Circadian rhythms: the food-entrainable oscillator (FEO)
Contents
Introduction
Mammals are exposed to a variety of environmental factors that have effect on their bodies. Many of these factors can be entrained; meaning can be brought into specific rhythms. In the following segments, the basics of the circadian rhythms, primary regulation from the suprachiasmatic nucleus, and the involvement of entrainable oscillators, specifically the food-entrainable oscillator are discussed. Furthermore, a brief insight is given to a few experiments outlining some of the functions and the effects of disruption to the food-entrainable oscillator.
Circadian rhythms
Circadian rhythms are rhythms within a living organism that are regular and cyclic with a period approximating 24 hours. These rhythms are present in every living organism, ranging from cyanobacteria to humans, making it possible for biological clocks to maintain synchrony between inputs, such as external effects, and outputs, such as the physiological processes and behaviour (Kent, 2014). The outputs from the environmental inputs affect processes within the living organism such as the metabolic state of the organism, cardiac functions, hormonal regulations and secretions, body temperature and several other physiological processes needed for the correct functioning of the body. This coordinating function between the external and internal environments makes it possible for the organism to optimize its energy expenditures, as well as, is vital to survival by assuring the correct time and place of the necessities, such as food seeking behaviour, hunting and avoidance of predators (Mohawk et al, 2012).
During a daily 24-hour cycle, the majority of environmental inputs an organism experiences, will change. Temperature, light and humidity, and the light and dark cycle of the organism remains the primary regulatory factors synchronizing the 24-hour long daily cycle, which in turn influence the internal processes occurring in the organism. The regulatory factors of the synchronization of the circadian rhythms are referred to as Zeitgebers (Gamble et al, 2014).
The pacemaker activity of the circadian rhythm is generated by the suprachiasmatic nucleus (SCN) in mammals. The SCN is located in the hypothalamus, situated dorsally to the chiasma optimum and its primary regulator is light (Moore, 2013). The light reaches the photoreceptor ganglionic-cells located on the retina of the eye, and directly stimulates the SCN. The specific oscillator within is termed the light-entrainable oscillator (LEO). Although the SCN is the main circadian oscillator in mammals, located in the central nervous system, there are peripheral oscillators present all around the body as well, indicating that the distribution of the oscillating activity of circadian rhythms cannot be localized to one single area. These peripheral oscillators are located in various organs and tissues generating processes such as gene expression, metabolic activity and rhythms. This makes it possible for us to investigate the clock-controlled genes: PER1, 2, CRY1, 2, CLOCK and BMAL1, which affect the physiological and behavioral rhythms in mammals (Takasu et al, 2012). The latter mentioned has been used to investigate the localization of the center of the oscillating activity.
The peripheral and central rhythms are synchronized by the SCN, making it possible for the body to have a close connection between the external environmental factors as an input, and the internal outputs affecting the physiology and behavior of the organism, all of which are controlled by daily cycles. When the SCN is impaired, not able to receive light, the primary Zeitgeber of the mammals will be neglected. This results in the disruption of the circadian rhythms, and the inability to synchronize the external environment with the internal behavioral and physiological processes.
Sustained desynchronization of circadian rhythms may lead to degenerative consequences in humans and animals. The immune system may deteriorate, hormone fluctuations may occur, insomnia and fatal consequences may result (Mohawk et al, 2012). Typical examples of disrupted circadian rhythms may be jet lag among humans and shift workers, where prolonged disruption may cause health effects such as higher susceptibility for cancer, diabetes and sleep disorders. In animals sustained desynchronization is often associated with fatality (Takasu et al, 2012).
Food-entrainable oscillator
As previously elaborated, under normal circumstances the SCN is responsible for time-related organization of the mammalian activity, and can be considered the internal master clock of the body (Schibler and Sassone-Corsi, 2004). Impairment can occur, for instance in animals with lesions of the SCN, resulting in disruption of the circadian rhythms. Two extra-SCN oscillators exist, namely the food-entrainable oscillator (FEO), which is entrained by periodic food availability, and the methamphetamine-sensitive circadian oscillator (MASCO), which is revealed by constant consumption of low-dose methamphetamine (a psycho-stimulant drug of abuse) (Pendergast et al, 2012). Both of these secondary Zeitgebers are capable of re-synchronizing the circadian outputs in the absence of the SCN. The role of the FEO specifically will be outlined in detail below.
