Itt írjon a(z) GHRelin_New-ról/ről
Current Ghrelin research
Contents
Introduction
Ghrelin is a 28 amino acid peptide produced mainly in the fundus of the stomach which stimulates growth hormone and hunger that was first discovered in 1999. It was first found to stimulate growth hormone release in rats however since its discovery numerous physiological effects of ghrelin have been observed (Wu & Kral, 2004). In this essay we will discuss the variety of physiological actions that ghrelin is involved in. Through our research of the literature we have determined some systems that ghrelin is involved in from the obvious growth hormone stimulation and hunger stimulation, to some less obvious interactions. We have shown in our essay that it is important hormone involved with alcoholism and obesity as well as being shown as a possible treatment for cancer and even Parkinson’s disease. We will start by discussing some important physiological actions that ghrelin is involved in.
Physiological actions of Ghrelin
As was mentioned before ghrelin is a gastric hormone that has orexigenic (appetite stimulant), adipogenic (fat forming), and somatotrophic properties (stimulates body growth), and is secreted mainly from the stomach. Recently discovered functions of ghrelin have shown that it may have further important physiological roles as well, one of these roles is as a brain-gut peptide. In this chapter we will look at the roles mentioned above and also some of the further functions that have been discovered and the possibilities for ghrelins further roles. Ghrelin has intense orexigenic and adipogenic properties, when an increase in food is required to produce more energy ghrelin generate signals to the hypothalamus to increase hunger. This clearly suggests that ghrelin acts centrally to regulate food intake, and plays a role in weight gain.
To further strengthen this argument it has been shown that rats injected with ghrelin increases food intake, and when injected with the antighrelin it drastically reduced food intake. It has been observed in patients who have undergone gastric bypass surgery have reduced levels of ghrelin possibly helping in further weight loss. It has similar effects on somatotrophs, as similarly rats injected with ghrelin also show a potent increase in growth hormone. This tells us that there is receptors for ghrelin present in the pituitary gland and gives evidence for ghrelins importance in stimulating growth as well as appetite.
There is evidence that ghrelin also has cardiovascular and immune function effects. Growth hormone secretagogue receptors (GHSR) have been found in the aorta and myocardium. In the case of cardiac cachexia, it has been observed that ghrelin has reduced left ventricular dysfunction and reduced cardiac cachexia in rats. In humans it was observed that it reduced cardiac afterload and increased output without an increase in heart rate. In the immune system ghrelin and the GHSR signalling systems were found in T-cells, B-cells and neutrophils. In ghrelin treated mice an increase in cytotoxic lymphocytes and reduction in tumour initiation was detected. Overall ghrelin plays a very important part in regulating food intake and growth rate, as can be observed with the effect it has on the hypothalamus and the pituitary gland. There is also good evidence to show that ghrelin can be used to help treat certain illnesses of the heart and immune system, and with its connection to the hypothalamus possibly the brain also. (Wu & Kral, 2004)
Ghrelin affect on appetite
The “central ghrelin signalling system” emerges therefore as a key physiological system implicitly involved in the control of food intake and reward behaviour and also as a potential therapeutic target for the control of obesity as well as disorders of the reward system that include eating disorders and substance use disorders. In rodents, acute injection of ghrelin, peripherally or centrally, induces a rapid orexigenic response , i.e. appetite stimulating (Asakawa et al., 2001; Wren et al., 2000). Ghrelin may also enhance non-homeostatic feeding by interacting with key reward systems (Egecioglu et al., 2010; Perello et al., 2010; Skibicka et al., in press-a, in press-b). The reward system, specifically the mesolimbic dopamine system, mediates a state of well-being of natural and chemical reinforcers; it enhances motivation behaviours such as food-seeking and is involved in the development of addiction to drugs of abuse (Engel et al., 1988; Hansen et al., 1991; Schultz et al., 1997). The identification of the reward circuits as a key target for ghrelin has therefore led to the unexpected discovery that the central ghrelin signalling system is required for reward induced by addictive drugs (Jerlhag et al., 2009; Kaur and Ryabinin, 2010; Tessari et al., 2007; Wellman et al., 2005) as well as palatable food (Egecioglu et al., 2010; Perello et al., 2010; Skibicka et al., in press-a, in press-b). The ghrelin receptor, GHS-R1A, has emerged therefore as a relevant therapeutic target for addictive behaviours.
