induced sexually dimorphic EOD signalling occurs - this being the time period at which courtship tends to occur. After midnight, the EOD re-stabilizes (Stoddard et al., 2006).
It is also worth noting, that androgens regulate the ability for melanocortin peptides to influence EOD waveforms, and in this way, steroid hormones act as master regulators (Allee et al. 2009).
Regulation by serotonin:
Serotonin also appears to play an important role in intermediate duration regulation (Stoddard et al., 2006). It probably works on the level of the hypothalamus or hypophysis since injections into electrocytes yield minor differences in EOD (Stoddard et al., 2003). It appears that serotonin binds to a 5HT1A-like receptor and ultimately reduces EOD amplitude and duration, likely by decreasing serum melanocortin peptide concentration, though this is yet to be confirmed (Stoddard et al., 2006).
Conclusion:
There are about 700 electric fish species known, distribution in saltwater and freshwater sheds light on their electric organ and ancestry. More research regarding species specific mechanisms such as the mentioned capactitance detection in Gymnotiformes, phosphorylation targets for the cAMP mediated mechanism for peptide hormone regulation, and phylogeny and the driving forces for the convergent evolution of the electric organ trait development.
References:
Articles:
- Watanabe A.; Takeda K. (1963). The change of discharge frequenct by A.C. stimulus in weak electric fish. Department of Physiology, Tokyo Medical and Dental University, Tokyo, Japan, 10. in Journal of Experimental Biology 1963 40: 57-66;
- Allee, S. J.; Markham, M. R.; Stoddard, P. K. (2009). Androgens enhance plasticity of an electric communication signal in female knifefish, Brachyhypopomus pinnicaudatus. Hormones and Behavior, 56(2), 264–273.
- Ayres Sá, L.; Aurea Menezes, M.; Mermelstein, C. (2001). Expression of muscle-specific myosin heavy chain and myosin light chain 1 in the electric tissue ofElectrophorus electricus(L.) in comparison with other vertebrate species. The Journal of Experimental Zoology, 290(3), 227–233.
- Babineau, D.; Longtin, A.; Lewis, J. E. (2006). Modeling the electric field of weakly electric fish. The Journal of Experimental Biology 209, 3636-3651 Published by The Company of Biologists 2006
- Baier, B.; Kramer, B. (2007). Electric communication during courtship and spawning in two sibling species of dwarf stonebasher from southern Africa, Pollimyrus castelnaui and P. marianne (Mormyridae, Teleostei): evidence for a non species-specific communication code? Behaviour, 144(1), 115–142.
- Belbenoit, P. (1985). Fine analysis of predatory and defensive motor events in Torpedo Maromorata (Pisces). Laboratoire de Physiologie Nerveuse, Departement de Neurophysiologie Sensorielle, C.N.R.S., 91190 Gif-Sur-Yvette, France and Institut de Biologie Marine, Universite de Bordeaux I, 33120 Arcachon, France, (J. exp. Biol. 121, 197-226 (1986) 197 Printed in Great Britain © The Company of Biologitts Limited 1986).
- Bennett, M. V.; Pappas, G. D.; Aljure, E.; Nakajima, Y. (1967). Physiology and ultrastructure of electrotonic junctions. II. Spinal and medullary electromotor nuclei in mormyrid fish. Journal of Neurophysiology, 30(2), 180–208.
- Bennett, M. V.; Pappas, G. D.; Giménez, M.; Nakajima, Y. (1967). Physiology and ultrastructure of electrotonic junctions. IV. Medullary electromotor nuclei in gymnotid fish. Journal of Neurophysiology, 30(2), 236–300.
- Caputi, Á. A.; Aguilera, P. A.; Pereira, A. C. (2011). Active Electric Imaging: Body-Object Interplay and Object’s “Electric Texture.” PloS One, 6(8), e22793.
- Caputi, A. A.; Budelli, R.; Grant, K.; Bell, C. C. (1998). The electric image in weakly electric fish: physical images of resistive objects in Gnathonemus petersii. The Journal of Experimental Biology, 201(Pt 14), 2115–2128.
- Carlson, B. A. (2003). Single-unit activity patterns in nuclei that control the electromotor command nucleus during spontaneous electric signal production in the mormyrid Brienomyrus brachyistius. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(31), 10128–10136.
Catania, K. C. (2015a). Electric Eels Concentrate Their Electric Field to Induce Involuntary Fatigue in Struggling Prey. Current Biology: CB, 25(22), 2889–2898.Current Biology 25, 2889–2898 Elsevier Ltd
- Catania, K. C. (2015b). Electric eels use high-voltage to track fast-moving prey. Nature Communications, 6, 8638.
- Dangelmayer, S.; Benda, J.;Grewe, J. (2016). Weakly electric fish learn both visual and electrosensory cues in a multisensory object discrimination task. Journal of Physiology-Paris, 110(3), 182–189.