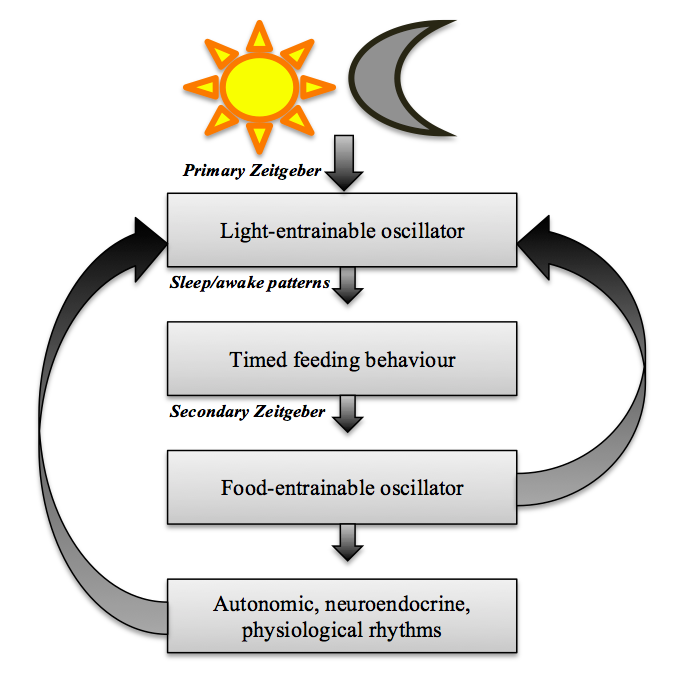
The FEO functions as a pacemaker, similar to the SCN, but is considered to be independent of the light-entrainable circadian oscillator, specifically in terms of location (Mohawk et al, 2012). As previously expressed, lesions made to the SCN will disrupt light-related rhythms, while food-entrained rhythms can not only survive, but also drive the light-entrained rhythms such as rest-activity during SCN ablation (Kent, 2014). Moreover, experiments have shown that the CLOCK, PER1 and 2 genes are not necessary factors of the FEO (Mohawk et al, 2012), and rather it is mediated by molecular time-keeping mechanisms. Nevertheless, the two oscillators are coupled by feedback mechanisms (Castro-Faúndez et al, 2016). The rhythms controlled by the LEO influence the timing of feeding behaviour, which in turn will entrain the FEO (Kent, 2014). In other words, mammals of a specific light-dark cycle will exhibit feeding behaviour accordingly. The diagram figure 1 below illustrates the mechanism of these feedback loops. As shown, both the effected rhythms (outputs) and the FEO itself, provide feedback to the oscillators.
|
For further clarification, the FEO can be considered a circadian oscillator (CO) due to the fact that it complies to the following properties: it requires a Zeitgeber for initial entrainment, the entrainment is limited to a window of time of approximately 22–29 hours, and even during prolonged food deprivation, the oscillation cycle will persist for several cycles, after which only gradual change is made to reset the anticipated feeding time (Blum et al, 2012). Independent to the LEO, the FEO is directly related to food availability. Entrainment of behavioral rhythms to food availability is reflected in the appearance of food anticipatory activity (FAA), which is the output of the FEO (Blum et al, 2012). This is brought about by timed or scheduled food restriction.