The mesolimbic dopamine pathway (Fig. 1) from the VTA to the N.Acc, that has a rather well-established role in incentive motivation (i.e. wanting) (Berridge and Robinson, 2003), is clearly an important component of the central ghrelin-responsive network. While Ghrelin was gaining status as a circulating hunger hormone, the work pinpointing the midbrain dopamine system as a target for ghrelin, led us to hypothesise that this system may have a role that extends beyond regulation of food intake and of energy balance homeostasis to include reward-seeking behaviour, not only for rewarding foods but also for other reward reinforcers such as alcohol and other drugs of abuse. Interestingly, we have now shown that ghrelin and its receptor have a role in drug-induced reward and specifically in alcohol dependence.
The endogenous peptide ghrelin also appears to have a role in drug-induced reward. Specifically, ghrelin administration (icv or into the LDTg or VTA) increases the intake of alcohol in mice and the ability of alcohol to induce a locomotor stimulation, accumbal dopamine release and to condition a place preference is reduced in ghrelin knockout mice (Jerlhag et al., 2009). Due to the prolific problems caused by obesity studies have generally focused on the impact that ghrelin, the orexigenic hormone, has on energy balance and feeding behaviour. But recently there has been a shift into the examination of the effects ghrelin has on the motivated reward-driven behaviour system, via action of the so called "cholinergic-dopaminergic reward link" (Dickson et al., 2011).It's affect on dopamine has been recently documented, dopamine is a neurotransmitter found in the reward pathway of the brain that is directly connected to the nucleus accumbens, i.e. the pleasure centre. Dopamine is particularly important in terms of motivation and the sensation of feeling good. The reward system includes food, but also alcohol and drugs. This food reward seeking behaviour has been described as non homeostatic, as it occurs in the absence of nutritional or calorific deficiency (De Vriese et al., 2010).
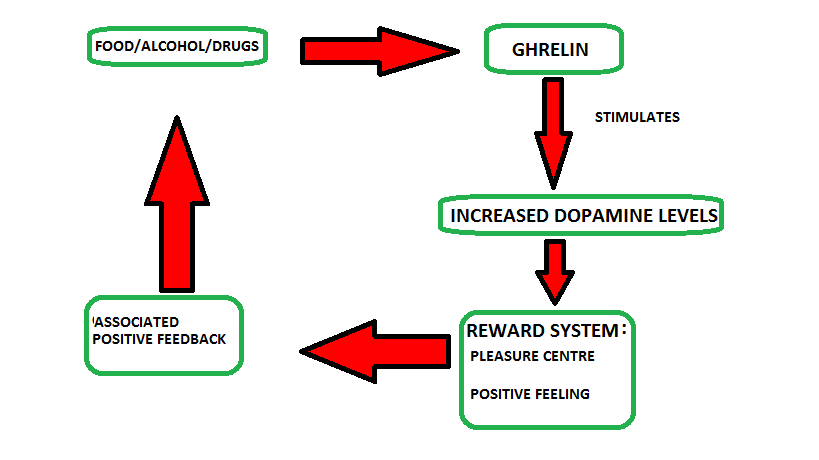
Figure 1: Ghrelin and the reward system.
(Adapted from De Vriese et al., 2010)
King et al., 2011 conducted a series of experiments, using the rat as the model organism, testing the effects of ghrelin, ghrelin receptor antagonist [Lys-3]-GHRP-6 and saline when injected via a cannulae to the ventral tegmental area (VTA). They tested the influence of ghrelin on motivation to obtain a highly palatable food source, i.e. chocolate pellets. The twenty-four adult males underwent diet restriction and were placed separately in a standard operant conditioning chamber (Colboum Instruments). Two retractable levers were present in the chamber and the rats were trained in a way that every correct press of a lever would result in a 45mg chocolate flavored food pellet reward. The experiment was set up in a way that the effort required to gain the pellet was increased after each reward, therefore increased effort was required for the same outcome. The efficacy the reinforcer (e.g. a food pellet) is defined in terms of the breakpoint (BP), the point in the experiment at which responding ceases (i.e. the final ratio completed by an animal) and reflects the maximum effort that the animal is willing to exert to obtain a particular reward (Hodos, 1961; Richardson and Roberts, 1996). After the rats were trained, the surgery was performed in which the rats received either ghrelin, ghrelin receptor antagonist [Lys-3]-GHRP-6 or saline. The rats were then tested again.