Dunlap, K. D.; Lynne McAnelly, M.; Zakon, H. H. (1997). Estrogen Modifies an Electrocommunication Signal by Altering the Electrocyte Sodium Current in an Electric Fish,Sternopygus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(8), 2869–2875.
- Elekes, K.; Szabo, T. (1981). Synaptology of the command (pacemaker) nucleus in the brain of the weakly electric fish, Sternarchus (Apteronotus) albifrons. Neuroscience, 6(3), 443–460.
Ferrari, M. B.; McAnelly, M. L.; Zakon, H. H. (1995). Individual variation in and androgen-modulation of the sodium current in electric organ. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 15(5 Pt 2), 4023–4032.
- Few, W. P.; Zakon, H. H. (2007). Sex differences in and hormonal regulation of Kv1 potassium channel gene expression in the electric organ: molecular control of a social signal. Developmental Neurobiology, 67(5), 535–549.
- Gallant, J. R.; Traeger, L. L.; Volkening, J. D.; Moffett, H.; Chen, P.-H.; Novina, C. D.; Sussman, M. R. (2014). Nonhuman genetics. Genomic basis for the convergent evolution of electric organs. Science, 344(6191), 1522–1525.
- Gottwald, M.; Bott, R. A.; von der Emde, G. (2017). Estimation of distance and electric impedance of capacitive objects in the weakly electric fish Gnathonemus petersii. The Journal of Experimental Biology, 220(17), 3142–3153.
- Grundfest, H. (1960). Electric Fishes. Scientific American, 203(4), 115–124.
- Hagedorn, M.; Heiligenberg, W. (1985). Court and spark: electric signals in the courtship and mating of gymnotoid fish. Animal Behaviour, 33(1), 254–265.
- Heiligenberg, W. (1975). Electrolocation and jamming avoidance in the electric fishGymnarchus niloticus (Gymnarchidae, Mormyriformes). Journal of Comparative Physiology ? A, 103(1), 55–67.
- Hopkins, C. D. (1974). Electric communication in the reproductive behavior of Sternopygus macrurus (Gymnotoidei). Zeitschrift Fur Tierpsychologie, 35(5), 518–535.
- Hopkins, C. D. (1988). Neuroethology of electric communication. Annual Review of Neuroscience, 11, 497–535.
- Ho, W. W.; Fernandes, C. C.; Alves-Gomes, J. A.; Smith, G. T. (2010). Sex differences in the electrocommunication signals of the electric fish Apteronotus bonapartii. Ethology: Formerly Zeitschrift Fur Tierpsychologie, 116(11), 1050–1064.
- Kramer, B. (1996). Electroreception and communication in fishes (Vol. 42, pp. VIII, 119). Stuttgart: Gustav Fischer.
- Kramer, B. (2009). Electric Organ Discharge. In: Binder, Marc D. und Hirokawa, Nobutaka und Windhorst, Uwe, (eds.) Encyclopedia of Neuroscience. Springer, Berlin u.a., S. 1050-1056. ISBN 978-3-540-23735-8 (Print), 978-3-540-29678-2
- Lissmann, H. W. (1951). Continuous electrical signals from the tail of a fish. Gymnarchus niloticus Cuv. Nature, 167(4240), 201–202.
- Liu, H.; Wu, M.-M.; Zakon, H. H. (2008). A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(37), 9173–9182.
- Markham, M. R. (2013). Electrocyte physiology: 50 years later. The Journal of Experimental Biology, 216(13), 2451–2458.
- Markham, M. R.; Stoddard, P. K. (2005). Adrenocorticotropic hormone enhances the masculinity of an electric communication signal by modulating the waveform and timing of action potentials within individual cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25(38), 8746–8754.
- Mills, A.; Zakon, H. H. (1991). Chronic androgen treatment increases action potential duration in the electric organ of Sternopygus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 11(8), 2349–2361.
- Stoddard, P. K.; Markham, M. R.; Salazar, V. L. (2003). Serotonin modulates the electric waveform of the gymnotiform electric fish Brachyhypopomus pinnicaudatus. The Journal of Experimental Biology, 206(Pt 8), 1353–1362.
Stoddard, P. K.; Zakon, H. H.; Markham, M. R.; McAnelly, L. (2006). Regulation and modulation of electric waveforms in gymnotiform electric fish. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 192(6), 613–624.
- Szabo, T. (1974). Anatomy of the Specialized Lateral Line Organs of Electroreception. In T. H. Bullock, A. Fessard, P. H. Hartline, A. J. Kalmijn, P. Laurent, R. W. Murray, … A. Fessard (Eds.), Electroreceptors and Other Specialized Receptors in Lower Vertrebrates (pp. 13–58). Berlin, Heidelberg: Springer Berlin Heidelberg.