Food anticipatory activity is expressed through increased locomotor activity and alertness several hours prior to anticipated feeding time, when food availability has been restricted to a particular time each day and adaption period has been allowed. In addition to increased behavioral activity, core temperature, ketogenesis, free fatty acid synthesis, and plasma levels of cortisone levels change prior to feeding. Further studies have shown that the secretion of metabolic hormones, such as ghrelin, a hunger hormone, is another output signal of the FEO, produced in the gastrointestinal tract in ghrelinergic cells of oxyntic glands (LeSauter et al, 2009). Ghrelin acts to enhance the feeding-related zeitgebers, so reductions of plasma ghrelin can weaken the FEO and disrupt circadian rhythmicity accordingly (Kent, 2014).
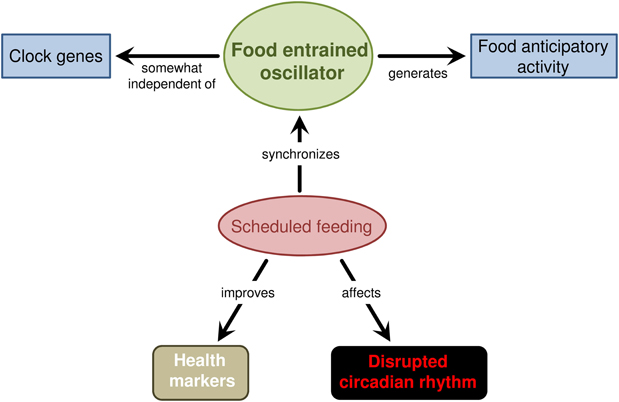
The diagram figure 2 below summarizes the fundamental concepts of the FEO. The FFA is generated by the FEO, which is synchronized by repeated, scheduled daily feeding. This scheduled feeding has also shown to have positive health effects, such as reduce tumor development in mice (Davidson, 2006), as well aid in disrupted circadian rhythms.
|
The anatomical location of the FEO is to present-day not confirmed and under ongoing investigation. Progress thus far has been made in identifying locations where the FEO is not, rather than where it is, e.g. the olfactory bulbs, ventromedial hypothalamus, paraventricular thalamic nucleus, and certain regions of the gastro-intestinal tract have been ruled-out as candidates through lesion studies (Mohawk et al, 2012). However, the ghrelin cells in parts of the gastro-intestinal tract as mentioned are of relevance, and hence considered to be further candidate loci. Furthermore, the dorsomedial hypothalamus (DMH), with influence from the parabrachial nucleus, is one candidate that has proven to interact with the SCN and is believed to entrain rhythms in accordance to daily feeding (Mohawk et al, 2012). Nevertheless, follow-up studies have provided controversial results, stating the DMH is not critical to the expression of FFA (Landry et al, 2007). These contradictions emphasize the still undetermined status of the FEO locus. It can be gathered that there is a possibility of several, central and peripheral, loci involved with a variety of output signals.
Experimental cases on laboratory animals
As mentioned earlier, the FEO is regulated by hormones and leads to food intake regulation and anticipation. These next examples summarize experiments, which expose the effects on specific hormone deprived mammals and the effects of different diets.
Glucocorticoids on peripheral Circadian Oscillators
Glucocorticoids are secreted by the adrenal gland, especially right before activity i.e. food intake; in nocturnal animals this would be at the onset of darkness as they are most active then (Dickmeis, 2008). The rhythmic secretion of this corticosteroid is controlled by the SCN. In adults, there are not many receptors for these corticosteroids in the SCN, making them ideal to test the effect of circadian oscillators (CO), among them the FEO. In this particular experiment the adrenal glands in rats were removed (bilateral adrenalectomy) to deprive the animals of glucocorticoids in order to examine the effects of deprivation on the CO. The SCN was kept as it regulates the circadian rhythms and without it, there would be no cycle detectable at all (Pezük et al, 2012).
The biological rhythmicity depends on negative feedback from the tissues to restart the cycles. In the study Glucocorticoids as Entraining Signals for Peripheral Circadian Oscillators (Pezük et al, 2012), a pair of known activators and corresponding proteins, which would elicit the negative response, to track the effects of glucocorticoid deprivation. After several weeks of rhythmic light-dark cycles and regular feeding, blood analysis and bioluminescence on different in vitro organs, showed that although both normal and glucocorticoid deprived subjects had the same environment, the lack of glucocorticoids significantly increased the negative feedback delay, thereby disrupting the natural body clock.