The experiment showed that food restricted rats infused with ghrelin increased their efforts to receive the reward, whereas those infused with ghrelin receptor antagonist did not increase their efforts. Ghrelin treated rats pressed the reinforcing lever 167 times on average to receive one chocolate pellet, whereas [D-Lys3]-GHRP-6 treated rats pressed the lever only 42.5 times on average to receive a similar reward. This experiment shows that the subject showed increased motivation to obtain the reward. This correlates with studies which have used a conditioned place preference (CPP), these test the rewarding properties of substances (e.g. cocaine, food, alcohol). In CPP, animals learn to associate one compartment with rewarding food (high fat, high sugar), and the other with regular chow. Animals will spend more time in the reward compartment, even in the absence of the reward. Ghrelin enhances the reward seeking response, animals treated with ghrelin spend increased time in the reward compartment (Perello M. et al., 2010).
Ghrelin affect on alcohol intake
Initially Jerlhag et al., 2009, carried out a study which showed that central ghrelin administration increased alcohol intake in mice,and in contrast, mice with ghrelin receptor (GHS-R1A) knocked out, and mice which had been treated with two different GHS-R1A antagonists showed reduced alcohol consumption. The ghrelin receptor, GHS-R1A, has emerged therefore as a relevant therapeutic target for addictive behaviours. Jerlhag et al., 2010 has shown that alcohols ability to increase accumbal dopamine release is absent in ghrelin knockout mice, in comparison to their wild-type littermates.Loco-motor activity is stimulated by drugs (including alcohol). Alcohol-induced loco-motor enhances dopamine concentration in the nucleus accumbens. Therefore Loco-motor stimulation is an indirect and supportive measure of dopamine levels, taking into account that other neurotransmitters also have an effect on alcohol induced loco-motor stimulation. The alcohol induced loco-motor stimulation was significantly lower in ghrelin knockout, than in wild-type mice. Showing that ghrelin knockout mice show a much lesser ability to increase the accumbal dopamine levels, and therefore that ghrelin may be required in order for alcohol to activate the mesolimbic dopamine system.
Ghrelin and Dopamine Function
Growth hormone secretagogue receptor (GHSR) is a type of Ghrelin receptor and is present in the substantia nigra pars compacta (SNpc) of the brain. The SNpc is located in the midbrain and has roles in motor function and reward. It is an area of the brain where the degeneration of dopamine cells can lead to Parkinson’s disease. It has been shown that obesity potentially plays a role in neurodegenerative diseases which has led to research into the effect ghrelin has on Parkinson’s disease to try and devise a possible treatment. In one particular study mice with GHSR’s only present in catecholaminergic cells (cells containing dopamine or norepinephrine) to demonstrate the effect ghrelin has on dopamine neurotransmitters. Numerous experiments were done on these mice, including experiments to determine binding of ghrelin to the SN region of the brain. Through further tests it was shown that the binding of ghrelin to SNpc cells increases the action potentials (firing rate) of these cells, increasing dopamine levels.