- Tan, E. W.; Nizar, J. M.; Carrera-G, E.; Fortune, E. S. (2005). Electrosensory interference in naturally occurring aggregates of a species of weakly electric fish, Eigenmannia virescens. Behavioural Brain Research, 164(1), 83–92.
- Tee-Van, J. (1948). Fishes of the western North Atlantic. Editorial board: editor-in-chief John Tee-Van [and others].
- Thompson, A.; Infield, D. T.; Smith, A. R.; Troy Smith, G.; Ahern, C. A.; Zakon, H. H. (2018). Rapid evolution of a voltage-gated sodium channel gene in a lineage of electric fish leads to a persistent sodium current. PLoS Biology, 16(3), e2004892.
- Turner, C. R.; Derylo, M.; de Santana, C. D.; Alves-Gomes, J. A.; Smith, G. T. (2007). Phylogenetic comparative analysis of electric communication signals in ghost knifefishes (Gymnotiformes: Apteronotidae). The Journal of Experimental Biology, 210(Pt 23), 4104–4122.
- Von der Emde, G.; Gebhardt, K.; Alt, W. (2012). Electric discharge patterns in group-living weakly electric fish, Mormyrus rume (Mormyridae, Teleostei). Behaviour, 149(6), 623–644.
Von der Emde, G. 2007 Biometric Sensors: Active Electroloction of Weakly Electric Fish as Model for Active Sensing in Techincal System. in Journal of Bionic Engineering Volume 4, Issue 2, June 2007, Pages 85-90
- Von der Emde, G. (2004). Distance and shape: perception of the 3-dimensional world by weakly electric fish. Journal of Physiology, Paris, 98(1-3), 67–80.
- Von der Emde, G.; Sena, L. G.; Niso, R.; Grant, K. (2000). The midbrain precommand nucleus of the mormyrid electromotor network. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 20(14), 5483–5495.
- Worm, M.; Kirschbaum, F.; Von der Emde, G. (2017). Social interactions between live and artificial weakly electric fish: Electrocommunication and locomotor behavior of Mormyrus rume proboscirostris towards a mobile dummy fish. PloS One, 12(9), e0184622.
- Zakon, H. H.; Lu, Y.; Zwickl, D. J.; Hillis, D. M. (2006). Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3675–3680.
Bibliography:
- Albert J, Crampton WGR. Electroreception and electrogenesis. In: Evans DH, Claiborne JB, editors. The physiology of fishes. Boca Raton: CRC Press; 2005. P.429-470
Bennett, M. V. L. (1971). Electric Organs. In W. S. Hoar & D. J. Randall (Eds.), Fish Physiology (Vol. 5, pp. 347–491). Academic Press.
- Darwin, C. (1869). On the Origin of Species by Means of Natural Selection: Or the Preservation of Favoured Races in the Struggle for Life.
Shark Trust; 2009. An Illustrated Compendium of Sharks, Skates, Rays and Chimaera. Chapter 1: The British Isles. Part 1: Skates and Rays. Torpedo Nobilana
- Squire, A.; Moller, P. (1982). Effects of water conductivity on electrocommunication in the weak-electric fish Brienomyrus niger (Mormyriformes). in Animal Behaviour, 30(2), 375–382.
Tricas, T. C.; Carlson, B. A. (2012). Chapter 41 - Electroreceptors and Magnetoreceptors. In N. Sperelakis (Ed.), in Cell Physiology SourceBook (Fourth Edition) (pp. 705–725). San Diego: Academic Press.
Websites:
Figures:
- Figure 1: Copyright Adil Viswanath
Figure 2: Caputi, Á. A., Aguilera, P. A., & Pereira, A. C. (2011). Active Electric Imaging: Body-Object Interplay and Object’s “Electric Texture.” PloS One, 6(8), e22793.
- (Recreated Kurt Scicluna)
Figure 3: https://commons.wikimedia.org/wiki/File:Eigenmannia_virescens.jpg
Figure 4: https://commons.wikimedia.org/wiki/File:Sternopygus_macrurus_by_Castelnau.jpg
- Figure 6: Catania, K. C. (2015a). Electric Eels Concentrate Their Electric Field to Induce Involuntary Fatigue in Struggling Prey. Current Biology: CB, 25(22), 2889–2898.
- (Recreated by Kurt Scicluna)
Tables:
- Table 1: Data derived from: Albert J, Crampton WGR. Electroreception and electrogenesis. In: Evans DH, Claiborne JB, editors. The physiology of fishes. Boca Raton: CRC Press; 2005. P.429-470
Table 2: Data derived from: Baier, B., & Kramer, B. (2007). Electric communication during courtship and spawning in two sibling species of dwarf stonebasher from southern Africa, Pollimyrus castelnaui and P. marianne (Mormyridae, Teleostei): evidence for a non species-specific communication code? Behaviour, 144(1), 115–142.