Disruption of natural feeding times in Mice
Mice are nocturnal animals, which means they primarily eat most of their food at night. Darkness triggers the release of hormones making them more active and hungry. If food is restricted during dark periods, FAA and wakefulness can be observed as the internal body clock starts preparing the body for food intake even though it is not available. Animals have to eat, at some point it doesn’t matter at what time food is available, if the animals are hungry they will eat despite it being outside their regular feeding periods (Pezük et al, 2012).
In the study Delayed Timing of Eating: Impact on Weight and Metabolism, an experiment conducted by Allison, Goel and Ahima, 2 groups of mice, both groups were held under same light and dark phases. Group 1 was denied food during dark phases and fed during light phases i.e. unnatural pattern for mice, while the group 2 was fed during the 8 hours of darkness. The results were as follows: Mice in group 1, were prone to obesity while the others were not affected by obesity, diabetes, liver disease and inflammation. Several studies have shown that FEO disruption in fat tissue due to a shift in food intake can lead to obesity and increased fat storage without any change in amount of daily movement and increased food intake (Allison et al, 2014).
High-fat diet effects on the FEO
Studies have found that corticosterone has a major role in the FEO as it is secreted before feeding (Luna-Moreno et al, 2012). Due to experiments with restricted feeding we know that the internal body clock knows when it is time for food, and therefore secretes corticosteroids before a meal to prepare the body for food ingestion. Rats have been observed to become more active during this time, also visiting the food dispenser more often. FAA means that even though food is not necessarily available, the body is getting ready to feed; this is one way of evaluating the effect of different foods on the FEO.
In the study Dietary fat and corticosterone levels are contributing factors to meal anticipation (Namvar et al, 2016), the effects of a high fat diet (HFF), compared to a standard laboratory chow were examined. Obesity has been associated with increased corticosterone levels, which in theory would disrupt the daily cycle. To arrive at results, rats were initially acclimatized, by having them under a strict, 12:12h light-dark cycle with standard laboratory chow available at any time (called ad libitum) for several weeks. After this initial period they were split in two groups, one group being changed to a high-fat diet, the other not. Then half of each group was subjected to a restricted amount of feeding time (RF). Hence four groups where examined: Rats fed on laboratory chow – ad libitum, rats fed on laboratory chow with restricted feeding time, rats fed on a high fat diet – ad libitum, and rate fed on a high fat diet with restricted feeding time.
The rats were monitored 80 minutes prior to feeding to assess the FAA. Rats were also placed in special chambers with infrared photocells, before and after food entrainment, to assess the different effects of the diets. Several physiological and behavioral criteria were analyzed, including activity, food intake, the number of food dispenser visits, respiratory quotient, oxygen and carbon dioxide consumption. After several weeks the four feeding groups showed significant differences in FAA, activity and weight. Regarding FAA, both groups with restricted feeding showed FAA behavior prior to their respective food times, this became more pronounced as the restricted feeding time continued for several weeks. Rats fed laboratory chow showed a greater FAA compared to high-fat diet rats with restricted feeding time, signifying that a high-fat diet dampens the effect of corticosterone on the body due to its constant increased corticosterone levels. Both ad libitum groups were used as controls to compare the activity/resting periods of the restricted feeding times groups. As the restricted feeding times lengthened, both restricted subject groups showed less resting time and more activity than their ad libitum counterparts.