It was determined that ghrelin also increases tyrosine hydroxylase (TH), a rate determining enzyme of dopamine biosynthesis, as well as the TH mRNA levels in the SNpc and the number of mitochondria in dopamine perikarya (cell body of neuron). The effect ghrelin has on the SNpc cells after MPTP (a neurotoxin which causes permanent symptoms of Parkinson’s disease) treatment, which showed that ghrelin decreases dopamine cell loss in the SNpc. This study showed that ghrelin has substantial effects on the SNpc area of the brain and could help develop a possible treatment for Parkinson’s disease in the future. Ghrelin increases the firing rate of SNpc dopamine neurons, increasing dopamine availability in the brain as the degeneration, possibly helping to control the amount of degeneration that is taking place. The authors do however state that ghrelin only offers neuroprotection during a physiological window period and is very dependent on metabolic rate. Despite this the research done here has made some very positive steps forward in tackling Parkinson’s disease. (Andrews et al., 2009)
Ghrelin effects on canine cancer
Ghrelin was first identified in 1999 as a natural signalling molecule for the growth hormone secretagogue receptor type (GHS-R) which is a G-protein receptor. (Kojima et al., 1999). Ghrelin and GHS-R are often co-expressed in multiple human tumours and related cancer cells which can indicate that the ghrelin/GHS-R axis may have an important role in tumour growth and progression. (Majchrzak et al., 2012) However, a role of ghrelin in canine tumours remains unknown. The objective of the study by Majchrzak et al., 2012, was to assess the presence of ghrelin and its receptor in canine mammary cancer and to examine the effect of ghrelin on cancer cells reproduction, migration and invasion. (Majchrzak et al., 2012) The expression of ghrelin and its receptor in canine mammary cancer cells was examined using immunohistochemistry. The expression of ghrelin was detected in both benign (non-invasive) and malignant (invasive) canine mammary tumours. The analysis showed that ghrelin immunoreactivity was significantly lower in benign tumours comparing to the malignant tumours. (Majchrzak et al., 2012) It was also shown that the presence of the ghrelin receptor varies in tumours depending on how malignant they are. The GHS-R expression level was higher in lower severity cancers when compared to higher grade cancers. (Majchrzak et al., 2012)
The next step of the study was to assess the role of ghrelin in cancer cell biology. According to Majchrzak et al, 2012 the result of ghrelin given in high doses inhibits cancer cell reproduction, whereas given at low doses increases proliferation. The fact that the examined cancer cells respond differently to the various ghrelin doses may be related to differences in internal ghrelin production levels. The migration and invasion ability of the cancer cells was also significantly increased when treated with ghrelin. The distinction between ghrelin doses that stimulate cells proliferation or and doses that increase cells migration can be explained by the fact that these different cellular activities are facilitated by different cellular pathways. Hence, different ghrelin doses are required to activate various cellular pathways. Majchrzak et al., 2012 concluded that inhibition of ghrelin/GHS-R axis may be of potential use in the treatment mammary cancers in dogs in the future.
The effects of Ghrelin on Traumatic Brain Injury
Traumatic Brain injury (TBI) and haemorrhagic shock often accompany each other due to multiple injuries. (Manley et al., 2001) Traumatic brain injury (TBI) can lead to many complications including gastrointestinal dysfunction. Ghrelin is a “gut-brain” hormone with multiple functions including anti-inflammation and the prevention of cell death. The purpose of the study was to determine whether ghrelin lessens brain injury in a rat model of TBI and uncontrolled haemorrhage (UH). (Qi et al., 2012) To study this, brain injury was induced by dropping a 450g weight from 1.5 m onto a steel helmet attached to the skull of male adult rats. 45 min after TBI/UH, ghrelin or normal saline was administered. Several tests were carried out to access sensory, motor and reflex functions. These included balancing on a beam, forelimb placing test and hindlimb placing test. (Qi et al., 2012) Ghrelin or normal saline was subcutaneously administered daily for 10 days after TBI/UH in further groups of animals. The animals were then further monitored for 28 days to record body weight changes, severity of brain injury and survival. (Qi et al., 2012) The results as seen in the figure below showed that ghrelin improved sensory, motor and reflex functions, and decreased mortality after TBI/UH. Therefore, ghrelin has a great potential to be further developed as an effective resuscitation approach for the trauma victims with brain injury and severe blood loss. (Qi et al., 2012) Traumatic brain injury (TBI) remains a major public health problem globally. (Coronado et al., 2011) Roughly 53,000 Americans die each year following traumatic brain injury (Coronado et al., 2011). However, no drugs are currently approved for the treatment of TBI. At present, emergency care for TBI focuses on making sure the person has an adequate oxygen and blood supply, maintaining blood pressure, and preventing any further injury to the head or neck. (Mayo Clinic, online).
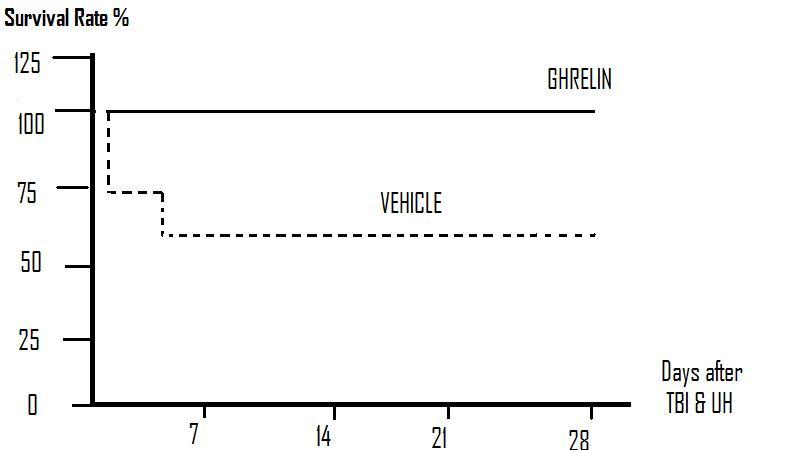
Figure 2: The survival rate after TBI and UH was 73% at day 1 and reduced to 66% from days 3–28. There was no mortality in ghrelin-treated TBI and UH animals during the 28 day observation period.
(Adapted from Qi et. al., 2012.)
References
Andrews Z.B., Erion D., Beiler R., Liu Z.W., Abizaid A., Zigman J., Elsworth J.D., Savitt J.M., DiMarchi R., Tschoep M., Roth R.H., Gao X.B., Horvath T.L. (2009): Ghrelin promotes and protects nigrostriatal dopamine function via an UCP2-dependent mitochondrial mechanism. The Journal of Neuroscience 29: (45), 14057 – 14065.
Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., Hemphill J.D., Centers for Disease Control and Prevention (2011): Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveillance Summaries 60: 1–32.
De Vriese C., Perret J., Delporte C., (2010): Focus on the short- and long-term effects of ghrelin on energy homeostasis. Nutrition 26: 579-584.
Dickson S.L., Hrabovszky E., Hansson C., Jerlhag E., Alvarez-Crespo M., Skibicka K.P., Molnar C.S., Liposits Z., Engel J.A., Egecioglu E., (2010): Blockade of central nicotine acetylcholine receptor signaling attenuate ghrelin-induced food intake in rodents. Neuroscience 171: (4), 1180–1186.
Hodos W., (1961): Progressive Ratio as a measure of reward strength. Science 134: 943–944.
Jerlhag E., Egecioglu E., Landgren S., Salome,N., Heilig M., Moechars D., (2009): Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci. U.S.A. 106: 11318–11323.
Jerlhag E., Landgren S., Egecioglu E., Dickson S.L., Jo¨rgen A., Engel J.A., (2010): The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol 45: 341-347.
King S.J., Isaacs A.M., O'Farrell E., Abizaid A., (2011): Motivation to obtain preferred foods is enhanced by ghrelin in the ventral tegmental area. Hormones and Behavior 60: 572–580.
Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K., (1999): Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature 402: 656–660.
Majchrzak K., Pawlowski K., Orzechowska E.J., Dolka I., Mucha J., Motyl T., Krol M. (2012): A role of ghrelin in canine mammary carcinoma cells proliferation, apoptosis and migration. BMC Veterniary Research 8: 170.
Majchrzak K., Szyszko K., Pawłowski K.M., Motyl T., Król M., (2012): A role of ghrelin in cancerogenesis. Polish Journal of Veterinary Sciences 15: 181–189.
Manley G., Knudson M.M., Morabito D., Damron S., Erickson V., Pitts L., (2001): Hypotension, hypoxia, and head injury: frequency, duration, and consequences. Archives of Surgery 136: 1118-23.
Mayo Clinic Staff. http://www.mayoclinic.com/health/traumatic-brain-injury/DS00552/DSECTION=treatments-and-drugs. [Online].
Perello M., Sakata I., Birnbaum S., Chuang J.C., Osborne-Lawrence S., Rovinsky S.A., (2010): Ghrelin increases the rewarding value of high-fat diet in an orexindependent manner. Biol Psychiatry 67: 880–6.
Richardson N.R., Roberts D.C.S., (1996): Progressive ratio schedules in drug self administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 66: 1–11.
Qi L., Xiaoxuan Cui X., Dong W., Barrera R., Nicastro J., Coppa G.F., Wang P., Wu R. (2012): Ghrelin Attenuates Brain Injury after Traumatic Brain Injury and Uncontrolled Hemorrhagic Shock in Rats. Molecular Medicine 18: 186-193
Wu, J.T., Kral, J.G. (2004): Ghrelin Integrative Neuroendocrine Peptide in Health and Disease. Annals of Surgery 238: 464 – 474.