Finally, regarding weight gain, at the end of the third week of acclimatization period where all subjects received unlimited food, no significant weight difference was observed between the normal fat and high fat diet. However, after restricted feeding treatment, both normal diet groups were considerably lighter than both high-fat diet groups. The differences in weight increased throughout the weeks of treatment.
|
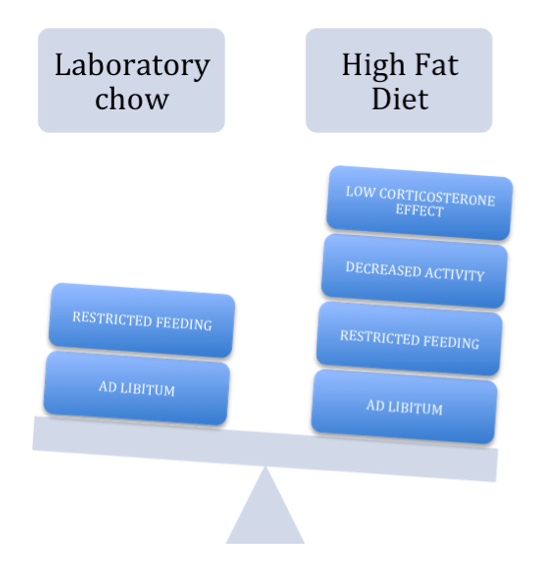
The diagram figure 3 above represents the gradual effects of a High Fat Diet. It lowers the corticosteroid effect, which has shown to decrease locomotoric activity. In regards to feeding times, both diets show an increased weight gain when given complete access to food rather than only at certain times.
In conclusion, and in comparison to the lifestyle of modern society, it can be stated that lack of restriction of food to certain times of the day, and unnaturally high-fat diet with processed food can be having an effect on our FEO and with that, our metabolism leading to increased weight gain and thus resulting problems. Furthermore, due to our constant eating, we become less active and hence, more prone to gaining weight.
Unnatural daily routine in humans
In our modern society, 20% of working people work outside of the 07:00-18:00 time frame, working evening/nights shifts. This leads to unnatural sleeping patterns, such only getting 4 hours of sleep a night. In earlier times, humans would rise with the sun and go to sleep as darkness fell. Investigations have been carried out and have shown that decreased sleep leads to increased food intake, and with it, decreased insulin sensitivity leading to several health issues such as diabetes and obesity. The study Metabolism and the Circadian Clock Converge written by Eckel-Mahan and Sassone-Corsi, investigates the effect on subjects that were only 4 hours of sleep each night compared to subjects that were allowed unrestricted sleep, over a 5-day period. Sleep deprivation resulted in a higher weight gain of 0.97kg compared to 0.11kg in well-rested candidates.
The study also observed that increased caloric value was also a side effect of lack of sleep. Sleep-deprived subjects took in an extra 553 calories, mostly from fat. Nevertheless, there is a range of further possibilities as to why reduced sleep and shiftwork results in weight gain. As people work later into the evening due to shift work, they are exposed to food and eating opportunities for longer periods of time resulting in wanting a greater amount of food in the evening, in addition to more gastric emptying at night rather than morning leading to increased caloric intake with less feeling of fullness. Disruption of FEO also affects the leptin metabolism, which leads to increased appetite, especially in the evening. These results led to the conclusion that the indirect effects of disruption of natural sleeping cycles have proven to result in weight gain and diabetes among other things.
Conclusion
The functioning of the circadian rhythms, therefore the functioning of the main pacemaker, the SCN, is essential for maintenance and control of neuroendocrine, autonomic and physiological processes. Synchronizing the external inputs with these outputs, all in the frame of a daily cycle are necessary for the well-being and survival of every living organism. Food is a critical source to all species, hence, understanding the force of its action through the food-entrainable oscillator, is of upmost importance. The fact it has the ability to drive even non-food related rhythms only emphasizes the relevance it has to body-environment synchronization. Experiments in this field have only just begun to outline the mechanisms of the FEO. With the confirmed identity of the FEO’s anatomic location for instance, possible interventions for aiding synchronization could be made. It is clear however, that in the present day of modern lifestyle, conflict with our natural body clock is arising. How this affects our domestic animals in the future is still a matter of investigation.
Acknowledgments
The authors thank Dr. István Tóth for comments made to the essay and guidance regarding the relevance of scientific information.
Notes
Figure 1 was composed by Emilia Jennings using Smart Art Graphics from Microsoft Office Word, and has been adapted from the work of Kent (2014).
Figure 2 is credited to the work of (Carneiro and Araujo 2012), obtained from an open-access article that permits the usage given proper citation.
Figure 3 was composed by Alejandro Bostroem using SmartArt Graphics from Microsoft Office Word, based on the work of Namvar et al (2016).
Works Cited
Allison K. C.; Goel N.; Ahima R. S. (2014) Delayed Timing of Eating: Impact on Weight and Metabolism. Current Obesity Reports 3(1): 91-100
Blum I. D.; Lamont E. W.; Rodrigues T.; Abizaid A. (2012) Isolating Neural Correlates of the Pacemaker for Food Anticipation. PLOS ONE 7(4): e36117
Carneiro B. T. S.; Araujo J. F. (2012) Food entrainment: major and recent findings. Frontiers in Behavioral Neuroscience 6(83): 1-4
Davidson A. J. (2006) Search for the feeding-entrainable circadian oscillator: a complex proposition. Editorial Focus (American Physiological Society) 290(6): 1524-1526
Dickmeis T. (2009) Glucocorticoids and the circadian clock. Journal of Endocrinology 200: 3-22
Eckel-Mahan K.; Sassone-Corsi P. (2013) Metabolism and the Circadian Clock Converge. American Physiological Society 93(1): 107-135
Gamble K. L.; Berry R.; Frank S. T.; Young M. E. (2014) Circadian clock control of endocrine factors. Nature Reviews Endocrinology 10(8): 466-475
Castro-Faúndez J.; Díaz J.; Ocampo-Garcés A. (2016) Temporal Organization of the Sleep-Wake Cycle under Food Entrainment in the Rat. Sleep 39(7): 1451-1465
Kent B. A. (2014) Synchronizing an aging brain: can entraining circadian clocks by food slow alzheimer’s disease? Frontiers in Aging Neuroscience 6(234): 1-7
Landry G. J.; Yamakawa G. R.; Webb I. C. (2007) The Dorsomedial Hypothalamic Nucleus Is Not Necessary for the Expression of Circadian Food-Anticipatory Activity in Rats. Journal of Biological Rhythms (Sage Journals) 22: 467-478
LeSauter J.; Hoque N.; Weintraub M.; Pfaff D. W. (2009) Stomach ghrelin-secreting cells as food-entrainable circadian clocks. PNAS 106(32): 13582-13587
Luna-Moreno D.; García-Ayala B.; Díaz-Muñoz M. (2012) Daytime restricted feeding modifies 24 h rhythmicity and subcellular distribution of liver glucocorticoid receptor and the urea cycle in rat liver. British Journal of Nutrition 108(11): 1-12
Mohawk J. A.; Takahashi J. S.; Green C. B. (2013) Central And Peripheral Circadian Clocks In Mammals. National Institutions of Health 35: 445-462
Moore R. Y. (2013) The Superchiasmatic Nucleus and the Circadian Timing System. Chronobiology: Biological timing in health and disease 119: 9-10
Namvar S.; Gyte A.; Denn M.; Leighton B.; Pig H. D. (2016) Dietary fat and corticosterone levels are contributing factors to meal anticipation. APS Journals 310(8): 711-723
Pendergast J. S.; Oda J. S.; Niswender K. D.; Yamazaki S. (2012) Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s). PNAS 109(35): 14218-14223
Pezük P.; Mohawk J. A.; Wang A. L.; Menaker M. (2012) Glucocorticoids as Entraining Signals for Peripheral Circadian Oscillators. Oxford Academic 153(10): 4775-4783
Schibler U.; Sassone-Corsi P. (2004) A Web of Circadian Pacemakers. Cell (Cell Press) 111(7): 919-922
Takasu N. N.; Kurosawa G.; Tokuda I. T.; Mochizuki A.; Todo T.; Nakamura W. (2012) Circadian Regulation of Food-Anticipatory Activity in Molecular Clock-Deficient Mice. PLOS ONE 7(11): 1-8